Abstract
Thirty-six female Sprague-Dawley rats were divided into two groups: oophrectomised (oestrogen deficient) rats and sham operated (oestrogen maintained) rats. Rats were sacrificed at six, ten, and 14 weeks. The rats were randomly chosen to have biomechanical evaluation on one side and histological evaluation on the other. Biomechanical testing was performed on an Instron machine to measure peak load. Histological sections were evaluated for cell proliferation, collagen-fibre organisation, fibroblast density, angiogenesis, inflammatory cells, chondroid and osseous metaplasia. Compared with the sham operated group, the oophrectomised group showed a lesser average maximum stress (42.9 N/m2 versus 33.7 N/m2) at six weeks, which was significant (p < .05). Succeeding weeks showed no significant biomechanical differences between the two groups. The sham operated group showed greater inflammatory response, which was statistically significant (p < 0.05), and also revealed greater cell proliferation and density. The results of this study revealed that endogenous oestrogen may improve healing of the Achilles tendon in rats.
Introduction
It is well known that women are more likely to sustain musculoskeletal injuries. Many studies reveal that sex differences in injury rates are apparent in ligaments. In vitro studies reveal that oestradiol has an inhibiting effect upon collagen formation in ligaments [11, 19]. Magnusson et al. showed that tendon adaptation to mechanic loading may differ between men and women [12]. However, current studies do not support gender-related differences in tendon injury rates [10] and little difference in ligament injuries [14].
In this study, it was postulated that serum oestrogen levels may play a role in sex-differentiated tendon healing and biomechanic properties in the tendon. We investigated whether an oestrogen deficient status affected tendon healing and tendon mechanical properties.
Materials and methods
Study design
Appropriate permits for the study were obtained and the experiments conformed to the Council Directive of the European Community. Adult female Sprague-Dawley rats were provided by the Medical and Surgical Research Centre of our University. Rats were acclimatised to caged laboratory conditions and were allowed to feed with a standard diet and water ad libitum. The room temperature and humidity were maintained at 20–24°C and at 50–60%, respectively. The light cycle was fixed at 12 hours.
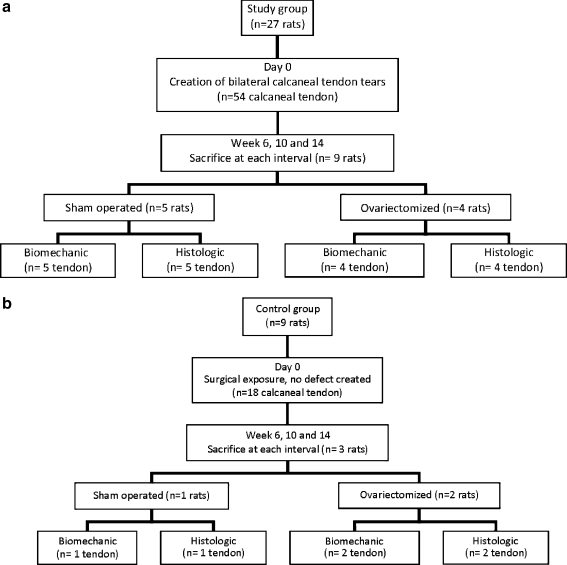
Thirty-six rats with a mean age of 12 months and mean weight of 289.4 ± 46.1 grams were used. All rats were divided randomly into two groups: oophrectomised (oestrogen deficient, n = 18) and sham operated (oestrogen maintained, n = 18). Complete bilateral Achilles tendon tears were surgically created in 27 rats (54 Achilles with tendon tears). The sham group consisted of nine rats (18 Achilles with intact tendons). The study design is demonstrated in Fig. 1a,b. The rats were randomly divided into three equal groups (12 rats in each group). Finally, rats were sacrificed at six, ten, and 14 weeks.
Fig. 1.
