Abstract
This study prospectively assessed the outcome of 134 cemented titanium stems and serum ion levels. The stems were polished (0.1 μm Ra) with circular cross section. At the end point, only one stem revision was performed for aseptic loosening, and two were planned due to subsidence greater than 5 mm. Non-progressive radiolucencies in zones 1 and 7 were observed in 16 hips at the cement-bone interface without osteolysis. Median serum titanium concentrations were below the detection limit (30 nmol/l) except in patients with failed stems. The overall stem survival rate was 97.7% at nine years, which is comparable to other series of cemented stems. The protective layer of titanium oxide coating the stem and a thick cement mantle may help resist aseptic loosening. In addition, satisfactory monitoring of the stem was reached using titanium serum level determination.
Résumé
Cette étude rapporte le suivi clinique prospectif d’une série de 134 tiges fémorales titane cimentées et l’évolution des taux de titane sérique. Les implants étaient en titane anodisé, lisses (0.1-microm Ra) et de section circulaire. Au recul moyen de 9 ans, le taux de survie était de 97,7%, ce qui est comparable aux autres séries de tiges cimentées. On notait une reprise pour descellement et deux révisions prévisibles pour un enfoncement supérieur à 5 mm. Des liserés non évolutifs étaient observés en zones 1 et 7 pour 16 hanches sans ostéolyse. La médiane du titane sérique était inférieure à la limite de détection (30 nmol/l) à l’exception des 3 échecs où les taux sont étaient nettement plus élevés. La couche protectrice d’oxyde de titane et l’épaisseur du manteau de ciment peuvent expliquer la résistance au descellement aseptique. Le taux de titane sérique semble intéressant pour la surveillance des tiges titanes cimentées.
Introduction
Titanium and its alloys are commonly used for orthopaedic implants such as screws, plates and spinal implants. This success is explained by the mechanical properties of these materials such as resistance to fatigue, low elasticity modulus to reduce stress shielding and biocompatibility [12]. Despite these properties, the use of polished cemented titanium stems is still debated [1, 25]. Titanium stems are thought to be highly sensitive to corrosion and micromotion leading to early aseptic loosening [15].
Like other metallic orthopaedic implants, titanium stems can release metal debris and ions that may also contribute to stem failure. Several previous studies investigated ion and particle release into the biological fluid from joint replacement, but to the best of our knowledge no series concerning cemented titanium stems are available [4, 6, 14].
In this prospective study, we hypothesised that satisfactory results could be reached with cemented polished titanium stems that have an oval cross section. In addition, the titanium ions released into the serum from the stems were measured up to the last point in time, in both unilateral and bilateral hip replacements, to monitor the behaviour of the stems.
Materials and methods
Demographic data From January 1997 to December 2000, we prospectively followed a consecutive series of 109 patients who underwent primary total hip replacements performed at our institution (134 joint replacements/25 bilateral). All patients provided informed consent for the long duration of this prospective clinical and biological study. They received complete information, including the goals and procedures for blood sampling.The inclusion criteria were strict; patients with orthopaedic titanium implants other than their hip stem, professional or dental exposure to titanium, previous conservative surgical hip procedures or renal disease were excluded. Additionally, all patients enrolled were under 60 years of age at the time of surgery. In our practice, this represented less than 20% of all primary total hip arthroplasties performed during the study period.The average follow-up was nine years, with follow-up ranging from seven to 11 years. The age at the time of arthroplasty ranged from 30 to 60 years (mean age: 54 years). The study included 53 women and 56 men; 68 right hips were treated and 66 left hips. Aetiologies were essentially symptomatic stage of osteonecrosis or primary arthritis. Patients were evaluated preoperatively and followed up with clinical and radiological examinations at regular intervals. Hip function results were rated according to the Harris hip score grading system in the preoperative period and at the latest follow-up [11].
