Abstract
Background
The role of the p38 mitogen-activated protein (MAP) kinase in acute pancreatitis pathogenesis is controversial. We hypothesize that p38 plays a role in regulating NF-κB activation in exocrine pancreatic cells.
Methods
AR42J cells incorporating an NF-κB-responsive luciferase reporter, with and without adenoviral transduction of DNp38, were stimulated with cholecystokinin (CCK) or tumor necrosis factor-α (TNF-α) prior to measuring NF-κB activation.
Results
CCK- or TNF-α-stimulated NF-κB-dependent gene transcription (luciferase assay) was substantially subdued by DNp38 expression. These findings were confirmed by electrophoretic mobility shift assay. Nuclear translocation of the p65 NF-κB subunit following agonist stimulation was evident (supershift). Characterization studies showed excellent adenoviral infection efficiency and cell viability in our AR42J cell model. Agonist-stimulated dose- and time-dependent p38 activation, with inhibition by DNp38 expression, was also confirmed.
Conclusion
The p38 MAP kinase regulates NF-κB pathway activation in exocrine pancreatic cells, and thus potentially plays a role in the mechanism of acute pancreatitis pathogenesis.
Key Words: AR42J cell, Acinar cell, Acute pancreatitis, p38 MAP kinase, Nuclear factor-κB, Cholecystokinin, Tumor necrosis factor-α, Adenoviral vector
Introduction
Although mitogen-activated protein (MAP) kinases are established mediators of cellular proinflammatory signaling pathways, the role of the p38 MAP kinase in acute pancreatitis pathogenesis is controversial [1,2]. Nuclear factor-κB (NF-κB), a transcription factor involved in the induction of many proinflammatory molecules, is reportedly involved in disease pathogenesis [3,4]. Tumor necrosis factor-α (TNF-α) and cholecystokinin (CCK) activate the NF-κB pathway and induce the expression of proinflammatory mediators in pancreatic acinar cells [5,6,7]. However, the key signaling mechanisms that underlie NF-κB activation in pancreatic acinar cells are as yet incompletely understood. We hypothesize that the p38 MAP kinase plays an important role in regulating NF-κB activation in exocrine pancreatic cells. To test this hypothesis, we expressed a dominant negative form of the p38 MAP kinase (DNp38) and evaluated its effect on NF-κB pathway activation in an exocrine pancreatic malignant cell line (AR42J cells). Our findings indicate that DNp38 expression reduces nuclear translocation and DNA binding of NF-κB and subdues NF-κB-dependent gene transcription following CCK or TNF-α stimulation in AR42J cells. These results provide further evidence for a fundamental role for p38 MAP kinase as a regulator of NF-κB pathway activation, which in turn emphasizes the potential importance of p38 MAP kinase in the mechanism of acute pancreatitis pathogenesis.
Materials and Methods
Materials
Rabbit polyclonal antibodies against total p38 MAPK (Cat. No. 9212) and total NF-κB p65 (Cat. No. 3034), as well as rabbit monoclonal antibody against phospho-p38 MAPK (Cat. No. 4511), were purchased from Cell Signaling (Danvers, Mass., USA). Rabbit polyclonal antibodies against NF-κB p50 (Cat. No. sc-7178x) and NF-κB p65 (Cat. No. sc-109x), and goat anti-rabbit IgG-HRP secondary antibody (Cat. No. sc-2004) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif., USA). Mouse monoclonal antibody against α-tubulin (Cat. No. CP06) was from Calbiochem (San Diego, Calif., USA). Mouse monoclonal antibody against TATA-binding protein (TBP) (Cat. No. ab818) was from Abcam (Cambridge, Mass., USA). AR42J cell line (Cat. No. CRL-1492) was from ATCC (Manassas, Va., USA). F-12K nutrient mixture Kaighn's Modification (Cat. No. 21127-022) was from Invitrogen (Grand Island, N.Y., USA). Protein Assay (Cat. No. 500-0006) was from Bio-Rad Laboratories (Hercules, Calif., USA). Replication-deficient adenoviruses expressing GFP (Ad.GFP), NF-κB-luciferase (Ad.NF-κB-luc) [8], and empty vector (Ad.EV) were purchased from the University of Iowa Vector Core Facility (Iowa City, Iowa, USA). Recombinant adenoviral vector containing the dominant negative form of murine p38α (Cat. No. ADV-105; TGY dual phosphorylation site at Thr180/Tyr182 replaced by AGF) was from Cell Biolabs, Inc. (San Diego, Calif., USA). Sulfated CCK-8 (Cat. No. C2175) was from Sigma (St. Louis, Mo., USA). Rat recombinant TNF-α (Cat. No. 510-RT-010) was from R&D Systems, Inc. (Minneapolis, Minn., USA). Oligonucleotides were from Integrated DNA Technologies (Coralville, Iowa, USA). Goat anti-mouse IgG (Cat. No. 31438) was from Pierce Biotechnology (Rockford, Ill., USA).
