Abstract
The objective of this research was to induce a new animal model of osteonecrosis of the femoral head (ONFH) by microwave heating and then repair with tissue engineered bone. The bilateral femoral heads of 84 rabbits were heated by microwave at various temperatures. Tissue engineered bone was used to repair the osteonecrosis of femoral heads induced by microwave heating. The roentgenographic and histological examinations were used to evaluate the results. The femoral heads heated at 55°C for ten minutes showed low density and cystic changes in X-ray photographs, osteonecrosis and repair occurred simultaneously in histology at four and eight weeks, and 69% femoral heads collapsed at 12 weeks. The ability of tissue engineered bone to repair the osteonecrosis was close to that of cancellous bone autograft. The new animal model of ONFH could be induced by microwave heating, and the tissue engineering technique will provide an effective treatment.
Résumé
L’objectif de cette étude est de constituer un modèle animal d’ostéonécrose de la tête fémorale (ONFH) par micro traumatismes et guérison par greffe. Matériel et méthode: les deux têtes fémorales de 84 lapins ont été soumises à des micro traumatismes à des températures variables. L’os a été utilisé pour la réparation de cette ostéonécrose. Un examen radiologique et histologique ont également été réalisés de façon à évaluer les résultats. Résultats: la tête fémorale traumatisée à 55 iàC pendant 10 minutes montre des modifications de densité osseuse avec apparition de kystes sur les radiographies avec une ostéonécrose et une réparation qui apparaît entre 4 et 8 semaines. 69% des têtes fémorales sont effondrées à 12 semaines. La réparation osseuse a été réalisée par de l’os spongieux. Conclusion: ce nouveau modèle animal permet de réaliser des micro traumatismes et montre que la réparation cellulaire peut être réalisée de façon effective.
Introduction
Osteonecrosis of the femoral head (ONFH) remains an important unsolved problem in the field of orthopaedic surgery. Left untreated, osteonecrosis generally progresses to femoral head collapse and subsequent secondary osteoarthritis [13, 19]. The only effective treatment for the collapsed femoral head is total hip replacement, but some papers have showed a higher frequency of failure in the long term [6, 8]; therefore, much focus has been placed on modalities aimed at preserving the femoral head contour [21]. The preserving surgical alternatives may include core decompression and osteotomy, as well as nonvascularised and vascularised bone grafting. But these procedures are far from perfect, with failure and some complications [2, 22]. Recently, tissue engineered bone has been advocated for the treatment of osteonecrosis before collapse of the head [7, 20].
Orthopaedic management of ONFH remains problematic, partly because of the inability to systematically compare treatments in an animal model whose natural history parallels the human in terms of progression to femoral head collapse [19]. Many animal models have been developed to study pharmacologically- or surgically-induced osteonecrosis, which included dogs [12], rabbits [14], rats [15], goats [20], and emus [19]. But there is no reliable animal model of the early stages of ONFH for the evaluation of new therapeutic approaches. In this study, a new model of ONFH was induced by microwave heating for the first time, and then the mimic core decompression in patients with early Ficat stages of ONFH was carried out and treated with tissue engineered bone in this new model. The results showed that tissue engineered bone provided an effective treatment for ONFH.
Materials and methods
Establishment of animal model of ONFH
Animals and operation
The study was approved by Kunming Medical College IACUC. Eighty-four adult New Zealand rabbits (3.10 ± 0.27 kg) were randomly divided into group I (heated at 50°C for ten minutes), group II (heated at 55°C for ten minutes), or group III (heated at 60°C for ten minutes). A hole was drilled from subcapital to the centre of both femoral heads under sterile conditions. The diameter of the drill track was 1.5 mm and the depth was 8 mm under the cartilage. A microwave antenna (GW-92C multifunction microwave machine, Gelande Tianjin Medical Device Corporation) was set into the centre of the femoral head through the drill track to heat the three groups.
Radiological analysis
The femoral heads of 48 rabbits (16 rabbits for each group) underwent roentgenographic examination to show the cystic change and collapse at two, four, six, eight, ten, and twelve weeks.
Histological analysis
Twelve rabbits were randomly selected from each group and sacrificed at two, four, eight, and twelve weeks postoperatively. Both heads were collected, fixed, decalcified, embedded and cut into 5 μm slides, stained with hematoxylin and eosin, and evaluated with light microscopy.
