Abstract
We analysed 17 patients with primary malignant bone tumour of the femur who underwent limb salvage surgery with the total femoral custom mega prosthesis during the period 1994–2008. The patients were in the age group of 12–73 years, with a mean age of 30.94 years. There were 14 males. The most common diagnosis was osteosarcoma. The average follow-up period was 54.05 months with the longest being 168 months. The average Musculoskeletal Tumour Society (MSTS) functional score was 66.6%. The two- to 14-year overall survival was 82.4%. Three patients died of disease and one patient required amputation. Complications encountered were deep infection and dislocation of the prosthesis.
Electronic supplementary material
The online version of this article (doi:10.1007/s00264-009-0737-x) contains supplementary material, which is available to authorized users.
Introduction
The femur is the most common site of malignant bone tumours. Limb salvage surgery is now an accepted modality of treatment for selected bone sarcomas of the extremities. With the advent of limb salvage surgery, the functional and oncological outcomes have proven to be better than limb ablation [11, 13, 17]. With the improving survival rates, the long-term behaviour and associated ratings of various methods of limb reconstruction have become an important issue. Total femoral replacement, although a major undertaking, provides a means of limb salvage.
The use of allografts [7, 10, 16] and allograft-prosthetic composites [7] for reconstruction of bony defects after resection have been well described. The complications associated with allografts or alloprosthetic composites limit their use in certain situations. Massive femoral tumours, tumours of the diaphysis and recurrent tumours after limb salvage surgery pose unique problems with respect to reconstruction options. The role of a total femoral endoprosthesis becomes vital in these situations. Progress in biomechanical engineering along with better surgical and chemotherapeutic techniques has increased the overall five-year survival rate after endoprosthetic replacement from 20% to 85%. These superior results along with minimal complications have established endoprosthetic replacement as a useful modality in the management of malignant bone tumours of the femur [14, 15].
In this article we present our experience over a decade with total femoral endoprosthetic replacement in malignant bone tumours.
Materials and methods
Between 1994 and 2006, 17 patients with primary malignant bone tumours involving part of or the entire femur underwent endoprosthetic reconstruction using total femoral custom mega prosthesis. There were 14 males and three females, with ages ranging from 12 to 73 years (average age of 30.94 years). Although the origin of the tumour was the proximal third of the femur in two cases, diaphysis in 12 cases and the distal third of the femur in three cases, all of our patients had involvement of more than one segment of the femur, which necessitated total femur replacement, as retaining any portion of the femur for anchorage of other prostheses would have proven inadequate and unstable. The most common diagnosis was osteosarcoma in 12 cases. Other diagnoses in our study were multiple myeloma in two cases and one case each of non-Hodgkin’s lymphoma, chondrosarcoma and Ewing’s sarcoma. Patient details are given in Table 1.
Table 1.
Patient details
| Patient no. | Age (y) | Gender | Histopath diagnosis | Stage | Pre-op chemo cycles | Pre-op RT | Follow-up (mo) | Functional resultsa (%) | Oncological results | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | M | Osteosarcoma | II A | 2 | No | 168 | 77 | NED | |
| 2 | 68 | M | Multiple myeloma | NA | 1 | Yes | 11 | 24 | Systemic spread | Died |
| 3 | 16 | M | Osteosarcoma | II A | 3 | No | 129 | 76 | NED | Dislocation |
| 4 | 30 | M | Non-Hodgkin | NA | 6 | No | 20 | 30 | Systemic spread | Died |
| 5 | 45 | M | Multiple myeloma | NA | 3 | No | 13 | 34 | Systemic spread | Died |
| 6 | 73 | M | Osteosarcoma | II A | 3 | No | 96 | 81 | NED | Infection |
| 7 | 23 | M | Osteosarcoma | II B | 3 | No | 68 | 78 | NED | |
| 8 | 63 | M | Chondrosarcoma | I A | 0 | No | 68 | 86 | NED | Dislocation |
| 9 | 12 | F | Ewings sarcoma | II A | 3 | Yes | 56 | 76 | NED | |
| 10 | 20 | M | Osteosarcoma | III A | 3 | No | 50 | 61 | Local recurrence | Amputation |
| 11 | 18 | M | Osteosarcoma | II A | 3 | No | 47 | 76 | NED | |
| 12 | 15 | M | Osteosarcoma | III A | 2 | No | 40 | 68 | NED | Infection |
| 13 | 19 | F | Osteosarcoma | III A | 3 | No | 38 | 77 | NED | |
| 14 | 15 | F | Osteosarcoma | II A | 3 | No | 36 | 66 | NED | |
| 15 | 37 | M | Osteosarcoma | II A | 3 | No | 27 | 76 | NED | |
| 16 | 20 | M | Osteosarcoma | II A | 3 | No | 26 | 76 | NED | |
| 17 | 25 | M | Osteosarcoma | III A | 3 | No | 26 | 71 | NED |
RT radiotherapy , NED no evidence of disease
aFunctional score according to the system of the Musculoskeletal Tumour Society [5]
All patients underwent standard radiographic evaluation and their disease was staged accordingly using the system of the American Musculoskeletal Tumour Society (Enneking’s system) [8]. Eight patients were in stage II A and three in stage III A. All patients also had clinical and radiological examination to detect any skip or concomitant lesions. The diagnosis was confirmed by needle biopsy in all patients. Pre-op chemotherapy was given in 16 patients and pre-op radiotherapy in two patients (one case of Ewing’s sarcoma and non-Hodgkin’s lymphoma).