Cell Culture
AR42J cells were maintained in F-12K medium supplemented with 20% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml), in poly-D-lysine-coated culture dishes. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Immunoblotting
For immunoblots using whole cell protein extracts, AR42J cells were lysed following stimulation in 100 μl of a modified RIPA buffer (50 mM Tris-Cl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% deoxycholate) on ice for 10 min. The soluble protein was collected by centrifugation at 15,000 g for 10 min at 4°C. Nuclear protein extracts were obtained following stimulation of cells using a commercial extraction kit (NE-PER Nuclear and Cytoplasmic Extraction Reagents, Cat. No. 78833, Pierce Biotechnology). Lysate protein concentrations were measured by Bradford assay (Bio-Rad), then aliquots containing either 40 μg (whole cell) or 10 μg (nuclear) of lysate were denatured in SDS sample buffer and boiled for 3 min. Proteins were subjected to SDS-PAGE on 12% gels and transferred to PVDF membranes. Blots were probed with primary antibody followed by HRP-conjugated secondary antibody. Proteins were visualized using chemiluminescent detection (ECL Plus Western blotting detection reagent, Cat. No. RPN2132, Amersham Pharmacia Biotech, Piscataway, N.J., USA). Membranes were then stripped for 20 min at 50°C with a mild stripping buffer (69.3 mM SDS, 125 mM Tris pH 6.8, 243 mM β-mercaptoethanol), and re-probed for sample loading control (total p38, α-tubulin, or TBP, as appropriate). Densitometry analysis of immunoblots was then performed using ImageJ software (Version 1.4, National Institutes of Health, Bethesda, Md., USA).
Time-Course and Dose-Response Studies
AR42J cells were seeded at a density of 1E6 cells/well in 6-well culture dishes and incubated for 24 h. To determine the optimal response time for p38 MAP kinase activation, cells were stimulated with 10 μM CCK or 10 ng/ml TNF-α for 0, 1, 3, 5, 10 or 20 min prior to harvest. For dose-response studies, the cells were stimulated with various doses of CCK or TNF-α for 5 min. Cells were then lysed in 100 μl modified RIPA buffer, and protein concentration was determined by Bradford assay. Immunoblotting using primary antibody against dually phosphorylated p38 (1:1,000 v/v) and HRP-conjugated secondary antibody was performed, followed by stripping and probing for total p38 (1:1,000 v/v).
Adenovirus Infection of AR42J Cells
To evaluate infection efficiency, AR42J cells were incubated with Ad.GFP (5 MOI) and imaged with an Olympus IX51 Inverted Florescent Microscope (Leeds Precision Instruments, Inc., Minneapolis, Minn., USA). To assess cellular injury and viability, AR42J cells were plated at a density of 5E5 cells/well in 12-well culture dishes and incubated with Ad.EV (5 MOI) or medium alone. After 24 and 48 h, cell viability was evaluated by ATP assay while cell injury was assessed by LDH assay (CellTiter-Glo Luminescent Cell Viability Assay, Cat. No. G7571; CytoTox 96 Non-Radioactive Cytotoxicity Assay, Cat. No. G1780; Promega, Madison, Wisc., USA).
Immunoblot Analysis of Cells Expressing DNp38
To test the effect of DNp38 expression on p38 MAP kinase activation, AR42J cells were plated at a density of 1E6 cells/well in 6-well culture dishes and infected with 5 MOI Ad.EV or Ad.DNp38. At 48 h post-infection, cells were stimulated with 10 μM CCK or 10 ng/ml TNF-α for 5 min and lysed in 100 μl of modified RIPA buffer. Whole cell lysate protein concentration was determined by Bradford assay and immunoblotting using primary antibody against dually phosphorylated p38 (1:1,000 v/v) and HRP-conjugated secondary antibody was performed, followed by stripping and re-probing for α-tubulin (1:2,000 v/v). To test the effect of DNp38 expression on NF-κB activation, AR42J cells were plated at a density of 1E6 cells/well in 6-well culture dishes and infected with 5 MOI of Ad.EV or Ad.DNp38. At 48 h post-infection, cells were stimulated with 100 nM CCK or 10 ng/ml TNF-α for 10 min. The nuclear fraction was extracted, protein concentration was determined, and 10-μg protein aliquots were subjected to immunoblotting using primary antibody against total p65 (1:1,000 v/v) and HRP-conjugated secondary antibody. The membranes were stripped and reprobed for TBP (1:2,000 v/v) as a nuclear protein loading control.
Electrophoretic Mobility Shift Assay (EMSA)
AR42J cells were plated at a density of 1E6 cells/well in 6-well culture dishes and infected with 5 MOI of either Ad.EV or Ad.DNp38. At 48 h post-infection, cells were stimulated with 100 nM CCK or 10 ng/ml TNF-α for 10 min. Nuclear proteins were extracted using a commercial extraction kit (Pierce Biotechnology), and lysate concentrations quantified by Bradford assay (Bio-Rad). EMSA was performed using a non-radioactive chemiluminescent kit (LightShift Chemiluminescent EMSA Kit, Cat. No. 20148, Pierce Biotechnology). The oligonucleotide probe for EMSA corresponded to a biotin 3′ end-labeled recognition site sequence specific to activated NF-B, where the NF-κB-binding site sequence is underlined: sense, 5′-AGTTGAGGGGACTTT-CCCAGGC-3′; antisense, 5′-GCCTGGGAAAGTCCCCTCAA-CT-3′. The complementary oligonucleotides were then annealed to generate a double-stranded DNA probe. For each binding reaction, 3 μg nuclear extract was used. Reaction mixtures were subjected to PAGE on 6% gels in 0.5× TBE buffer at 100 V. DNA was transferred to positively charged nylon membranes, crosslinked for 10 min at 254 nm using a UV transilluminator, and then probed with HRP-conjugated streptavidin primary antibody (1:300 v/v). EMSA supershift analysis of activated NF-κB subunit composition was performed by adding 2 μg of the appropriate primary antibody to the binding reactions for 15 min at room temperature prior to the addition of the DNA probe.