Tissue engineered bone repair ONFH
Preparation of the biphasic bioactive ceramic bone (BBCB)
Fresh cancellous bone was obtained from porcine vertebral bone and cut into small blocks of 5 mm3. The raw bone blocks were processed with a series of physical and chemical methods [9], resulting in biphasic porous bioceramic bone (BPBB). Then 600 mg bone morphogenetic protein (BMP) solution was mixed with 96.00 IU basic fibroblast growth factor (bFGF). The 48 BPBBs were dipped into this mixture under pressure aspiration and freeze dried to prepare the biphasic bioactive ceramic bone (BBCB). Each BBCB contained 10 mg BMP and 1600IU bFGF; they were sterilised with ethylene oxide and preserved for study.
Preparation of the tissue-engineered bone
Bone marrow mesenchymal stem cells (BMSCs) were isolated from the femoral bone shaft of New Zealand rabbits [18]. The bone marrow was washed out from the femoral medullary cavity and centrifuged at 800 r/min for ten minutes, the upper liquid was discarded, and the rest of the cells were suspended in Dulbecco’s Modified Eagle’s Medium (DMEM, 100 U/mL penicillin, 100 μg/mL streptomycin, 15% foetal bovine serum, 0.01 mol/L HEPES), then incubated at 37°C in 5% CO2. When the primary cells grew to 80–90% confluence, they were harvested and passaged at the rate of 1:2. At the end of the third passage, BMSCs were harvested and cryopreserved until use. Then 24 BBCBs of 5 mm3 were placed in the six culture plates (four BBCBs in each plate). After four hours, the third passage of BMSCs were seeded onto BBCBs, and each BBCB was seeded 20 μL cells at the density of 1 × 107cells/mL. They then continued to culture in the CO2 incubator for another four hours to construct tissue engineered bone (BBCB/BMSCs).
Experimental animals and groups
Forty-eight New Zealand adult rabbits (3.10 ± 0.27 kg) were randomly divided into groups A, B, C, and D (12 rabbits for each group). Both femoral heads were induced into osteonecrosis at 55°C in ten minutes, which was found to be the optimum temperature and time. At six to eight weeks, the X-rays showed cystic change, and they were then repaired with different materials.
Implantation procedure
After curettage of the necrotic bone in the femoral head under sterile conditions, there were approximately 50% bone defects left in the head. The defects were then repaired with various materials in the different groups: group A with BBCB/BMSCs, group B with BBCB, group C with autogenous cancellous bone, and group D with nothing and used as the untreated control group. All the rabbits were raised separately postoperatively.
Radiological analysis
All femoral heads were evaluated with X-ray at two, four, eight, and twelve weeks.
Histological analysis
The rabbits were sacrificed at two, four, eight, and twelve weeks postoperatively. Both femoral heads were fixed, decalcified embedded, and cut into 5 μm slides. Stained with hematoxylin and eosin, the new bone formation area was evaluated from three slides of each specimen through the HPIAS-1000 image analysis system and light microscopy. The percentage of new bone formation was calculated by the following formula: area of new bone ÷ area of observation × 100%.
Statistical analysis
The femoral head collapse rates according to roentgenography examinations in the three groups at 12 weeks were compared with chi-square test. One-way analysis of variance (ANOVA) was used to compare the percentage of new bone formation (means±SD) in groups A, B, C, and D. A value of P < 0.05 was considered to be statistically significant.
Results
Establishment of animal model of ONFH
Radiological analysis
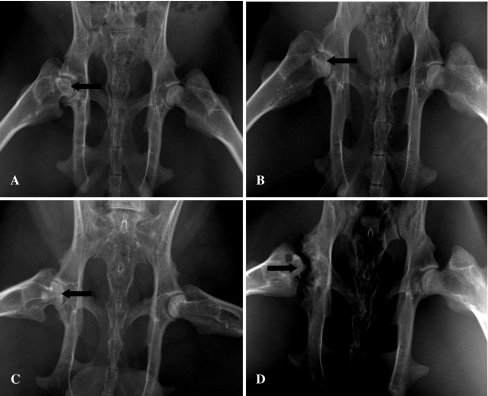
In groups I, II, and III, there were no changes in X-ray photography at two weeks postoperatively. In group I, some femoral heads showed lower density at four to six weeks, the cystic changes appeared at eight to ten weeks, while some heads collapsed at 12 weeks. In group II, the most femoral heads showed lower density at four weeks, and cystic changes appeared at six to eight weeks. More heads collapsed and flattened at 10–12 weeks. In group III, all the femoral heads showed abnormal changes at four to eight weeks, and some femoral heads developed severe deformity and collapse in X-ray photography at 10–12 weeks (Fig. 1A–D). The chi-square test results of collapse rate of the femoral head at 12 weeks (Table 1) showed that group III had the highest rate (93.8%, P < 0.05) and group I had the lowest (18.8%, P < 0.05), while the collapse rate in group II was in between (68.8%, P < 0.05).