Study design demonstrated in (a) study groups (b) control groups
Surgical intervention
The first day of the study, all rats were anaesthetised with a combined ketamine (50 mg/kg) and xylazine (5 mg/kg) intra-peritoneal injection. Rats were shaved and aseptically prepared. In the oophrectomy group, bilateral ovariectomies were performed. The sham operated group underwent the same surgical procedure exposing the ovaries but keeping them in the same position. In addition, Achilles tendon tears were performed in the rats. Bilateral surgical exposure involved a 1-cm-long longitudinal midline incision in the skin overlying the Achilles tendon and central to the calcaneus insertion. Full-thickness tendon defects were created transversely at the level of the calcaneal insertion. A gap of approximately 1 cm occurred between the two ends of the Achilles tendons when the ankle was brought to a neutral position after being cut by the Achillotomy technique, and the free ends of the tendon were not sutured. The control group underwent the same operative exposure of the Achilles tendon without tear creation. The skin was closed with absorbable 3-0 monocryl suture (Ethicon).
The animals were sacrificed with an intraperitoneal injection of 200 mg/kg thiopental at six, ten, and 14 study weeks. Each rat was evaluated biomechanically on one side and histologically on the other side. The sides were randomly determined.
Each specimen was carefully dissected and the muscle was removed at the musculotendinous junction, leaving the tendinous part and calcaneus intact.
Efficacy of ovariectomy
Rats become sexually mature at age six weeks and enter menopause between the ages of 15 and 18 months [3]. From the onset of sexual maturity up to menopause, the mean cycle length in the female rat is four days [4, 13]. Oestrogen has a half-life of between three and eight hours. According to these records, after the oophrectomy in the first study group, sacrifice was planned at six weeks. In addition, the concentration of serum 17B-oestradiol was analysed by radioimmunoassay.
Macroscopic assessment
Rats were sacrificed and surgical exposures were made during each study week.
Biomechanical testing
Specimens were stored at −20°C until testing. During biomechanical assessments, all samples were to test Achilles tendons tensile strength using electromagnetic testing equipment (Instron Tensometer, Model 8874, Automated Materials Testing System, Instron Corporation, Canton, Massachusetts, USA). The room temperature and humidity were controlled at 20°C ± 1 and at 40%, respectively, during biomechanical analysis. The fresh-frozen specimens were thawed at room temperature on the day of biomechanical analysis and kept moist with normal saline throughout testing. The calcaneus was placed on a model stone (Amberok, dental model stone) with its long axis in the horizontal plane. Specimens were prepared in the same shape and were kept in position for at least 12 minutes and allowed to set. The proximal end of the Achilles tendon was fixed between two pieces of sandpaper and clamped vertically in a custom-made cryoclamp. The system was loaded to 250 N with a displacement rate of 5 mm/min. The ultimate loads (loads at failure) were determined for specimens.
Histology
Tissue samples were fixed in 10% neutral buffered formalin overnight then dehydrated with alcohol. The fixed tissue was processed, embedded in paraffin, and sectioned at 3 micrometers. Finally, tissue sections were stained with haematoxylin-eosin according to standard protocols for evaluation of inflammation, fibroblast density, vascularity, chondroid and osseous metaplasia. To investigate collagen fibre orientation, Masson-Trichrome stain was used. Proliferating cell nuclear antigen (PCNA) assay was performed to measure cell proliferation.
Fibroblast density, inflammation, vascularity and collagen fibre orientation were graded on a four-point grading system on the following scale: 0 indicates a normal appearance, 1 indicates a slightly abnormal appearance, 2 a moderately abnormal appearance and 3 a markedly abnormal appearance.
The presence or absence of chondroid and osseous metaplasia was recorded, together with percentage of PCNA positivity determined.
For each staining technique, two slides were prepared. The area of specimen showing the most advanced pathological changes was selected, and the worst possible results for each slide were used in this study. Slides were examined by the same pathologist under light microscope (Olympus Bx50) and read blindly.