NF-κB Luciferase Assay
NF-κB-dependent gene transcription was evaluated by measuring luciferase activity related to an NF-κB-responsive luciferase reporter. AR42J cells were plated at 5E5 cells/well in 12-well culture dishes and infected with Ad.NF-κB-luc (5 MOI). The cells were stimulated with 10 μM CCK or 10 ng/ml TNF-α for 6 or 24 h prior to harvesting 48 h post-infection for luciferase assay. To evaluate the role of p38 activation in NF-κB-dependent gene transcription, AR42J cells were co-infected with 5 MOI of Ad.NF-κB-luc plus 5 MOI of either Ad.DNp38 or Ad.EV for 48 h. Cells were stimulated with 10 μM CCK or 10 ng/ml TNF-α for 6 h prior to harvest. NF-κB-mediated gene transcription was evaluated by measuring luminescence (Luciferase Assay System, Cat. No. E1501, Madison, Wisc., USA).
Statistical Analysis
One-way ANOVA or the paired t test were used (SigmaStat Software, SPSS, Inc., Chicago, Ill., USA). Three wells were studied in each group and results expressed as mean ± SEM. p values <0.05 were considered significant.
Results
Stimulation with CCK or TNF-α Activates p38 MAP Kinase in AR42J Cells
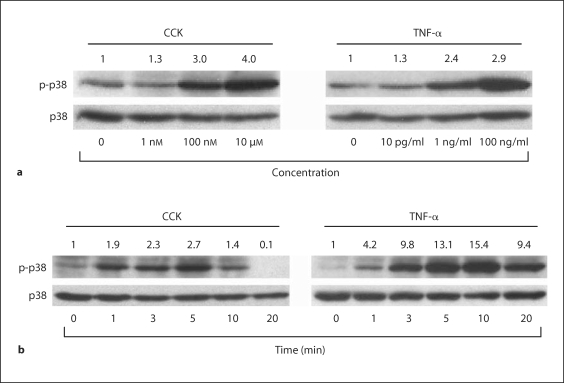
The p38 MAP kinase in AR42J cells is activated by CCK or TNF-α in a time- and dose-dependent manner (fig. 1). Robust p38 MAP kinase activation occurs 5 min after stimulating with CCK concentrations of 100 nM and above, or TNF-α concentrations of 1 ng/ml and above. Peak p38 MAP kinase activation occurs 5 min following CCK stimulation and 10 min following TNF-α stimulation. Activation of p38 MAP kinase was compared to total p38 expressed in each sample.
Fig. 1.
Dose response and time course of p38 activation in AR42J cells following CCK or TNF-α stimulation. a Cells were stimulated with increasing concentrations of either CCK or TNF-α for 5 min and immunoblots performed using antibody specific to phosphorylated p38. Dose-dependent p38 activation following CCK stimulation occurred at 100-nM concentrations and above, while TNF-α stimulated p38 activation occurred at 1-ng/ml doses and above. b AR42J cells were stimulated with 10 μM CCK or 10 ng/ml TNF-α for various periods of time from 0 to 20 min and immunoblots were performed using antibody specific to phosphorylated p38. Time-dependent p38 activation peaked at 5 min following CCK stimulation, and 10 min following TNF-α stimulation. In all panels, densitometry ratios normalized to total p38 expression are provided above each lane with the control value represented as 1.0.
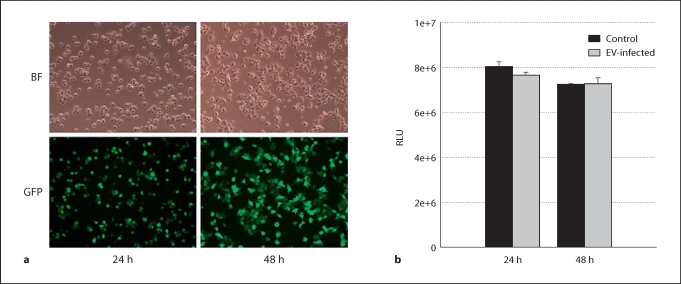
High Efficiency of Adenoviral Infection of AR42J Cells Is Not Associated with Detectable Cell Injury or Death
Cells infected with Ad.GFP show close to 90% infection efficiency at 48 h post-infection (fig. 2a). AR42J cells infected with Ad.EV for up to 48 h show no significant difference in cell viability compared to uninfected cells, as determined by ATP assay (fig. 2b); LDH assay showed no difference between infected and uninfected cells, indicating that 48 h of adenoviral infection does not cause detectable injury (data not shown).
Fig. 2.
Adenovirus infection of AR42J cells. a Cells were infected with 5 MOI of Ad.GFP, then imaged at 24 and 48 h post-infection. Comparison of bright field (BF) filter view (total number of cells) to the FITC filter view (GFP-expressing cells) for the same fields of cells showed close to 90% infection efficiency by 48 h. b AR42J cells were infected with 5 MOI of Ad.EV or left untreated (control), and cell viability evaluated at 24 h and 48 h post-infection by ATP assay. Results show no significant difference in cell viability in Ad.EV-infected cells in comparison to controls at either 24 h or 48 h. ANOVA, p < 0.05; n = 3 wells/group; RLU = relative light units.