Fig. 1.
Some femoral heads showed cystic degeneration or collapsed and flattened in X-ray photography after induced by microwave. A In group I, the cystic change (arrow) appeared in some femoral heads at eight to ten weeks. B In group II, the cystic change (arrow) appeared in some femoral heads at six to eight weeks. C In group II, some femoral heads collapsed and flattened (arrow) at 10–12 weeks. D In group III, some femoral heads experienced severe deformity and collapsed (arrow) at 10–12 weeks
Table 1.
Collapse rates of femoral head at 12 weeks postoperatively in three groups (n = 32)*
| Group | Collapsed | Non-collapsed | Collapse rate (%) |
|---|---|---|---|
| I | 6 | 26 | 18.8 |
| II | 22 | 10 | 68.8 |
| III | 30 | 2 | 93.8 |
* The collapse rate of the femoral head in group III was the highest (93.8%, P < 0.05), in group I the lowest (18.8%, P < 0.05), and group II was between them (68.8%, P < 0.05)
Histological analysis
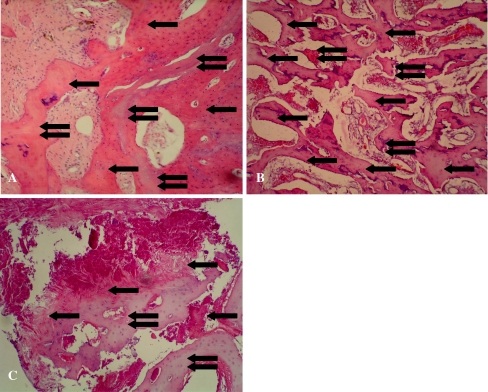
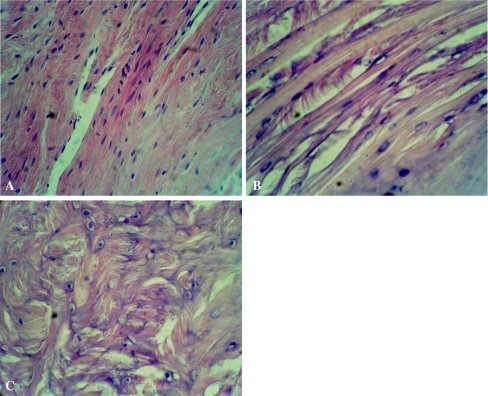
Different changes over time were found in the histological examination in three groups. At two weeks, some necrotic trabecular bone appeared and partially absorbed in groups I and II. In group III, more trabecular bone underwent necrosis and partially absorbed. At four weeks, the necrosis and repair occurred simultaneously in group II; some new bone formed with remaining necrotic trabecular bone debris. In group I, more new bone formed and surrounded the necrotic tissue. In group III, the trabecular bone developed even more necrosis with new formed bone (Fig. 2A–C). At eight weeks and 12 weeks in group I, the necrotic trabecular bones were completely absorbed and replaced by new trabecular bone; the cartilage maintained normal shape and layering. In group II, more necrotic trabecular bone appeared and then partially absorbed at eight weeks. At 12 weeks the bone repair stopped, the trabecular bone underwent necrosis and absorbed more, the cartilage matrix diminished, and the cartilaginous tissue layer was not clear and tidy. In group III, the bone repair stopped at eight weeks and the trabecular bone continued to undergo necrosis and absorbed, some empty space formed, the cartilage matrix reduced more, and the layer of cartilaginous tissue was deranged at 12 weeks (Fig. 3A–C).
Fig. 2.