Prostheses The cemented femoral stem was collarless, had an oval cross section and was straight (Alizé® Fournitures Hospitalières, Quimper, France). The implant was made of titanium alloy (TiA16V4) with a polished surface coated with titanium oxide (TiO2) obtained by anodisation [19]. The thickness of the titanium oxide layer was 1/10,000 μm. Six different sized stems were available.This modular stem was combined with a 28-mm Metasul® femoral head (Centerpulse-Zimmer, Warsaw, IN, USA) using a 12–14, 5°43 taper. Tapers were inspected individually before micro-threading, using an electro-pneumatic system (High pressure electronic pneumo transducer 150015, Solex Metrologie, La Boisse, France) for the taper angle (precision, 1 min), the diameter at the base and the diameter at the summit (precision, 1 μm). This inspection was counter-checked by a unit control on a tridimensional machine with 1-μm precision. The micro-threading was inspected using a projected side view with a 20× enlargement for the pace, profile and dimension of the threading (Pexit 14 VS Profile Profector, Pixit Dorsey Gage, Cambridge, UK). Compatibility between cones and heads was controlled and guaranteed by the manufacturers. All of the sockets were “sandwich” cemented Metasul® cups (Weber cups, Centerpulse-Zimmer, Warsaw, IN, USA). Palacos Genta® cement (Schering-Plough, Brussels, Belgium) was used to cement both components in all cases.
Surgical technique All procedures were performed following the standard procedure at our institution via an anterolateral approach on an orthopaedic table. In order to obtain a complete and thick cement mantle, the canal was over-reamed by 2 mm. Then the femoral canal was washed, brushed and distally occluded by a resorbable femoral plug (Synplug®, Zimmer, Warsaw, IN, USA. The cement was inserted retrogradely using a gun.
Radiological evaluation and clinical assessment Anteroposterior (AP) and lateral radiographs of each hip were available before and immediately after surgery, six weeks after discharge from the hospital and at three months, six months and one year and then yearly thereafter.We defined radiographic loosening of the cup as the presence of radiolucent lines measuring at least 2 mm according to DeLee-Charnley zones, axial cup migration of > 5 mm or > 5° of change in cup inclination on the AP radiographs of the pelvis [5].Parameters investigated on the femoral side included presence and progression of radiolucent lines according to Gruen et al., calcar resorption or atrophy, subsidence, periprosthetic osteolysis and cortical hypertrophy [9]. Loosening of the stem was defined as a migration exceeding 3 mm or a continuous radiolucent line greater than 2 mm. Heterotopic ossifications, if present, were graded according to Brooker et al. [3].
Titanium serum level determination In order to determine titanium release from the femoral stem, dosages were determined in two patient groups: those with unilateral replacements and those with bilateral replacements. Blood samples were taken just before implantation and at three months, six months and one year and then yearly thereafter until the last end point was reached.To avoid metallic contamination, blood samples were drawn using a sampling kit specifically dedicated to trace element determination: a needle for S-Monovette® (ref. 85.1162.400) and 7.5 ml S-Monovette® Lithium Heparin for Trace Metal analysis (ref. 01.1604.400) from Sarstedt (Marnay, France). Two Monovettes were sampled and numbered in sampling order. After centrifugation, aliquots of plasma were placed in metal-free plastic tubes (two tubes/sample) and frozen at −20°C. All metal measurements were performed on two samples in order to check for any contamination.Titanium was measured in blood plasma diluted by inductively coupled optical emission spectrometry (ICP-OES) on a JY24 spectrometer® (Jobin Yvon, Longjumeau, France). The detection limit (DL) was 30 nmol/l of plasma. Concentrations under the DL were set at half the DL value (15 nmol/l) to allow for statistical calculation by convention.Meanwhile, chromium and cobalt were also determined in the serum from the same samples. Cobalt and chromium were determined by electrothermal atomic absorption spectrometry on a simultaneous SIMAA 5100 spectrometer® UNTIL 2004, and then 6100 (Perkin Elmer, Courtabœuf, France). The DLs were 3 nmol/l for cobalt and 1 nmol/l for cromium. Seronorm Levels I and II (Sero, distributed by Ingen, Rungis, France) were analysed in each analytical run as internal quality controls. The serum titanium level was expressed in nmol/l (1 nmol/l = 47.9 ng/l = 0.0479 µg/l = 0.0479 ppb).
Statistical analysis
Survival analyses were calculated according to the Kaplan-Meier method. Loosened stems (revised or not) were considered as end points. For each point in time, the median as well as the 25th and 75th percentiles of serum titanium concentrations were calculated in the unilateral and bilateral replacement groups. Continuous data were tested for normal distribution using the Kolmogorov-Smirnov test. Normally distributed data were analysed with t-tests.
In order to test for any difference between small stems and larger ones regarding subsidences or radiolucencies, we selected two subgroups of stems within the unilateral hip replacement group. The first subgroup was composed of stems with a size of 1 to 3. In the second subgroup, the sizes of the stems ranged from 4 to 6. A non-parametric Wilcoxon test was performed to detect any difference in these two subgroups. Statistical significance was set at p < 0.05.
All statistical analyses were carried out with Prism 3 (GraphPad Software, Inc., San Diego, CA, USA).