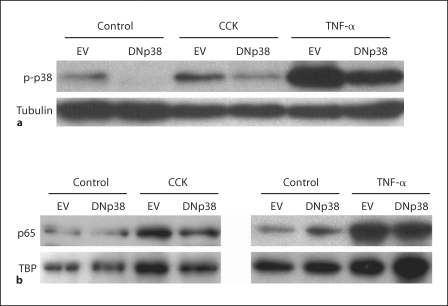
CCK- or TNF-α-Induced p38 MAP Kinase Activation in AR42J Cells Is Diminished by DNp38 Expression
To assess the effect of DNp38 expression on p38 MAP kinase activation following CCK or TNF-α stimulation, AR42J cells were infected with Ad.EV or Ad.DNp38 prior to stimulation. Densitometry analysis of whole-cell lysate immunoblots shows a 40% decrease in CCK-induced p38 MAP kinase activation in cells expressing DNp38 compared to controls and, similarly, a 46% reduction in signal with DNp38 expression following TNF-α stimulation (fig. 3a). The substantial attenuation of CCK- or TNF-α-induced p38 MAP kinase activation in AR42J cells infected with Ad.DNp38 indicates that sufficient DNp38 is expressed in our model to achieve functional inhibition of the p38 MAP kinase pathway.
Fig. 3.
Effect of DNp38 expression on p38 and NF-κB activation following CCK or TNF-α stimulation in AR42J cells. a Cells were infected with 5 MOI of Ad.EV or Ad.DNp38 for 48 h, then stimulated for 5 min with 10 μM CCK or 10 ng/ml TNF-α. Immunoblots of whole cell lysates were performed using an antibody specific to phosphorylated p38. Densitometry analysis of the immunoblots normalized to tubulin expression shows that p38 is activated following CCK or TNF-α stimulation, and that this activation is attenuated by the expression of DNp38 by 40% in CCK-stimulated cells, and 46% in TNF-α-stimulated cells. b AR42J cells were infected with 5 MOI of Ad.EV or Ad.DNp38 for 48 h, then stimulated for 10 min with either 100 nM CCK or 10 ng/ml TNF-α. Immunoblots of nuclear extracts were performed using an antibody specific to the p65 NF-κB subunit. Densitometry analysis of immunoblots normalized to TBP expression shows that NF-κB is activated following CCK or TNF-α stimulation, and that this activation is attenuated by the expression of DNp38 by 27% in CCK-stimulated cells and 35% in TNF-α-stimulated cells.
p38 MAP Kinase Regulates CCK- or TNF-α-Induced NF-κB Activation in AR42J Cells
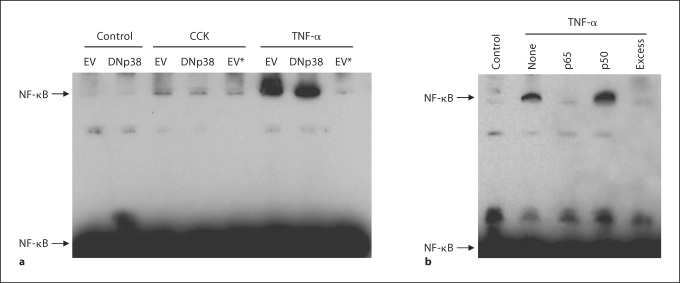
To test the effect of DNp38 expression on NF-κB activation following CCK or TNF-α stimulation, AR42J cells were infected with Ad.EV or Ad.DNp38 prior to stimulation. Densitometry analysis of immunoblots of the nuclear extracts shows a 27% decrease in p65 NF-κB subunit expression in the nucleus of CCK-stimulated cells expressing DNp38 compared to controls (fig. 3b). A corresponding 35% reduction in p65 expression in the nucleus following TNF-α stimulation is also seen when DNp38 is expressed. EMSA analysis of the nuclear extracts shows a marked decrease in activated NF-κB in cells expressing DNp38 following CCK or TNF-α stimulation (fig. 4a). Cells infected with Ad.EV show an increase in NF-κB DNA:protein complex formation following either CCK or TNF-α stimulation in comparison to unstimulated controls. Cells infected with Ad.DNp38 and stimulated with CCK or TNF-α show a decrease in NF-κB DNA:protein complex formation in comparison to the Ad.EV-infected cells. Adding a 200-fold excess of unlabeled NF-κB DNA almost completely blocks the interaction of NF-κB protein and DNA, showing that the shifted band is indeed specific. As CCK- or TNF-α-induced NF-κB activation in AR42J cells is diminished by DNp38 expression, our findings indicate that the p38 MAP kinase regulates NF-κB activation in this model.
Fig. 4.