Some necrotic tissue and new formed bone coexisted simultaneously at four weeks postoperatively. A In group I, more new bone(single arrow) surrounded the necrotic tissue (double arrows; hematoxylin and eosin stain; original magnification, ×100). B In group II, the necrosis and repair occurred simultaneously, some new bone formed (single arrow) with necrotic trabecular bone debris (double arrows) left (hematoxylin and eosin stain; original magnification, ×100). C In group III, the trabecular bone tissue went more necrosis (single arrow), and some new bone (double arrows) formed (hematoxylin and eosin stain; original magnification, ×100)
Fig. 3.
The cartilage showed different structure changes in the three groups in histology at 12 weeks postoperatively. A In group I, the cartilage remained normal shape and layering (hematoxylin and eosin stain; original magnification, ×100). B In group II, the cartilage matrix reduced and the cartilaginous tissue layer was not so clear and tidy (haematoxylin and eosin stain; original magnification, ×100). C In group III, the cartilage matrix reduced more and the layer of cartilaginous tissue was deranged (hematoxylin and eosin stain; original magnification, ×100)
Tissue engineered bone repair ONFH
Radiological analysis
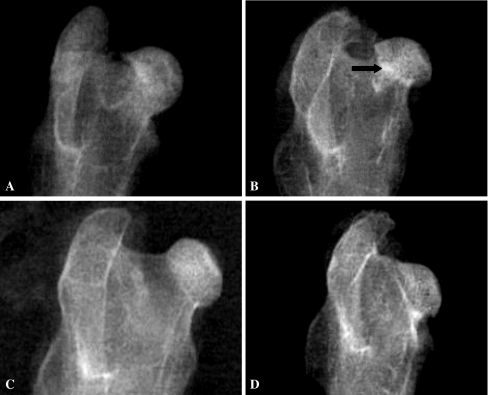
The density of grafted materials showed changes postoperatively in X-ray photography. At two weeks, the grafted materials showed high density in groups A and B, while the boundary between the grafted materials and host bone was clear. In group C, the density of grafted bone was close to the host bone with a clear boundary. In group D, empty defects existed in the femoral head. At four weeks, the density of grafted materials and boundary became lower in group A. In group B, the grafted materials still showed high density and the boundary was clear. In group C, the density of the grafted area was not uniform and the boundary became blurred. In group D, there were clear empty defects. At eight weeks, the density of grafted materials and the boundary become much lower in group A. The density of grafted materials was not uniform, and the boundary still existed in group B. The density of the grafted area close to host bone, and the boundary became much more blurred in group C. In group D, the empty defects still existed. At 12 weeks in group A, the density and structure of the grafted area were close to the host bone and there was no femoral head collapse. In group B, the grafted area showed little high density, at the boundary there was much blurring, and there was no femoral head collapse. In group C, the density and structure of the grafted area close to the host bone and there was no femoral head collapse. In group D, empty defects still existed and some femoral heads collapsed and deformed (Fig. 4A–D).
Fig. 4.
The density of the grafted area showed some different changes at 12 weeks postoperatively in X-ray photography. A In group A, the density and structure of the grafted area close to the host bone and there was no femoral head collapse. B In group B, the grafted area showed little high density (arrow) and there was no femoral head collapse. C In group C, the density and structure of the grafted area closed to the host bone and there was no femoral head collapse. D In group D, empty defects still existed in the femoral head and some femoral heads collapsed and deformed
Histological analysis
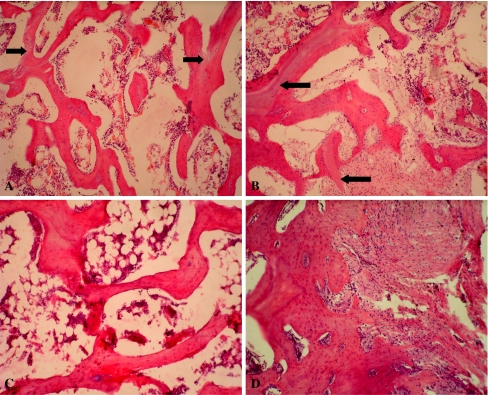
The grafted materials formed new bone and repaired the osteonecrosis area of the femoral heads postoperatively over time. At two weeks, more fibrous tissue and new blood capillaries grew well into the pores of BBCB in groups A and B, while some fibrous tissue grew into the pores of autogenous grafted bone in group C. In group D, some fibrous tissue grew into the defects. At four weeks, in group A, more new osteoid bone tissue and blood capillary grew in the pores of BBCB. In group B, some osteoid bone tissue and fibrous tissue grew in the pores of BBCB. In group C, some new bone formed by creeping substitution for the grafted bone. In group D, some fibrous tissue grew and filled the defects. At eight weeks, much more new bone and new blood vessels formed; the most BBCB was absorbed in group A. Some new bone formed and BBCB was partially absorbed in group B. Much more new bone was formed and was remodelling, and little grafted bone debris was left in group C. More fibrous tissue formed and filled the defects in group D. At 12 weeks, in group A, the new bone was remodelling and formed the formal bone structure, little BBCB debris was left. In group B, the most new bone formed and was remodelling; BBCB debris still remained. In group C, the new bone finished remodelling and its structure close to the normal bone. In group D, more fibrous tissue grew into the defects while the defects still existed (Fig. 5a-d).
