Abstract
We performed repeated dual-energy X-ray absorptiometry (DEXA) measurements over five years in a homogeneous patient population to study the effect of a cemented stem on proximal femoral bone remodelling. Data from 88 patients (88 hips) implanted with total hip arthroplasty (THA) prostheses were extracted from three randomised studies. Femoral bone mineral density (BMD) was measured using a Lunar DPX-IQ densitometer for five years postoperatively. At one year the BMD changes had decreased between −2.0% [region of interest (ROI) 1] and −11.5% (ROI 7). During the follow-up period the BMD initially increased during the second year and thereafter decreased again in ROIs 5, 6 and 7. The loss of BMD at five years was more pronounced in region 7 (12.9%) and decreased with increasing age, total hip replacement (THR) on the right side and decreasing weight of the patient. We found that after the initial phase of early bone loss a period of recovery follows. Thereafter the BMD decreases again, which probably reflects the normal ageing of bone after uncomplicated cemented THA.
Résumé
Nous avons réalisé des examens avec des mesures répétées de densité osseuse (DEXA) sur une période de 5 ans dans une population homogène de patients ayant bénéficié de la mise en place d’une prothèse fémorale cimentée avec évaluation de remodelage osseux. Nous avons extrait les données de 88 patients (88 hanches) de trois études randomisées. La densité minérale osseuse fémorale a été mesurée par densitomètre Lunar DPX-IQ durant les cinq années post-opératoires. A un an, la densité minérale osseuse diminue de −2% (zone 1) et de −11,5% (zone 7). Durant la période d’observation la densité minérale osseuse augmente au cours de la deuxième année et puis diminue à nouveau en zones 5, 6 et 7. La perte de densité osseuse à cinq ans est moins importante en zone 7 (12,9%) et diminue lorsque l’âge augmente notamment du côté droit et en fonction du poids des patients. Nous avons ainsi montré, qu’après une phase initiale de déminéralisation osseuse il existe une phase de reminéralisation puis la densité minérale osseuse diminue à nouveau ceci étant probablement dans les prothèses totales de hanches non compliquées le reflet de l’âge.
Introduction
The bone mineral density (BMD) changes in the proximal femur, which occur after total hip replacement (THR), may vary depending on patient-related factors, implant geometry, implant stiffness, surface structure and fixation [10, 22].
The quality of periprosthetic bone has been assessed with radiography, computed tomography and magnetic resonance imaging. With these methods, however, only qualitative data on periprosthetic bone can be obtained [11]. The technical improvements in dual-energy X-ray absorptiometry (DEXA) have made it possible to accurately measure the quantity of bone near a metallic implant [11, 12, 14]. Recently quantitative computed tomography (QCT)-assisted osteodensitometry has also been shown to be useful in assessing the in vivo structural bone changes after total hip arthroplasty (THA) [20, 21].
Previous prospective studies of postoperative changes of BMD have observed that the greatest bone loss occurs during the first six months after surgery and in the proximal part of the femur [15, 18]; thereafter continuous decrease of BMD has been reported, but it is unclear if this decrease only represents normal ageing or also depends on a more lengthy effect of load changes caused by the inserted femoral stem [1, 2, 18, 24].
The purpose of this study was to evaluate the longitudinal changes of BMD during the follow-up period and to what extent gender, age at operation, weight, side operated, stem size, postoperative BMD and stem subsidence as measured with radiostereometric analysis (RSA) influenced the observed bone remodelling.
Materials and methods
Data from 88 patients (88 hips) implanted with THA prostheses were extracted from three randomised studies including 182 patients (222 hips) designed to study new and undocumented bone cements or polyethylene (PE). All patients were followed with RSA and DEXA measurements for five years. The selection of patients was based on diagnosis (primary osteoarthritis), use of the same stem design (Spectron Primary, Smith and Nephew, Memphis, TN, USA) and same type of bone cement (Palacos cum gentamicin, Schering Plough, Wehrheim Germany). In patients who had bilateral THAs, only the first hip replacement was included. There were 30 men and 58 women (88 hips) with a median age 60 years (range: 37–78 years) and weight of 75 kg (range: 45–116 kg). In 48 patients the right hip and in 40 patients the left hip were replaced.
The Spectron Primary prosthesis is a straight stem made of cobalt-chromium (Co-Cr) alloy and has a 12/14 global taper. Its proximal one third is grit blasted [surface roughness (Ra): 2.8 μm]. The distal part is smoother (0.7 μm) and a centraliser is attached to the tip of the stem; 28-mm femoral heads made of Co-Cr were used in all hips. Standard (n = 62) and high offset (n = 26) stems were used. The manufacturer had supplied the femoral stems with three titanium towers each with a tantalum marker (ø 0.8 mm). Further markers were inserted into the proximal femur. Three stem sizes were used (size 1/2/3: n = 20/41/27).