Effect of DNp38 expression on NF-κB binding following CCK or TNF-α stimulation in AR42J cells. a Cells were infected for 48 h with 5 MOI Ad.EV or Ad.DNp38, then stimulated for 10 min with 10 μM CCK or 10 ng/ml TNF-α. EMSA immunoblot analysis of nuclear extracts shows increased formation of NF-κB DNA:protein complexes following stimulation with either CCK or TNF-α. Expression of DNp38 prior to stimulation attenuated NF-κB DNA:protein complex formation in comparison to Ad.EV-infected cells. Control reactions where 200-fold excess unlabeled NF-κB DNA was added prior to addition of NF-κB-biotin DNA, denoted by an asterisk (∗), show decreased DNA:protein interaction in comparison to the addition of NF-κB-biotin DNA alone. b AR42J cells were stimulated for 10 min with 10 ng/ml TNF-α. Nuclear extracts were incubated with 2 μg primary antibody specific to NF-κB subunits p50 or p65 prior to the addition of the DNA probe to the EMSA-binding reaction. Immunoblot analysis shows that the addition of antibody specific to the p65 NF-κB subunit interacted with activated NF-κB protein as seen by a loss of the shifted NF-κB DNA:protein complex, while antibody specific to the p50 NF-κB subunit did not diminish or supershift the band. Additionally, there was no formation of specific NF-κB DNA:protein complexes in either the unstimulated control lane or the lane containing a 200-fold excess of unlabeled NF-κB DNA probe.
EMSA supershift analysis was performed using antibodies specific to the NF-κB p50 and p65 subunits. Antibody specific to the p65 NF-κB subunit interacted with activated NF-κB protein as seen by a loss of the shifted NF-κB DNA:protein complex that accompanied TNF-α stimulation, while antibody specific to the p50 NF-κB subunit did not diminish or supershift the band (fig. 4b). Therefore, activation of the p65 subunit of NF-κB is involved in this model.
p38 MAP Kinase Regulates CCK- and TNF-α-Induced NF-κB-Dependent Gene Transcription in AR42J Cells
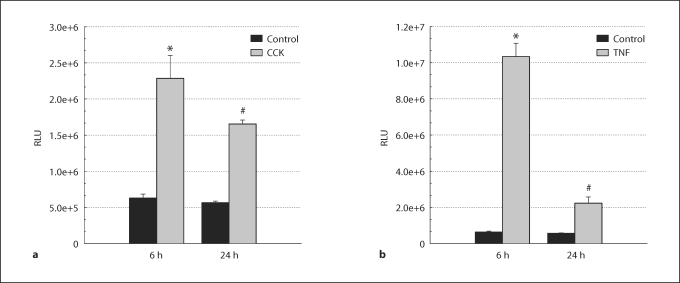
To determine the time course of CCK- or TNF-α-induced increases in NF-κB-dependent gene transcription, AR42J cells were infectedwith Ad.NF-κB-luc and then stimulated with CCK or TNF-α (fig. 5). Cells stimulated with CCK showed a 3.6-fold increase in NF-κB transcriptional activity after 6 h compared to unstimulated controls, which then dropped to a 2.9-fold increase 24 h following stimulation. With TNF-α stimulation, transcriptional activity showed a 16.4-fold increase at 6 h compared to unstimulated controls, which dropped to a 3.9-fold increase at 24 h. Therefore, we selected the 6-hour stimulation peak to evaluate the effect of DNp38 expression on NF-κB-dependent gene transcription in AR42J cells following agonist stimulation. Cells were co-infected with Ad.NF-κB-luc and Ad.EV, or Ad.NF-κB-luc and Ad.DNp38, and then stimulated for 6 h with either CCK or TNF-α (fig. 6). CCK-stimulated cells showed a 4.3-fold increase in luciferase activity in Ad.EV-infected cells compared to unstimulated controls, which decreased by 68% in DNp38-expressing cells. Cells stimulated with TNF-α exhibited a 13.1-fold increase in luciferase activity in Ad.EV-infected cells in comparison to unstimulated controls, which decreased by 54% in DNp38-expressing cells. These results indicate that p38 MAP kinase activation plays a key role in CCK- or TNF-α-induced NF-κB-dependent gene transcription in AR42J cells.
Fig. 5.
Time course of CCK- or TNF-α-induced NF-κB-dependent gene transcription in AR42J cells. Cells infected with 5 MOI Ad.NF-κB-luc were stimulated with 10 μM CCK or 10 ng/ml TNF-α for 6 or 24 h prior to harvesting 48 h post-infection. a CCK-stimulated cells showed a 3.6-fold increase in NF-κB transcriptional activity after 6 h compared to unstimulated controls, which dropped to a 2.9-fold increase at 24 h. b TNF-α-stimulated cells showed a 16.4-fold increase in luciferase activity at 6 h compared to unstimulated controls, which dropped to a 3.9-fold increase in activity at 24 h. ∗ Significant difference from the unstimulated empty vector control group, ANOVA, p <0.05. # Significant difference from the stimulated empty vector group, paired t test, p <0.05. n = 3 wells per experimental group; RLU = relative light units.
Fig. 6.
Effect of DNp38 expression on NF-κB-dependent gene transcription in AR42J cells stimulated with CCK or TNF-α. Cells were co-infected with 5 MOI of Ad.NF-κB-luc and also 5 MOI of either Ad.EV or Ad.DNp38 for 48 h, then stimulated with 10 μM CCK or 10 ng/ml TNF-α for 6 h prior to harvest. a Cells stimulated with CCK showed a 4.3-fold increase in luciferase activity in Ad.EV-infected cells compared to unstimulated controls, which then decreased by 68% in DNp38-expressing cells. b Cells stimulated with TNF-α exhibited a 13.1-fold increase in transcriptional activity in Ad.EV-infected cells in comparison to unstimulated controls, which decreased by 54% in DNp38-expressing cells. ANOVA, p <0.05. ∗ Significant difference from the unstimulated empty vector control group. # Significant difference from the stimulated empty vector group. n = 3 wells per experimental group; RLU = relative light units.