Fig. 5.
The implanted materials almost completely formed new bone and repaired the osteonecrosis area at 12 weeks postoperatively. A In group A, the new bone was remodelling and formed the formal bone structure; little BBCB debris (arrow) was left (hematoxylin and eosin stain; original magnification, ×100). B In group B, the most new bone formed and was remodelling, BBCB debris (single arrow) was still left (hematoxylin and eosin stain; original magnification, ×100). C In group C, the new bone finished remodelling and its structure close to the normal bone (hematoxylin and eosin stain; original magnification, ×100). D In group D, much more fibrous tissue grew into the defects, the defects still existed (hematoxylin and eosin stain; original magnification, ×100)
New bone forming area analysis
The new bone forming areas of the four groups were analysed by ANOVA at four, eight, and 12 weeks postoperatively. There was no significant difference between groups A and C (P > 0.05), which showed that the bone forming ability of BBCB/BMSCs was close to that of autogenous cancellous bone. There was significant difference between groups A and B (P < 0.05), which showed that the bone forming ability of BBCB/BMSCs was superior to BBCB (Table 2).
Table 2.
Results of new bone forming area quantitative analysis (mean±SD) %
| Postoperative | Group A (n = 16) | Group B (n = 16) | Group C (n = 16) | Group D (n = 16) |
|---|---|---|---|---|
| 4 weeks | 7.41 ± 1.87 | 4.63 ± 2.48 | 7.67 ± 2.17 | 0.00 ± 0.00 |
| 8 weeks | 13.36 ± 2.12 | 8.52 ± 1.59 | 13.61 ± 2.91 | 0.00 ± 0.00 |
| 12 weeks | 38.56 ± 3.23 | 22.41 ± 2.39 | 39.75 ± 3.13 | 0.00 ± 0.00 |
The new bone forming area was analysed by ANOVA at four, eight, and 12 weeks postoperatively. The results showed that the new bone forming area had no significant difference between groups A and C (P > 0.05), but there was significant difference between groups A and B, groups B and C, groups A and D, groups B and D, and groups C and D (P < 0.05)
Discussion
Microwave heating can devitalise bone tumour cells and tissue, and it has been used to treat bone tumours in clinics for a long time [3, 4], but no microwave use has been attempted to date to induce animal models of ONFH. Microwave heating is internal heating; it can not generate ionisation, and the heating power can be focussed without heat diverging; thus, it was easily confined in the local tissue resulting in uniform tissue devitalisation within the microwave radiation field. Therefore, we were able to use microwave heating to induce animal models of ONFH successfully for the first time in this study.
A successful standard of an animal model of ONFH is that the cells die in the femoral head, which is followed with repair reaction [23]. In our study in group A, there was partial osteonecrosis at two weeks, but the necrotic tissue had been repaired at eight weeks and 12 weeks, the head collapse rate was the lowest, so this group could not be used as the optimal model. In group C, bone repair stopped at eight weeks and the trabecular bone underwent necrosis and absorbed continuously. The collapse rate was the highest in group C; this also could not be used as the optimal model. While in group B, there were some low density and cystic changes at four to eight weeks, the osteonecrosis and repair occurred simultaneously, the bone tissue underwent necrosis continuously with time, and 69% of the femoral head collapsed at 12 weeks; this is close to the clinical femoral head collapsed rate of 80% [11]. Thus, we found group B to be the optimal model; it was easy to control the heating temperature and time with low animal death rate, high success rate, and good repeatability. In effect, we could induce an animal model of ONFH by controlling the heating temperature and time precisely.