Palacos cement was pre-chilled at 8°C. A third-generation cementing technique (brush, high-pressure lavage, distal plugging of the femoral canal, tamponades soaked in epinephrine solution, retrograde injection of cement into femur and cement pressurisation) was used. The hip was approached through an anterolateral approach with the patient on the side. All patients were treated at the same hospital (Sahlgrenska University Hospital) by 15 surgeons. The patients were mobilised the day after the operation and were allowed as much weight-bearing as was tolerated.
RSA examinations were made with the patient supine between three and seven days postoperatively, after six months, and at one, two and five years. The uniplanar technique with the calibration cage positioned under the examination table was used [16]. Subsidence of the stems was measured.
Femoral BMD was measured using a Lunar DPX-IQ densitometer (Lunar Corporation, Madison, WI, USA) one week postoperatively and at one, two and five years. During scanning the patient was placed in the supine position. A foot brace and knee supports were used to obtain a standardised position. Femoral scan acquisition was started approximately 2.5 cm distal to the tip of the femoral stem. The scan continued proximally up to 2 cm above the tip of the greater trochanter. The orthopaedic software (Lunar DPX IQ V 4.6, Lunar Corporation, Madison, WI, USA) automatically excluded the soft tissues and the metallic stem. It divided the proximal femur into 7 Gruen regions of interest (ROI) based on implant length excluding the tip of the greater trochanter. In our analysis we expanded this region manually to also include this part of the trochanter.
Twenty patients were examined twice with DPX-IQ with a mean of eight days (range: 1–18 days) between the two examinations to evaluate the precision of the measurements. The intraobserver and interobserver errors were evaluated in a further 20 examinations. The precision, the intraobserver error and the interobserver error were expressed as the coefficient of variation (CV) according to the formula: 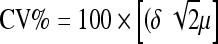 for each ROI, where δ represents the standard deviation of the difference between the paired DEXA measurements and μ is the overall mean of all BMD measurements for each individual ROI [25] (Table 1).
for each ROI, where δ represents the standard deviation of the difference between the paired DEXA measurements and μ is the overall mean of all BMD measurements for each individual ROI [25] (Table 1).
Table 1.
Precision, intraobserver and interobserver error of the BMD measurements
| ROI | Precision | Intraobserver error | Interobserver error |
|---|---|---|---|
| 1 | 4.55 | 0.63 | 1.60 |
| 2 | 2.55 | 1.05 | 0.93 |
| 3 | 2.03 | 0.14 | 0.33 |
| 4 | 2.37 | 0 | 0.26 |
| 5 | 1.74 | 0.13 | 0.32 |
| 6 | 2.92 | 2.40 | 2.61 |
| 7 | 3.72 | 1.36 | 6.45 |
Values expressed in CV% (coefficient of variation), ROI region of interest
We used non-parametric tests (Wilcoxon signed rank test, Mann-Whitney test) and stepwise linear regression analysis. All calculations were made using SPSS Version 13 (SPSS Inc., Chicago, IL, USA).
Results
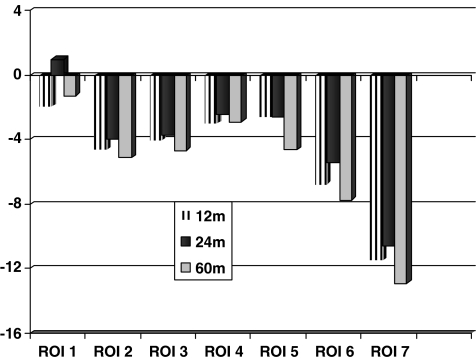
The BMD changes between the postoperative examination and the one-year follow-up varied between −1.97% in ROI 1 and −11.5% in ROI 7 (Fig. 1). This reduction of the mean BMD (g/cm2) between the postoperative and one-year examination was significant in all regions except in region 1 (the greater trochanter; p value in regions 2–7: < 0.001, Wilcoxon signed rank test, Table 2). The mean periprosthetic BMD of all seven regions was 2.06 g/cm2 at the postoperative examination and decreased to 1.95 g/cm2 at one year (p < 0.001, Table 2). During the second year the mean value of BMD increased in all regions (Table 2). Comparison of data obtained at one and two years postoperatively revealed significantly increased BMD in ROIs 1, 2 and 6 (p < 0.03 Wilcoxon signed rank test, Fig. 1). The mean periprosthetic BMD of all seven regions had, at the end of the second year, increased to 1.97 g/cm2. Between two and five years the BMD decreased in almost all of the regions and especially medially (ROIs 5, 6 and 7, p < 0.01, Fig. 1). At five years the mean periprosthetic BMD of all regions had decreased from 2.06 g/cm2 postoperatively to 1.94 g/cm2. During this period the most pronounced reduction was recorded in ROI 7 (−12.9%, Fig. 1).