Discussion
NF-κB is a key regulator of the cellular acute inflammatory response [3,4,7]. Inhibition of NF-κB improves survival in experimental acute pancreatitis, while expression of NF-κB in the pancreas in vivo is associated with pancreatic injury [3,7]. However, the signaling mechanisms responsible for NF-κB activation in exocrine pancreatic cells are not fully understood. In this study, we present our new findings that demonstrate the important role of the p38 MAP kinase in regulating NF-κB activation and NF-κB-dependent gene transcription following CCK or TNF-α stimulation of AR42J cells. We have characterized certain important aspects of the AR42J malignant pancreatic exocrine cell line. We have performed time-course and dose-response studies of CCK- and TNF-α-stimulated activation of p38 MAP kinase, shown impressive infection rates with the adenoviral vector, and demonstrated that our experimental conditions do not result in detectable cell injury or cell death. We have validated our novel in vitro model of AR42J cells that incorporates an NF-κB-responsive luciferase reporter, by showing a correlation between nuclear translocation of NF-κB (EMSA) and NF-κB-dependent gene transcription following agonist stimulation (luciferase reporter assay). Both nuclear translocation of NF-κB and NF-κB-dependent gene transcription are evident following agonist stimulation of AR42J cells and both are substantially subdued by expression of DNp38. In addition, we have also shown that the p65 subunit of NF-κB – but not the p50 subunit – is activated in AR42J cells stimulated by CCK or TNF-α. As NF-κB-dependent gene transcription is known to be associated with production of proinflammatory mediators, and we have shown a key role for the p38 MAP kinase as a regulator of NF-κB pathway activation, our findings have important implications in the mechanism of acute pancreatitis pathogenesis.
MAP kinases are critical components in signal transduction from cell surface receptors to the nucleus, but their role in acute pancreatitis pathogenesis has only recently received some attention [2,9,10,11,12]. Certain studies have suggested that p38 MAP kinase activation could either exacerbate acute pancreatic inflammation or in fact protect against it [2,10]. One group illustrated a protective role for p38 MAP kinase by finding that its inhibition exacerbates cerulein-induced pancreatitis in rats [2]. Our findings, and those of others, suggest that it is the activation and not the inhibition of p38 MAP kinase that may exacerbate acute pancreatitis. By demonstrating inhibition of NF-κB-dependent gene transcription following expression of DNp38, we have shown in a previous study that the p38 MAP kinase regulates activation of the NF-κB pathway in isolated pancreatic acinar cells [13], and we have confirmed the same phenomenon in AR42J cells in the present study. However, the conflicting reports illustrate that there is as yet no clear consensus as to the role p38 MAP kinase plays in acute pancreatitis pathogenesis and this underlines the importance of further investigations to clarify this controversy.
The AR42J cell line is often used in the investigation of signaling mechanisms that may mimic those seen in pancreatic acinar cells [14,15,16,17]. AR42J cells offer many advantages in the study of signaling mechanisms in the pancreas, such as the ease of cell culture maintenance, high transduction efficiency with adenoviral vectors, and the endogenous expression of receptors for many agonists such as CCK and TNF-α. We have previously used AR42J cells infected with Ad.NF-κB-luc to evaluate the role of another MAP kinase, extracellular regulated kinase (ERK), in modulating NF-κB-dependent gene transcription [15]. In CCK- or TNF-α-stimulated AR42J cells, expression of DN-ERK2 significantly diminished NF-κB-dependent luciferase activity [15].
NF-κB is a transcription factor that regulates the transcription of many proinflammatory mediators such as cytokines, chemokines, oxygen-derived free radicals, adhesion molecules, and inducible effector enzymes [4,7,18]. In the classical NF-κB pathway, NF-κB is present in the cytosol of quiescent cells in an inactive state complexed with IκB [8]. Following cell surface receptor agonist stimulation, the inhibitory subunit IκB undergoes phosphorylation and degradation, which allows activated NF-κB to translocate to the nucleus. Upon entering the nucleus, the activated NF-κB binds to response elements to induce the transcription of several proinflammatory mediators. Since it is present in the cytosol in an inactive form, NF-κB is known as a rapid activating transcription factor as no protein synthesis is required in order to activate it following stimulation.
In the present report, we show that either CCK or TNF-α stimulation of AR42J cells activates both p38 MAP kinase and NF-κB. The activation of NF-κB following stimulation is confirmed by translocation of the p65 NF-κB subunit to the nucleus, increased DNA binding by NF-κB as shown by EMSA of nuclear fractions, and the substantial increase in NF-κB-dependent gene transcription as measured by luciferase activity. In cells expressing DNp38, we see substantial decreases in activation of both p38 MAP kinase and NF-κB after agonist stimulation in comparison to controls. DNp38 expression dampens nuclear translocation of the p65 NF-κB subunit, DNA binding by NF-κB, and NF-κB-dependent gene transcription in agonist-stimulated AR42J cells. These findings support the view that p38 MAP kinase regulates proinflammatory transcription factors such as NF-κB in pancreatic exocrine cells. This is significant because of the roles that CCK and TNF-α play in exacerbating acute inflammation in experimental models of acute pancreatitis [1,19,20,21,22,23]. Investigations using the donor rat model have shown that bile-pancreatic juice exclusion from gut exacerbates duct ligation-induced acute pancreatitis and also increases pancreatic phosphorylation of stress kinases (JNK, ERK, p38), activation of NF-κB, and production of proinflammatory mediators [15,24,25,26,27]. Also, in keeping with our findings in the present report in AR42J cells, activation and nuclear translocation of NF-κB in duct ligation-induced acute pancreatitis in rats involves the p65 subunit but not the p50 subunit [11].