Statistical analysis
Data were evaluated using SPSS statistical software package 13.0 for Windows (SPSS Inc, Chicago, IL). Descriptive statistics were calculated, including frequencies, mean, standard deviation, and minimum and maximum values. Mann-Whitney U test was applied to biomechanical analysis. Because the data value is continuous, the Mann-Whitney U test was used to determine the significance of the percentage of cell proliferation and the histological sum grade. The chi-square test was used to ascertain the association between the study groups histological category during each study week. Results were considered statistically significant at p < .0.05.
Results
Evaluation of serum oestrogen levels
The mean serum 17B-oestradiol levels were 25.5 pg/ml (range 15–42 pg/ml) on the first day of the study. Serum oestrogen levels were <10 pg/ml before sacrifice at six, ten and 14 weeks. The decrease of oestradiol level corresponded with the previous report [15].
Macroscopic assessment
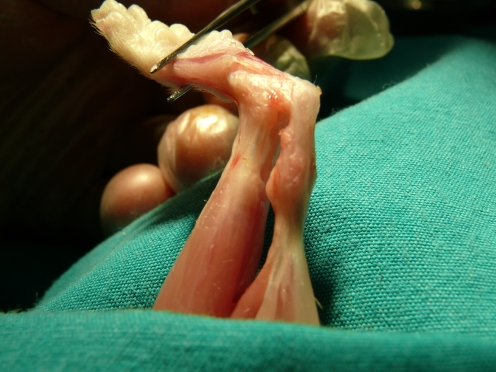
Regardless of the study week, the gross appearance of the tendon after the defect was similar in all rats. It showed intact tendon with thickened appearance (Fig. 2). No macroscopic differences were seen with or without oophrectomy.
Fig. 2.
Macroscopic assessment of intact tendon with thickened appearance
Biomechanical results
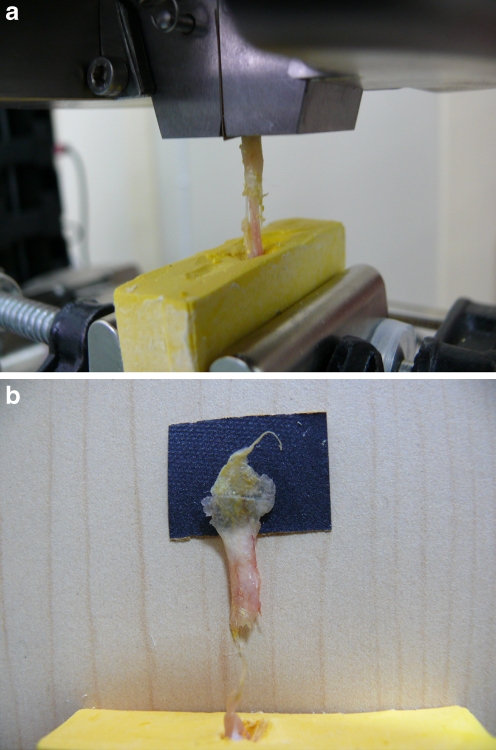
During biomechanical testing, all specimens were tested successfully. The expected load deformation curves were seen in tendon tissue. The tendon failed at mid-substance in both the study and control groups (Fig. 3a,b). The biomechanical results of the study group with Mann-Whitney U test showed that at six weeks, tendon ultimate loads to failure were significantly lower in the oophrectomised group than in the sham operated group (p < .0.05). In the succeeding study weeks, ultimate loads to failure were obtained very similarly and no significant differences were found between the groups (p > .0.05). All of these values were inferior to the control group (Table 1).
Fig. 3.
a Set-up for biochemical analysis of the Achilles tendon. b At the completion of testing, the tendon visibly ruptured at midsubstance
Table 1.