Resection and reconstruction
Resection of the tumour was performed through the lateral approach in all patients. The vastus intermedius muscle was sacrificed. The sciatic nerve (both tibial and peroneal components) was identified and preserved. A sleeve of the vastus lateralis and the rectus femoris were preserved to aid in hip flexion. The hip was dislocated after excising the capsule. The proximal tibia was osteotomised and reamed for press fit insertion of the tibial component. After securing haemostasis and dissecting all soft tissues around it, the femur with tumour was removed in toto. Wide margins of resection were obtained in all of our patients.
The defect following resection was reconstructed with the total femoral custom mega prosthesis. The prosthesis was secured using PMMA cement in the tibia and a bipolar modular cup was used to articulate with the acetabulum. The glutei and remaining vastii were sutured to the illiotibial band. A 316L stainless steel prosthesis was used in 15 patients and a titanium prosthesis in two patients.
The prosthesis
The total femoral custom mega prosthesis replaces two different but related joints, namely, the hip and knee. This is an indigenously manufactured prosthesis, manufactured in Chennai, India. The dimensions of the prosthesis were calculated using radiographs of the normal limb. The prosthesis used was a single monoblock system with no provisions for lengthening. The size of the femoral head used was the standard 28 size along with an appropriately sized bipolar acetabular cup. The prosthesis includes a bipolar cup, tube in tube mid-shaft component and a rotating hinge knee. The design of the prosthesis has remained largely unchanged over the last 14 years. A figure depicting the prosthesis and its various components is given in Fig. 1.
Fig. 1.

Total femoral custom mega prosthesis
Results
The patients were evaluated monthly for the first three months, then biannually for the first year and annually thereafter. The functional and oncological outcomes were assessed at every visit with physical and radiological examination. The minimum follow-up was 26 months and the maximum follow-up was 168 months.
Functional outcome
Functional outcome of the patients was assessed using the modified rating system of the Musculoskeletal Tumour Society [5], the average of which was 66.6% (Table 1). Thirteen patients had no evidence of disease up to the last follow-up. Three patients had metastatic lesions and eventually died at 11, 20 and 13 months, respectively. Three patients (nos. 9, 12 and 14) had shortening ranging from 2–3.5 cm which was corrected by appropriate footwear. The remaining patients did not have any limb length discrepancies.
Complications
Two patients had deep infection which resolved with wound debridement and IV antibiotics. Two patients had dislocation of the prosthesis at the hip, unrelated to the site of the primary tumour which was treated by open reduction. There were no mechanical complications in our study. One patient had local recurrence and eventually underwent amputation at 18 months follow-up. He is now alive with a disease free interval of 61 months.
Survivorship analysis
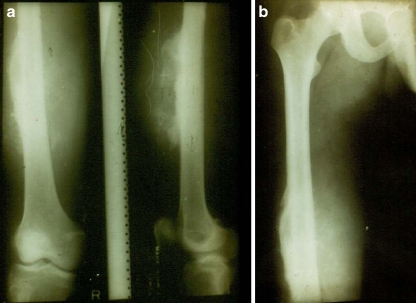
The survivorship analysis was done using the Kaplan-Meier survivorship method and found to be 82.4%. The preoperative and 14-year follow-up radiographs of a patient are shown in Figs. 2 and 3.
Fig. 2.
a,b Preoperative radiographs of patient no. 8
Fig. 3.
a Eight-year follow-up radiograph of patient no. 8. b Eight-year follow-up clinical picture of patient no. 8
Discussion
Use of the custom mega prosthesis for limb reconstruction has been well documented [14, 15]. Indications for total femoral reconstruction are rare [2–4], but this radical procedure will be mandatory in skip lesions or when there is a massive intramedullary extension of a diaphyseal sarcoma.