Results
Complications
Complications included a deep vein thrombosis without pulmonary embolism in one patient. Early and recurrent dislocations in two patients were noticed. Another patient suffered one anterior dislocation after he fell on the stairs five years after implantation. The dislocation was reduced by orthopaedic manoeuvres without recurrence. No patient had an infection or a periprosthetic fracture.
Revisions
The two recurrent dislocations required revisions due to impingement between the titanium femoral neck and the edge of the chromium-cobalt insert of the cup, one month and six months, respectively, after the implantation. At the time of revision, the components were not loosened; black staining of the joint space was observed due to titanium release from femoral neck notches. In both cases, the patients’ serum titanium levels were high due to the release of titanium from the femoral neck lesions. All of the components were revised with the same polished cemented stem and a cemented polyethylene cup.
Eight revisions were performed for loosened Metasul® cemented cups with radiolucencies greater than 2 mm and osteolysis. In the first three cases, hips were revised using new metal-on-metal or polyethylene cups with respect to the femur. In view of the high cobalt-chromium serum levels and the local conditions in two revision cases, the bearing surfaces were converted to alumina-on-alumina using a new cementless stem. In the last three cases, the hips were revised using alumina-on-alumina with sleeved femoral heads (CeramTec, Plochingen, Germany) as the taper and the stem fixation were intact.
One case of progressive subsidence due to poor cement technique required a unipolar femoral revision using a cementless stem five years after implantation. One prosthesis was revised due to persistent and unexplained pain. At the time of the revision, we found no abnormalities apart from a massive and macroscopic metallosis of the joint. The cobalt serum level was increased more than 20-fold compared with the DL. The implants were changed, and new metal-on-metal bearing surfaces were again implanted, but did not resolve the symptoms.
Clinical results
The mean Harris hip score improved significantly (p < 0.05) from 39 (range: 15–68) preoperatively to 91 (range: 83–97) at the ultimate follow-up.
Radiological results
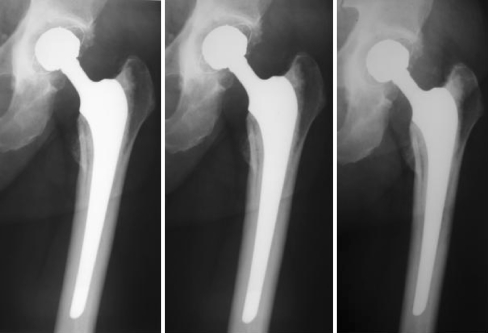
We observed four cases with osteolysis and 16 cases with progressive radiolucencies at the cement-bone interface on the acetabular side. Three stem subsidences due to poor cement technique were identified on the early postoperative radiographs. Two were less than 10 mm and slowly progressive with time requiring potential revision in the future (Figs. 1 and 2). The third was of more concern (subsidence greater than 10 mm) and was revised, as mentioned previously. It was associated with osteolysis in zone 6 of DeLee and Charnley [5].
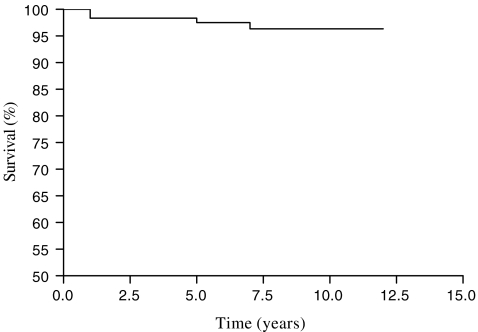
Fig. 1.
Survivorship of loosened stems as the end point
Fig. 2.
After 10 years of follow-up, typical evolution of the stem could be seen in this series showing controlled subsidence
Non-progressive femoral radiolucent lines were present in zone 1 at the cement-prosthesis interface in ten hips and had spread into zone 7 in six more hips. We did not observe a femoral hypertrophic reaction around the distal stem or calcar resorption.
No statistically significant difference (p > 0.05) was observed between small and large titanium stems regarding subsidences (p = 0.74) and radiolucency frequencies (p = 0.96).
Periarticular ossification, according to the method of Brooker et al. [3], was observed in 30% of the hips; 8% were types III and IV.
Serum titanium concentration
In both the unilateral and bilateral replacement groups, the median titanium concentration was constant and within range and always below the DL of 15 nmol/l (Tables 1 and 2).
Table 1.