Ultimate loads ± standard deviations (in Newtons) to failure
| Groups | Six weeks | Ten weeks | Fourteen weeks |
|---|---|---|---|
| Study groups | |||
| Sham operated | 42.9 ± 8.5 | 32.8 ± 3.7 | 33.9 ± 8.9 |
| Oophrectomised | 33.7 ± 8.1 | 33.8 ± 11.5 | 34.1 ± 10.2 |
| Control groups | |||
| Sham operated | 58.2 | 44.4 | 50 |
| Oophrectomised | 45.8 ± 9.0 | 51.3 ± 1.1 | 55.2 ± 1.4 |
Histological results
The distribution of the histological grades for fibroblast density, inflammation, vascularity and collagen fibre orientation of the study groups are shown in Table 2. Within each specific category at every study week, the chi-square test showed association between the oophrectomised and sham operated groups; in all the variables there were no significant differences (p > .0.05).
Table 2.
Distribution of tendon histological grades
| Six weeks | Ten weeks | Fourteen weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histological grade | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Sham operated group | ||||||||||||
| Fibroblast density | 2 | - | 2 | 1 | - | - | 4 | 1 | - | - | 4 | 1 |
| Inflammation | - | 1 | 3 | 1 | - | 1 | 2 | 2 | - | 5 | - | - |
| Vascularity | 2 | 1 | 2 | - | - | 1 | 3 | 1 | 1 | 3 | 1 | - |
| Collagen fibre orientation | 1 | 3 | 1 | - | 1 | 3 | 1 | - | - | 3 | 2 | - |
| Oophrectomised group | ||||||||||||
| Fibroblast density | 3 | 1 | - | 2 | 2 | - | - | 1 | 3 | - | ||
| Inflammation | 2 | 1 | 1 | - | 1 | 3 | - | - | 2 | 2 | - | - |
| Vascularity | - | 1 | 3 | - | - | 3 | 1 | - | 1 | 3 | - | - |
| Collagen fibre orientation | 1 | 2 | 1 | - | - | 1 | 3 | - | - | - | 4 | - |
The mean histological sum-grade and standard deviations of both study groups are shown in Table 3. The Mann-Whitney U test was used to determine the sum-grade difference between the oophrectomised and sham operated study groups at each histological category. The results revealed the mean histological sum grade of the inflammatory response of the no-oophrectomy study group was greater than the oophrectomised groups. The mean histological sum grade of collagen fibre orientation was greater in the oophrectomised groups than in the sham operated groups. Over time, collagen contents decreased in the presence of oestrogen. Only inflammatory response difference between the study groups was statistically different (p < .0.05).
Table 3.
Summary of the mean histological sum grades
| Parameter | Sham operated group | Oophrectomised group |
|---|---|---|
| Fibroblast density | 1.8 ± 0.7 | 1.8 ± 0.4 |
| Inflammation | 1.7 ± 0.6 | 0.7 ± 0.1 |
| Vascularity | 1.3 ± 0.6 | 1.3 ± 0.5 |
| Collagen fibre orientation | 1.1 ± 0.2 | 1.6 ± 0.5 |
| Total tendon histologic grade | 6.0 ± 1.2 | 5.3 ± 0.4 |
The worst grading result was used for each situation. Values are given as mean grade ± standard deviation
In the sham operated group, the results revealed 12 of the 15 specimens (80%) with chondroid metaplasia and ten of the 15 specimens (66.7%) with osseous metaplasia. In the oophrectomised group, results showed ten of the 12 specimens (83.3 %) with chondroid metaplasia and eight of the 12 specimens (66.7%) with osseous metaplasia. None of the control specimens showed chondroid or osseous metaplasia. According to the chi-square test, neither the presence of osseous metaplasia nor chondroid metaplasia were significant between the study groups (p > 0.05).
Cellular proliferation was higher in the sham operated groups than in the oophrectomised groups during the last two study weeks. Cellular proliferation decreased over time in both groups. The Mann-Whitney U test did not show significant differences between the groups studied (p > .0.05).
Discussion
Tendinopathies are common complications in sports- and work-related medicine. A number of factors such as ergonomic considerations, genetics, gender, and fitness levels could play a role in either the initiation of such tendinopathies or their progression. Tendon disorders are responsible for significant morbidity and disability lasting several months despite appropriate management. The management of tendon injury is a challenge for physicians. Therefore, the factors that affect tendon healing are extremely significant [17].