Reconstructive options such as osteoarticular allografts and allo-prosthetic composites have disadvantages of increased infection rates and nonunion (3.7–11%) [1, 5, 6, 9]. Other studies of prosthetic replacement of the femur have had increased infection rates (16%) when compared to 11% in our study [12]. Breakage of prosthesis was not encountered in our study, compared to other reports that quote as high as 1–4% [10]. As expected, there is a certain limitation of movement at the hip and knee.
This series presents custom mega prosthesis as a reliable method of reconstruction following resection of extensive malignant tumours of the femur. Advantages of this method are early functional recovery rate, relatively low complication rate and a high level of emotional acceptance. The knee functions well postoperatively while the bipolar hip is easier to insert and more stable than a conventional acetabular cup.
Conclusion
This study presents the total femoral custom mega prosthesis as a viable option for limb reconstruction. The success of this type of surgery depends on careful patient selection, meticulous surgical technique and better prosthetic design performed in a specialist centre.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video depicting the bending and rotating hinge mechanism of the Total femoral prosthesis.
References
- 1.Blunn GW, Wait ME, Lilley P, Walker PS (1991) The reactions of tissues to titanium wear generated from massive segmental bone defect prosthesis. In: Brown KLB (ed) Complications of limb salvage. Prevention, management and outcome. Montreal, ISOLS, pp 429–432
- 2.Nerubay J, Katznelson A, Tichler T. Total femoral replacement. Clin Orthop Rel Res. 1998;229:143–148. [PubMed] [Google Scholar]
- 3.Morris HG, Capanna R, Campanacci D, Ben M, Gasbarrini A. Modular endoprosthetic replacement after total resection of femur for malignant bone tumours. Intl Orthop. 1994;18:90–95. doi: 10.1007/BF02484417. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura S, Kusuzaki K, Murata H, Takeshita H, Hirata M. More than ten years follow-up of two patients after total femur replacement for malignant bone tumour. Intl Orthop. 2000;24:176–178. doi: 10.1007/s002640000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schindler OS, Cannon SR, Briggs TW, Blunn GW. Use of expandable total femoral prosthesis in children with malignant bone tumours. Clin Orthop Rel Res. 1998;357:157–170. doi: 10.1097/00003086-199812000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Scholz J, Dupka M, Rottger K. Metal-cancellous bone femoral component and total femoral endoprosthesis for surgical treatment of malignant tumours. Biomed Tech (Berl) 1991;36:320–324. doi: 10.1515/bmte.1991.36.12.320. [DOI] [PubMed] [Google Scholar]
- 7.Neider E, Engelbrecht E, Steinbrink K, Keller A. Modular system for the total femoral replacement of the femur-endo-model. Chirurg. 1983;54:391–399. [PubMed] [Google Scholar]
- 8.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Rel Res. 1986;204:9–24. [PubMed] [Google Scholar]
- 9.Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long term results of allograft replacement in the treatment of bone tumours. Clin Orthop. 1996;324:86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Unwin PS, Cannon SR, Grimer RJ, et al. Aseptic loosening in cemented custom mega prosthesis for malignant bone tumours of lower limb. J Bone Joint Surg [Br] 1996;78:5–13. [PubMed] [Google Scholar]
- 11.Davis A, Devlin M, Griffin A, Wunder J, Bell R. Functional outcome in amputation versus limb sparing of patients with lower extremity sarcoma: a matched case- control study. Arch Phys Med Rehabil. 1999;80(6):615–618. doi: 10.1016/S0003-9993(99)90161-2. [DOI] [PubMed] [Google Scholar]
- 12.Belthur MV, Grimer RJ, Carter SR, Tillman RM (2000) Infection after extensible endoprosthetic replacement for bone tumours in children. J Bone Joint Surg 82B:255–260
- 13.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb salvage treatment versus amputation for osteosarcoma of the distal end of femur. J Bone Joint Surg. 1986;68:1331–1337. [PubMed] [Google Scholar]
- 14.Natarajan MV, Sivaseelam A, et al. Distal femoral tumours treated by resection and custom mega prosthetic replacement. Intl Orthop. 2005;29(5):309–313. doi: 10.1007/s00264-005-0677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natarajan MV, Bose JC. Proximal femoral reconstruction with custom mega prosthesis. Intl Orthop. 2003;27:175–179. doi: 10.1007/s00264-003-0451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matejovsky Z, Jr, Matejovsky Z, Kofranek I. Massive allografts in tumour surgery. Int Orthop. 2006;30:478–483. doi: 10.1007/s00264-006-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heisel C, Kinkel S, Bernd L, Ewerbeck V. Megaprostheses for the treatment of malignant bone tumours of the lower limbs. Int Orthop. 2006;30:452–457. doi: 10.1007/s00264-006-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Video depicting the bending and rotating hinge mechanism of the Total femoral prosthesis.