Although we have demonstrated a link between MAP kinase activation and subsequent NF-κB pathway activation, other pathways independent of MAP kinase pathways probably also participate in NF-κB activation and proinflammatory mediator production following agonist stimulation of exocrine pancreatic cells. This is suggested by our finding that DNp38 expression did not completely block CCK- or TNF-α-induced NF-κB DNA binding or transcriptional activity. For instance, others have shown that agonist-stimulated NF-κB activation in acinar cells can be mediated by protein kinase C and protein kinase D1 [7,28].
In the present report, we have analyzed the role that the p38 MAP kinase plays in regulating NF-κB activation in the short term (10 min). However, studies have shown evidence of two phases of NF-κB activation in response to agonist stimulation due to the role of the inhibitory subunit IκB [29,30]. IκB is composed of the subunits IκBα and IκBβ, which maintain NF-κB in its quiescent form in the cytosol by masking its nuclear localization sequence. IκBα is rapidly but transiently degraded in pancreatitis (first phase), while IκBβ degrades much more slowly (second phase). We have shown evidence for the involvement of p38 in mediating the initial phase of NF-κB activation, but have yet to determine if it plays a role in long term activation. Clarification of these aspects of the role of p38 MAP kinase in NF-κB activation will be useful prior to embarking onto in vivo studies in experimental models of pancreatitis.
In summary, we have shown that following CCK or TNF-α stimulation of AR42J cells, p38 MAP kinase is activated in a time- and dose-dependent manner and is accompanied by NF-κB pathway activation. We have shown that expression of DNp38 attenuates CCK- or TNF-α-induced p38 activation, nuclear translocation and DNA binding of NF-κB, and NF-κB-dependent gene transcription in AR42J cells. These findings provide evidence for the key role of the p38 MAP kinase in the regulation of NF-κB pathway activation in exocrine pancreatic cells and potentially in the mechanism of acute pancreatitis pathogenesis.
Acknowledgements
This material is based upon work supported in part by the following research awards (to Dr. I.S.): (a) VA Merit Review Award, the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development), Washington, D.C., (b) Grant R01 DK-071731, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health, Bethesda, Md., and (c) American Recovery and Reinvestment Act of 2009 – Supplemental Award to NIH R01 DK-071731.
References
- 1.Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76–83. doi: 10.1016/s0002-9610(97)00240-7. [DOI] [PubMed] [Google Scholar]
- 2.Fleischer F, Dabew R, Goke B, Wagner AC. Stress kinase inhibition modulates acute experimental pancreatitis. World J Gastroenterol. 2001;7:259–265. doi: 10.3748/wjg.v7.i2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satoh A, Shimosegawa T, Fujita M, Kimura K, Masamune A, Koizumi M, Toyota T. Inhibition of nuclear factor-κB activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253–258. doi: 10.1136/gut.44.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-κB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448–457. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- 5.Han B, Ji B, Logsdon CD. CCK independently activates intracellular trypsinogen and NF-κB in rat pancreatic acinar cells. Am J Physiol. 2001;280:C465–C472. doi: 10.1152/ajpcell.2001.280.3.C465. [DOI] [PubMed] [Google Scholar]
- 6.Han B, Logsdon CD. CCK stimulates mob-1 expression and NF-κB activation via protein kinase C and intracellular Ca2+ Am J Physiol. 2000;278:C344–C351. doi: 10.1152/ajpcell.2000.278.2.C344. [DOI] [PubMed] [Google Scholar]
- 7.Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR, Jr, Shimosegawa T, Pandol SJ. PKC-δ and -∊ regulate NF-κB activation induced by cholecystokinin and TNF-α in pancreatic acinar cells. Am J Physiol. 2004;287:G582–G591. doi: 10.1152/ajpgi.00087.2004. [DOI] [PubMed] [Google Scholar]
- 8.Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Jr, Ritchie TC, Hunninghake GW, Zandi E, Engelhardt JF. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-α secretion through IKK regulation of NF-κB. J Biol Chem. 2001;276:30188–30198. doi: 10.1074/jbc.M102061200. [DOI] [PubMed] [Google Scholar]
- 9.Schafer C, Williams JA. Stress kinases and heat shock proteins in the pancreas: possible roles in normal function and disease. J Gastroenterol. 2000;35:1–9. doi: 10.1080/003655200750024443. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Murphy C, Denham W, Botchkina G, Tracey KJ, Norman J. Evidence of a central role for p38 MAP kinase induction of tumor necrosis factor-α in pancreatitis-associated pulmonary injury. Surgery. 1999;126:216–222. [PubMed] [Google Scholar]
- 11.Samuel I, Zaheer S, Nelson JJ, Yorek MA, Zaheer A. CCK-A receptor induction and p38 and NF-κB activation in acute pancreatitis. Pancreatology. 2004;4:49–56. doi: 10.1159/000077067. [DOI] [PubMed] [Google Scholar]