Previous studies have revealed tendon healing can be stimulated by several growth factors (e.g. platelet-derived growth factor [PDGF], transforming growth factor [TGF]-beta, insulin-like growth factor [IGF]-1, vascular endothelial growth factor [VEGF], bone morphogenetic proteins [BMPs] like growth differentiation factor [GDF]-5, -6, -7) or by a thrombocyte concentrate (PRP) [1].
There is increased awareness of gender vulnerability of certain tissues that can lead to pathological stages. Oestrogen has been, to date, the primary hormone investigated to play a potential role in musculoskeletal disease and injury. Research has identified oestrogen as being important to the homeostasis of many musculoskeletal tissues, often with an incomplete understanding of the role this hormone plays on tissue structure and function, since sex and collagen content are known to affect mechanical properties of some connective tissue [12]. It is reasonable to believe these factors might be causing variability in the properties of tendons.
It is well known that tendons have the highest tensile strength of any soft tissue because their main constituent is collagen, one of the strongest proteins, and collagen fibres are arranged parallel to the direction of the tensile force [9]. A few studies have investigated the biomechanical properties of tendons in response to gender differences. Fisher et al. showed that collagen concentration decreases after treatment with oestrogen in rat tail tendons [5]. Bryant et al. revealed that long-term exposure to attenuated oestrogen in users resulted in a decrease in Achilles tendon strain in humans, which was thought to be attributed to the effects of endogenous oestrogen on collagen synthesis [2]. Contrary to this, Goldstein et al. reported that the tendons from females were found to be significantly stiffer in uniaxial tension than those from males [6]. The results from this study showed that collagen contents were associated with oestrogen status, although tendon mechanical properties were not related to oestrogen deficient status.
Experiments may indicate that sex hormones impact on tendon cell biology and modify neuropeptide responsiveness. Hart et al. demonstrated the preservation of mRNA for oestrogen receptors in a number of tendons from the rabbit. In addition, oestrogen receptors have been identified in human tendons, although their function is not clear [7]. When oestrogen is withdrawn, there is a negative impact on many tissues that can lead to increased disease. There is debate regarding the healing potential of the tendon tissue. Interestingly, inflammatory responses are attenuated in many females during pregnancy [8]. Similarly, this study’s results demonstrate that cell proliferation and inflammatory response are greater in the presence of endogenous oestrogen. Unlike previously published studies, it demonstrates that supraphysiological levels of oestrogen treatment decreased cell proliferation [16, 18].
Our results show that there is not a direct effect of endogenous oestrogen deficiency of Achilles tendon mechanical properties in the rat model. On the contrary, it was concluded that oestrogen may improve tendon healing. To our knowledge, this is the first experimental study to investigate the effect of endogenous oestrogen in both tendon healing and tendon biomechanical properties. Further clinical and experimental studies are clearly necessary to definitively determine the oestrogen influence on tendon healing and mechanical properties.