BMSCs is the most hopeful clinical application prospect seeding cell of tissue engineering [1], and BMP could induce and promote the osteogenic potential of BMSCs [5]. bFGF could also promote the proliferation and osteogenic potential of BMSCs [10], and bFGF can also augment the healing of bone grafts by enhancing vascularisation through the up-regulation of VEGF [17]. Thus, we seeded the BMSCs into BBCB which contained BMP and bFGF and co-culture to construction tissue engineered bone (BBCB/BMSCs). This was then implanted to repair the osteonecrosis model of the femoral head induced by microwave heating. In our study, we found that the ability to repair ONFH with BBCB/BMSCs was close to that of autologous cancellous bone grafting, which is the gold standard for repairing bone defects [16]. The main reason is that BMP can induce BMSCs into osteoblasts and form new bone to repair the osteonecrotic tissue, and the bFGF could promote the osteoblasts to proliferate and stimulate more blood capillaries to grow into the new bone and osteonecrosic tissue, thus supplying more nutrition to repair the osteonecrosic tissue. Our results indicate that there is a great potential to repair ONFH with tissue engineered bone, and the animal model induced by microwave heating is a very useful model for treatment study.
Acknowledgments
This study was supported by grants from NIAMS (no. 5R03AR052479), Aircast Foundation (no. P20RR024484), and Natural Science Foundation of Yunnan Province (nos. 2005C0070M and 2007C0003R).
Footnotes
This study was approved by Kunming Medical College IACUC, and the principles of laboratory animal care were followed during the animal experiments.
The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
References
- 1.Bajada S, Harrison PE, Ashton BA, Cassar-Pullicino VN, Ashammakhi N, Richardson JB. Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation. J Bone Joint Surg Br. 2007;89:1382–1386. doi: 10.1302/0301-620X.89B10.19103. [DOI] [PubMed] [Google Scholar]
- 2.Dailiana ZH, Gunneson EE, Urbaniak JR. Heterotopic ossification after treatment of femoral head osteonecrosis with free vascularized fibular graft. J Arthroplasty. 2003;18:83–88. doi: 10.1054/arth.2003.50000. [DOI] [PubMed] [Google Scholar]
- 3.Fan QY, Ma BA, Qlu XC, Li YL, Ye J, Zhou Y. Preliminary report on treatment of bone tumors with microwave-induced hyperthermia. Bioelectromagnetics. 1996;17:218–222. doi: 10.1002/(SICI)1521-186X(1996)17:3<218::AID-BEM7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Fan QY, Ma BA, Zhou Y, Zhang MH, Hao XB. Bone tumors of the extremities or pelvis treated by microwave-induced hyperthermia. Clin Orthop Relat Res. 2003;406:165–175. doi: 10.1097/00003086-200301000-00026. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa H, Kitoh H, Sugiura F, Ishiguro N. The effect of recombinant human bone morphogenetic protein-2 on the osteogenic potential of rat mesenchymal stem cells after several passages. Acta Orthop. 2007;78:285–292. doi: 10.1080/17453670710013816. [DOI] [PubMed] [Google Scholar]
- 6.Johnsen SP, Sorensen HT, Lucht U, Soballe K, Overgard S, Pedersen AB. Patient-related predictors of implant failure after primary total hip replacement in the initial, short- and long-terms. A nationwide Danish follow-up study including 36,984 patients. J Bone Joint Surg Br. 2006;88:1303–1308. doi: 10.1302/0301-620X.88B10.17399. [DOI] [PubMed] [Google Scholar]
- 7.Kawate K, Yajima H, Ohgushi H, Kotobuki N, Sugimoto K, Ohmura T, Kobata Y, Shigematsu K, Kawamura K, Tamai K, Takakura Y. Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs. 2006;30:960–962. doi: 10.1111/j.1525-1594.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 8.Koch PP, Tannast M, Fujita H, Siebenrock K, Ganz R. Minimum ten year results of total hip arthroplasty with the acetabular reinforcement ring in avascular osteonecrosis. Int Orthop. 2008;32:173–179. doi: 10.1007/s00264-006-0303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin FH, Liao CJ, Chen KS, Sun JS. Preparation of a biphasic porous bioceramic by heating bovine cancellous bone with Na4P2O7.10H2O addition. Biomaterials. 1999;20(5):475–484. doi: 10.1016/S0142-9612(98)00193-8. [DOI] [PubMed] [Google Scholar]