Serum titanium levels in the unilateral hip replacement group
| Percentile | 3 months | 6 months | 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | 9 years |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25th | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Median | 15 | 15 | 15 | 25 | 15 | 15 | 15 | 15 | 15 | 15 | 20 |
| 75th | 15 | 27 | 25.5 | 57.5 | 63 | 77 | 21.5 | 15 | 27 | 25.5 | 40 |
Table 2.
Serum titanium levels in the bilateral hip replacement group
| Percentile | 3 months | 6 months | 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | 9 years |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25th | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 20 |
| Median | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| 75th | 15 | 15 | 15 | 15 | 15 | 15 | 24.5 | 25 | 19 | 35.5 | 32 |
Failed stems, as shown in Table 3, caused the highest titanium serum levels at their end point in the series, and titanium levels were much higher than the DL ranging from 196 to 1,274 nmol/l.
Table 3.
Serum titanium levels in the group with failed stems
| Type of stem failure | Ti level (nmol/l) at the end point | |
|---|---|---|
| Case 1 | Loosening (subsidence > 10 mm) | 392 |
| Case 2 | Impingement and dislocation | 1,274 |
| Case 3 | Impingement and dislocation | 876 |
| Case 4 | Loosening (subsidence > 5 mm) | 196 |
| Case 5 | Loosening (subsidence > 5 mm) | 252 |
Titanium serum levels remained below the DL in cases with loosened cups.
Survival rate
Overall stem survival (loosened, revised or not) was 97.7% at nine years (95% confidence interval: 95.4–99.5%) (Fig. 1).
Discussion
Use of cemented titanium alloy stems remains extremely controversial and has caused some surgeons to renounce them [1, 25]. Some series showed a large rate of early aseptic loosening, usually when the stem was rough and cemented [7, 15, 23]. Scholl et al. reported a 88% revision rate at a mean of 6.6 years due to loosened stems, and 30% of cases showed a significant osteolysis of the proximal femur [24].
Two factors are suggested that explain the early stem failure for the cemented titanium solutions. Firstly, the high elasticity of titanium and the excessive stresses in the mantle of the cement could lead to micromotion and debonding of the stem [12]. Micromovements at the cement-stem interface may be responsible for cement mantle breakage and generation of titanium debris inducing necrosis and osteolysis [23]. The failure risk could be higher for smaller stems due to their greater elasticity, and in men who are physically active, while titanium stems with a larger diameter may be more successful [15]. In our series, we did not observe this relationship. The second factor in early stem failure for the cemented titanium implants could be corrosion affecting the cemented, titanium alloy, stem surface. The implant could be deeply damaged by micromotion at the cement-stem interface leading to a progressive abrasion, which later induces a surface corrosion. Retrieval studies on loosened titanium alloy cemented stems report severe corrosion with associated typical crevices [25, 26]. Scholl et al. found such abrasions in corroded areas in all stem revisions with radiographic osteolysis at the same location [24]. Tissues were stained black with granuloma, including titanium wear particles. These findings suggest that corrosion could initiate an inflammatory foreign body reaction that is responsible for the osteolysis of the adjacent bone and aseptic loosening as seen with polyethylene wear particles.
The results of this prospective series are not in accordance with these observations. The overall survival rate of the stems was 97.7% at nine years, which makes the failure rate consistent with the survival rate at ten years of other cemented stems as reported in the Swedish Hip Registry [22]. In the past, satisfactory results have been reported in the literature with cemented titanium stems. Known as the “French paradox”, the stems had a rectangular cross section, filling the medullary canal of the femur as much as possible with the largest implant associated with a thin cement mantle [10, 18]. Survivorship ranged from 97% at ten years to 85% at 20 years in these series [20].
Irrespective of their design or surface coating, all stems have been shown to move inside of their cement mantle in the first years after implantation [17]. Obviously, a polished surface limits the abrasion due to micromotion and ipso facto reduces production of active biological debris. On the other hand, it is established that the roughness of the stem directly influences the amount of debris produced and the rate of femoral loosening [23]. Better results have been obtained after implantation of titanium alloy stems with a polished surface compared with a rough surface with regard to the aseptic loosening rate [18]. Resistance to abrasion and corrosion of titanium implants depends on a thin and highly protective surface of oxide [19]. The protective, passive, titanium oxide film (TiO2) is obtained by anodisation during the manufacturing process. This titanium oxide surface protects against exposure to air or other oxidising elements. If scratches occur, this passive layer is supposed to heal and restore itself.