References
- 1.Aspenberg P. Stimulation of tendon repair: mechanical loading. GDFs and platelets. A mini-review. Int Orthop. 2007;31(6):783–789. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryant AL, Clark RA, Bartold S, Murphy A, Bennell KL, Hohmann E, Marshall-Gradisnik S, Payne C, Crossley KM. Effects of estrogen on the mechanical behavior of the human Achilles tendon in vivo. J Appl Physiol. 2008;105(4):1035–1043. doi: 10.1152/japplphysiol.01281.2007. [DOI] [PubMed] [Google Scholar]
- 3.Durbin PW, Williams MH, Jeung N, Arnold JS. Development of spontaneous mammary tumors over the life-span of the female Charles River (Sprague-Dawley) rat: the influence of ovariectomy, thyroidectomy, and adrenalectomy-ovariectomy. Cancer Res. 1966;26(3):400–411. [PubMed] [Google Scholar]
- 4.Evans HM, Long JA. Characteristic effects upon growth, oestrus and ovulation Induced by the intraperitoneal administration of fresh anterior hypophyseal substance. Proc Natl Acad Sci USA. 1922;8(3):38–39. doi: 10.1073/pnas.8.3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer GM. Comparison of collagen dynamics in different tissues under the influence of estradiol. Endocrinology. 1973;93(5):1216–1218. doi: 10.1210/endo-93-5-1216. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein SA, Armstrong TJ, Chaffin DB, Matthews LS. Analysis of cumulative strain in tendons and tendon sheaths. J Biomech. 1987;20(1):1–6. doi: 10.1016/0021-9290(87)90261-2. [DOI] [PubMed] [Google Scholar]
- 7.Hart DA, Archambault JM, Kydd A, Reno C, Frank CB, Herzog W (1998) Gender and neurogenic variables in tendon biology and repetitive motion disorders. Clin Orthop Relat Res (351):44–56 [PubMed]
- 8.Hart DA, Reno C. Pregnancy alters gene expression in normal synovium: influence of age and parity. J Rheumatol. 1999;26(8):1775–1784. [PubMed] [Google Scholar]
- 9.Hoffmann A, Gross G. Tendon and ligament engineering in the adult organism: mesenchymal stem cells and gene-therapeutic approaches. Int Orthop. 2007;31(6):791–797. doi: 10.1007/s00264-007-0395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lian OB, Engebretsen L, Bahr R. Prevalence of jumper's knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33(4):561–567. doi: 10.1177/0363546504270454. [DOI] [PubMed] [Google Scholar]
- 11.Liu SH, Al-Shaikh RA, Panossian V, Finerman GA, Lane JM. Estrogen affects the cellular metabolism of the anterior cruciate ligament. A potential explanation for female athletic injury. Am J Sports Med. 1997;25(5):704–9. doi: 10.1177/036354659702500521. [DOI] [PubMed] [Google Scholar]
- 12.Magnusson SP, Hansen M, Langberg H, Miller B, Haraldsson B, Westh EK, Koskinen S, Aagaard P, Kjaer M. The adaptability of tendon to loading differs in men and women. Int J Exp Pathol. 2007;88(4):237–240. doi: 10.1111/j.1365-2613.2007.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandl AM, Zuckerman S. The reaction of the ovaries and adrenal glands of female rats to ovarian and muscle homografts. J Endocrinol. 1951;7(4):344–348. doi: 10.1677/joe.0.0070344. [DOI] [PubMed] [Google Scholar]
- 14.Mountcastle SB, Posner M, Kragh JF, Taylor DC. Gender differences in anterior cruciate ligament injury vary with activity: epidemiology of anterior cruciate ligament injuries in a young, athletic population. Am J Sports Med. 2007;35(10):1635–1642. doi: 10.1177/0363546507302917. [DOI] [PubMed] [Google Scholar]
- 15.Persky AM, Green PS, Stubley L, Howell CO, Zaulyanov L, Brazeau GA, Simpkins JW. Protective effect of estrogens against oxidative damage to heart and skeletal muscle in vivo and in vitro. Proc Soc Exp Biol Med. 2000;223(1):59–66. doi: 10.1046/j.1525-1373.2000.22308.x. [DOI] [PubMed] [Google Scholar]
- 16.Seneviratne A, Attia E, Williams RJ, Rodeo SA, Hannafin JA. The effect of estrogen on ovine anterior cruciate ligament fibroblasts: cell proliferation and collagen synthesis. Am J Sports Med. 2004;32(7):1613–1618. doi: 10.1177/0363546503262179. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Jt Surg Am. 2005;87(1):187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 18.Wentorf FA, Sudoh K, Moses C, Arendt EA, Carlson CS. The effects of estrogen on material and mechanical properties of the intra- and extra-articular knee structures. Am J Sports Med. 2006;34(12):1948–1952. doi: 10.1177/0363546506290060. [DOI] [PubMed] [Google Scholar]
- 19.Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA (2001) Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop Relat Res (383):268-281 [DOI] [PubMed]