- 12.Murr MM, Yang J, Fier A, Gallagher SF, Carter G, Gower WR, Norman JG. Regulation of Kupffer cell TNF gene expression during experimental acute pancreatitis: the role of p38-MAPK, ERK1/2, SAPK/JNK, and NF-κB. J Gastrointest Surg. 2003;7:20–25. doi: 10.1016/s1091-255x(02)00053-7. [DOI] [PubMed] [Google Scholar]
- 13.Williard DE, Twait E, Yuan Z, Carter AB, Samuel I. NF-κB-dependent gene transcription in CCK- and TNF-α-stimulated isolated acinar cells is regulated by p38 MAP kinase. Am J Surg. 2010 doi: 10.1016/j.amjsurg.2009.12.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiehne K, Herzig KH, Folsch UR. Differential activation of p42ERK2 and p125FAK by cholecystokinin and bombesin in the secretion and proliferation of the pancreatic amphicrine cell line AR42J. Pancreatology. 2002;2:46–53. doi: 10.1159/000049448. [DOI] [PubMed] [Google Scholar]
- 15.Samuel I, Tephly L, Williard DE, Carter AB. Enteral exclusion increases MAP kinase activation and cytokine production in a model of gallstone pancreatitis. Pancreatology. 2008;8:6–14. doi: 10.1159/000114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Seo J, Kim H, Chung JB, Kim KH. Signal transduction of cerulein-induced cytokine expression and apoptosis in pancreatic acinar cells. Ann NY Acad Sci. 2003;1010:104–108. doi: 10.1196/annals.1299.017. [DOI] [PubMed] [Google Scholar]
- 17.Satoh A, Gukovskaya AS, Edderkaoui M, Daghighian MS, Reeve JR, Jr, Shimosegawa T, Pandol SJ. Tumor necrosis factor-α mediates pancreatitis responses in acinar cells via protein kinase C and proline-rich tyrosine kinase 2. Gastroenterology. 2005;129:639–651. doi: 10.1016/j.gastro.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Algul H, Tando Y, Schneider G, Weidenbach H, Adler G, Schmid RM. Acute experimental pancreatitis and NF-κB/Rel activation. Pancreatology. 2002;2:503–509. doi: 10.1159/000066090. [DOI] [PubMed] [Google Scholar]
- 19.Mole DJ, McFerran NV, Diamond T. Differential preservation of lipopolysaccharide-induced chemokine/cytokine expression during experimental pancreatitis-associated organ failure in rats shows a regulatory expressed phenotype. Pancreatology. 2008;8:478–487. doi: 10.1159/000151775. [DOI] [PubMed] [Google Scholar]
- 20.Sempere L, Martinez J, de Madaria E, Lozano B, Sanchez-Paya J, Jover R, Perez-Mateo M. Obesity and fat distribution imply a greater systemic inflammatory response and a worse prognosis in acute pancreatitis. Pancreatology. 2008;8:257–264. doi: 10.1159/000134273. [DOI] [PubMed] [Google Scholar]
- 21.Szabo G, Mandrekar P, Oak S, Mayerle J. Effect of ethanol on inflammatory responses. Implications for pancreatitis. Pancreatology. 2007;7:115–123. doi: 10.1159/000104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Criddle DN, McLaughlin E, Murphy JA, Petersen OH, Sutton R. The pancreas misled: signals to pancreatitis. Pancreatology. 2007;7:436–446. doi: 10.1159/000108960. [DOI] [PubMed] [Google Scholar]
- 23.Hofner P, Balog A, Gyulai Z, Farkas G, Rakonczay Z, Takacs T, Mandi Y. Polymorphism in the IL-8 gene, but not in the TLR4 gene, increases the severity of acute pancreatitis. Pancreatology. 2006;6:542–548. doi: 10.1159/000097363. [DOI] [PubMed] [Google Scholar]
- 24.Samuel I. Bile and pancreatic juice exclusion activates acinar stress kinases and exacerbates gallstone pancreatitis. Surgery. 2008;143:434–440. doi: 10.1016/j.surg.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuel I, Yorek MA, Zaheer A, Fisher RA. Bile-pancreatic juice exclusion promotes Akt/NF-κB activation and chemokine production in ligation-induced acute pancreatitis. J Gastrointest Surg. 2006;10:950–959. doi: 10.1016/j.gassur.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Samuel I, Zaheer S, Zaheer A. Bile-pancreatic juice exclusion increases p38MAPK activation and TNF-α production in ligation-induced acute pancreatitis in rats. Pancreatology. 2005;5:20–26. doi: 10.1159/000084486. [DOI] [PubMed] [Google Scholar]
- 27.Samuel I, Toriumi Y, Zaheer A, Joehl RJ. Mechanism of acute pancreatitis exacerbation by enteral bile-pancreatic juice exclusion. Pancreatology. 2004;4:527–532. doi: 10.1159/000080527. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J, Lugea A, Zheng L, Gukovsky I, Edderkaoui M, Rozengurt E, Pandol SJ. Protein kinase D1 mediates NF-κB activation induced by cholecystokinin and cholinergic signaling in pancreatic acinar cells. Am J Physiol. 2008;295:G1190–G1201. doi: 10.1152/ajpgi.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Y, Brasier AR. Mechanism for biphasic rel A. NF-κB1 nuclear translocation in tumor necrosis factor α-stimulated hepatocytes. J Biol Chem. 1997;272:9825–9832. doi: 10.1074/jbc.272.15.9825. [DOI] [PubMed] [Google Scholar]
- 30.Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-κB is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003;278:2294–2303. doi: 10.1074/jbc.M207129200. [DOI] [PubMed] [Google Scholar]