In our series, we experienced three femoral subsidences that could be clearly explained by a poor cementing technique. The cementing technique did not follow the “French paradox” guidelines; our cement mantle had to be complete and greater than 2 mm thickness using a pressurised cementing technique. Studies focused on stress at the interfaces demonstrated minimal micromotion of the stem when the cement mantle was 3–4 mm thick around either a titanium or a more rigid, cobalt-chromium implant [18, 22]. Moreover, in these studies, high micromovement occurred when the cement was thinner than 2 mm and there was no difference in micromotion or debonding between a titanium and a cobalt-chromium stem [18, 22]. A finite-element analysis study identified factors influencing cement strains of the femoral component [8]. The authors of this study found that mantle thickness had the greatest effect on cement strains and suggested that a cement mantle thickness of 2.5–5.0 mm was optimal.
This series also investigated the matter of serum ion levels for monitoring the behaviour of cemented titanium femoral stems as previously performed with chromium or cobalt [2, 13].
Although a few studies had analysed titanium release from femoral stems previously, it was difficult to compare their results with ours because of differences in the type of stems (design, coating, etc.), fixation, mean follow-up and units [4, 6, 14]. In our study, medians were constant in range up to a mean follow-up of nine years in both unilateral and bilateral hip replacement groups and were always under the DL. The low serum concentrations observed are reassuring with regard to some concerns about potential titanium toxicity [16]. Although titanium is considered safe except for its potential osteolytic activity, it circulates throughout the body and particles have been found in hair, lungs, brain, urine and serum [16].
Several previous reports have shown significantly increased levels in cases of failed arthroplasty [4, 21]. Leopold et al. reported a failed patellar component in total knee arthroplasty, with elevated serum titanium at least 20 times higher than normal values [16, 21]. The highest titanium serum concentrations of our series were found in cases of mechanical complications such as stem fixation (subsidences) or neck impingement. These findings are in accordance with previous studies that monitored metal release. They could also emphasise the ability of polished and titanium oxide-coated surfaces to resist against corrosion and micromotion.
In conclusion, this series shows that satisfactory results can be achieved using cemented, polished titanium alloy stems with an oval cross section. A thick cement mantle combined with a polished, anodised surface may play a major role in minimising the debris source and the stress at the interfaces.
The high rate of acetabular loosening in this series of cemented cups questions the potential role of titanium debris. We could not find an argument for this hypothesis. We did not observe relationships between acetabular loosening and high titanium serum levels; head-neck modularity did not seem responsible. Both the head and taper were under the specifications and we did not observe significant lesions in the contact areas at the time of the revision. In addition, titanium serum levels found in this study showed satisfactory monitoring of the stems.
References
- 1.Barrack Rl. Early failure of modern cemented stems. J Arthroplasty. 2000;15:1036–1050. doi: 10.1054/arth.2000.16498. [DOI] [PubMed] [Google Scholar]
- 2.Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Serum cobalt levels after metal-on-metal total hip arthroplasty. J Bone Joint Surg Am. 2003;85-A:2168–2173. doi: 10.2106/00004623-200311000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed] [Google Scholar]
- 4.Buly Rl, Huo MH, Salvati E, Brien W, Bansal M. Titanium wear debris in failed cemented total hip arthroplasty: an analysis of 71 cases. J Arthroplasty. 1992;7:315–323. doi: 10.1016/0883-5403(92)90056-V. [DOI] [PubMed] [Google Scholar]
- 5.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 6.Dorr LD, Bloebaum R, Emmanual J, Meldrum R. Histologic, biochemical, and ion analysis of tissue and fluids retrieved during total hip arthroplasty. Clin Orthop Relat Res. 1990;261:82–95. [PubMed] [Google Scholar]