- 10.Lisignoli G, Fini M, Giavaresi G, Nicoli AN, Toneguzzi S, Facchini A. Osteogenesis of large segmental radius defects enhanced by basic fibroblast growth factor activated bone marrow stromal cells grown on non-woven hyaluronic acid-based polymer scaffold. Biomaterials. 2002;23:1043–1051. doi: 10.1016/S0142-9612(01)00216-2. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, Urbaniak JR. Osteonecrosis of the hip: management in the 21st century. Instr Course Lect. 2003;52:337–355. [PubMed] [Google Scholar]
- 12.Malizos KN, Quarles LD, Seaber AV, Rizk WS, Urbaniak JR. An experimental canine model of osteonecrosis: characterization of the repair process. J Orthop Res. 1993;11:350–357. doi: 10.1002/jor.1100110306. [DOI] [PubMed] [Google Scholar]
- 13.Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors. Clin Orthop Relat Res. 1998;355:S314–335. doi: 10.1097/00003086-199810001-00032. [DOI] [PubMed] [Google Scholar]
- 14.Motomura G, Yamamoto T, Miyanishi K, Jingushi S, Iwamoto Y. Combined effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits. Arthritis Rheum. 2004;50:3387–3391. doi: 10.1002/art.20517. [DOI] [PubMed] [Google Scholar]
- 15.Peled E, Bejar J, Zinman C, Boss JH. Vasculature deprivation-induced osteonecrosis of rats’ femoral heads associated with the formation of deep surface depressions. Arch Orthop Trauma Surg. 2007;127:369–374. doi: 10.1007/s00402-006-0258-7. [DOI] [PubMed] [Google Scholar]
- 16.Rosenwasser MP, Garino JP, Kierna HA, Michelsen CB. Long term follow up of thorough debridement and cancellous bone grafting of the femoral head for avascular necrosis. Clin Orthop Relat Res. 1994;306:17–27. [PubMed] [Google Scholar]
- 17.Rabie AB, Lu M. Basic fibroblast growth factor up-regulates the expression of vascular endothelial growth factor during healing of allogeneic bone graft. Arch Oral Biol. 2004;49:1025–1033. doi: 10.1016/j.archoralbio.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Savarino L, Baldini N, Greco M, Capitani O, Pinna S, Valentini S, Lombardo B, Esposito MT, Pastore L, Ambrosio L, Battista S, Causa F, Zeppetelli S, Guarino V, Netti PA. The performance of poly-epsilon-caprolactone scaffolds in a rabbit femur model with and without autologous stromal cells and BMP4. Biomaterials. 2007;28:3101–3109. doi: 10.1016/j.biomaterials.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Troy KL, Lundberg HJ, Conzemius MG, Brown TD. Habitual hip joint activity level of the penned EMU (Dromaius novaehollandie) Iowa Orthop J. 2007;27:17–23. [PMC free article] [PubMed] [Google Scholar]
- 20.Tang TT, Lu B, Yue B, Xie XH, Xie YZ, Dai KR, Lu JX, Lou JR. Treatment of osteonecrosis of the femoral head with hBMP-2-gene-modified tissue-engineered bone in goats. J Bone Joint Surg Br. 2007;89:127–129. doi: 10.1302/0301-620X.89B1.18350. [DOI] [PubMed] [Google Scholar]
- 21.Yuan B, Liu Z. Treatment of osteonecrosis of the femoral head: combination of operation and multiple cellular mediators. Med Hypotheses. 2007;68:502–505. doi: 10.1016/j.mehy.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Yoon TR, Song EK, Rowe SM, Park CH. Failure after core decompression in osteonecrosis of the femoral head. Int Orthop. 2001;24:316–318. doi: 10.1007/s002640000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto T, Hirano K, Tsutsui H, Sugioka Y, Sueishi K. Corticosteroid enhances the experimental induction of osteonecrosis in rabbits with Schwartzman reaction. Clin Orthop Relat Res. 1995;316:235–243. [PubMed] [Google Scholar]