- 7.Ebramzadeh E, Normand PL, Sangiorgio SN, Llinas A, Gruen TA, McKellop HA, Sarmiento A. Long-term radiographic changes in cemented total hip arthroplasty with six designs of femoral components. Biomaterials. 2003;24:3351–3363. doi: 10.1016/S0142-9612(03)00187-X. [DOI] [PubMed] [Google Scholar]
- 8.Estok DM, 2nd, Orr TE, Harris WH. Factors affecting cement strains near the tip of a cemented femoral component. J Arthroplasty. 1997;12:40–48. doi: 10.1016/S0883-5403(97)90045-0. [DOI] [PubMed] [Google Scholar]
- 9.Gruen TA, McNeice GM, Amstutz C. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 10.Hamadouche M, Boutin P, Daussange J, Bolander ME, Sedel L. Alumina-on-alumina total hip arthroplasty: a minimum 18.5-year follow-up study. J Bone Joint Surg Am. 2002;84-A:69–77. [PubMed] [Google Scholar]
- 11.Harris WH. Osteolysis and particle disease in hip replacement. A review. Acta Orthop Scand. 1994;65:113–123. doi: 10.3109/17453679408993734. [DOI] [PubMed] [Google Scholar]
- 12.Head WC, Bauk DJ, Emerson RH., Jr Titanium as the material of choice for cementless femoral components in total hip arthroplasty. Clin Orthop Relat Res. 1995;311:85–90. [PubMed] [Google Scholar]
- 13.Jacobs JJ, Skipor AK, Campbell PA, Hallab NJ, Urban RM, Amstutz HC. Can metal levels be used to monitor metal-on-metal hip arthroplasties? J Arthroplasty. 2004;19(8 Suppl 3):59–65. doi: 10.1016/j.arth.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs JJ, Skipor AK, Patterson LM, Hallab NJ, Paprosky WG, Black J, Galante JO. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80:1447–1458. doi: 10.2106/00004623-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Jergesen HE, Karlen JW. Clinical outcome in total hip arthroplasty using a cemented titanium femoral prosthesis. J Arthroplasty. 2002;17:592–599. doi: 10.1054/arth.2002.32697. [DOI] [PubMed] [Google Scholar]
- 16.Kasai Y, Iida R, Uchida A. Metal concentrations in the serum and hair of patients with titanium alloy spinal implants. Spine. 2003;28:1320–1326. doi: 10.1097/00007632-200306150-00018. [DOI] [PubMed] [Google Scholar]
- 17.Kelley SS, Fitzgerald RH, Jr, Rand JA, Ilstrup DM. A prospective randomized study of a collar versus a collarless femoral prosthesis. Clin Orthop Relat Res. 1993;294:114–122. [PubMed] [Google Scholar]
- 18.Langlais F, Kerboull M, Sedel L, Ling RS. The ‘French paradox’. J Bone Joint Surg Br. 2003;85:17–20. doi: 10.1302/0301-620X.85B1.13948. [DOI] [PubMed] [Google Scholar]
- 19.Lappalainen R, Santavirta SS. Potential of coatings in total hip replacement. Clin Orthop Relat Res. 2005;430:72–79. doi: 10.1097/01.blo.0000150000.75660.ff. [DOI] [PubMed] [Google Scholar]
- 20.Mouel S, Allain J, Goutallier D. 10-year actuarial analysis of a cohort of 156 total hip prostheses of a cemented polished aluminum/polyethylene alloy (in French) Rev Chir Orthop Reparatrice Appar Mot. 1998;84:338–345. [PubMed] [Google Scholar]
- 21.Leopold SS, Berger RA, Patterson L, Skipor AK, Urban RM, Jacobs JJ. Serum titanium level for diagnosis of a failed, metal-backed patellar component. J Arthroplasty. 2000;15:938–943. doi: 10.1054/arth.2000.6632. [DOI] [PubMed] [Google Scholar]
- 22.Malchau H, Herberts P, Eisler T, Garellick G, Soderman P. The Swedish Total Hip Replacement Register. J Bone Joint Surg Am. 2002;84-A(Suppl 2):2–20. doi: 10.2106/00004623-200200002-00002. [DOI] [PubMed] [Google Scholar]
- 23.Mcgrath LR, Shardlow DL, Ingham E, Andrews M, Ivory J, Stone MH, Fisher J. A retrieval study of capital hip prostheses with titanium alloy femoral stems. J Bone Joint Surg Br. 2001;83:1195–1201. doi: 10.1302/0301-620X.83B8.9874. [DOI] [PubMed] [Google Scholar]
- 24.Scholl E, Eggli S, Ganz R. Osteolysis in cemented titanium alloy hip prosthesis. J Arthroplasty. 2000;15:570–575. doi: 10.1054/arth.2000.6618. [DOI] [PubMed] [Google Scholar]
- 25.Thomas SR, Shukla D, Latham PD. Corrosion of cemented titanium femoral stems. J Bone Joint Surg Br. 2004;86:974–978. doi: 10.1302/0301-620X.86B7.14812. [DOI] [PubMed] [Google Scholar]
- 26.Willert HG, Brobäck LG, Buchhorn GH, Jensen PH, Köster G, Lang I, Ochsner P, Schenk R. Crevice corrosion of cemented titanium alloy stems in total hip replacements. Clin Orthop Relat Res. 1996;333:51–75. [PubMed] [Google Scholar]