Fig. 1.
Mean changes of bone mineral density (%) at 1, 2 and 5 years for each region of interest (ROI)
Table 2.
The mean bone mineral density (g/cm2, SD) in seven regions and total area during the 5-year follow-up
| Gruen region | Postop. (n = 88) | 1-year follow-up (n = 88) | 2-year follow-up (n = 88) | 5-year follow-up (n = 88) |
|---|---|---|---|---|
| 1 | 1.47 (0.29) | 1.44 (0.32) | 1.48 (0.30)a | 1.44 (0.28) |
| 2 | 2.11 (0.30) | 2.00 (0.34)b | 2.01 (0.34)a | 1.99 (0.35) |
| 3 | 2.27 (0.25) | 2.18 (0.27)b | 2.19 (0.27) | 2.16 (0.29)a |
| 4 | 2.45 (0.47) | 2.34 (0.31)b | 2.35 (0.31) | 2.35 (0.33) |
| 5 | 2.19 (0.23) | 2.13 (0.26)b | 2.14 (0.22) | 2.09 (0.30)a |
| 6 | 2.04 (0.26) | 1.90 (0.26)b | 1.93 (0.26)a | 1.88 (0.30)a |
| 7 | 1.88 (0.30) | 1.67 (0.31)b | 1.68 (0.31) | 1.64 (0.32)a |
| Total | 2.06 (0.20) | 1.95 (0.20)b | 1.97 (0.20)a | 1.94 (0.24)a |
Significant changes as compared to previous BMD value:
ap < 0.05
bp < 0.001
A stepwise linear regression analysis was used to evaluate the influence of age, gender, weight, stem size, stem subsidence, operated side and postoperative BMD in region 7 on the change of BMD in ROI 7 up to five years.
The BMD in ROI 7 decreased with increasing age at operation (p = 0.002) and low weight (p = 0.02). Patients operated on the right side showed more reduction than those operated on the left (p = 0.02, R2 = 0.21)
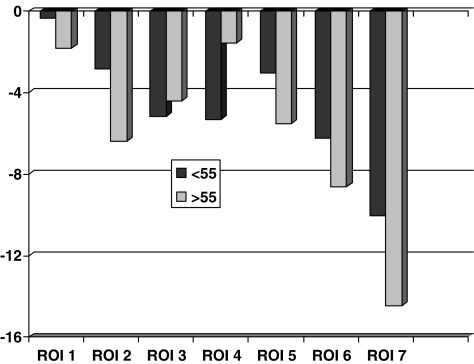
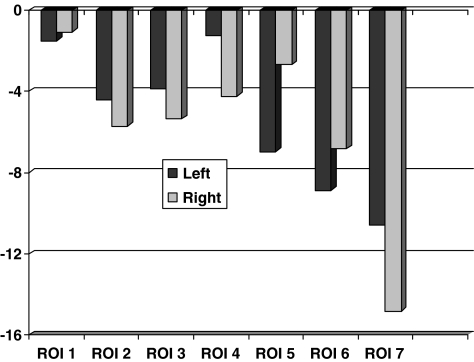
A separate comparison of the BMD changes in individual regions between patients up to 55 years (n = 31) and those that were older (n = 57) at operation revealed increased loss in the older group in region 7 at five years (> 55 years: −14.4%, ≤ 55 years: −10.0%, p = 0.04; Mann-Whitney U test, Fig. 2). Comparison between patients operated on the right or left side revealed increased loss of BMD on the right side in region 7 at five years (right side: −14.8%, left side: −10.6%, p = 0.009; Mann-Whitney U test, Fig. 3).
Fig. 2.
Changes of bone mineral density (%, mean) at 5-year examination according to age
Fig. 3.
Changes of bone mineral density (%, mean) at 5-year examination for right and left hips
The mean stem subsidence increased from −0.058 mm at one year to −0.18 mm at the five-year follow-up, but did not seem to affect the pattern of bone remodelling in any of the regions. The mean subsidence on the right (−0.21 mm) and on the left (−0.15 mm) did not differ (p = 0.44, Mann-Whitney test).
The precision for the DEXA measurements varied between 1.74 and 4.55% while the intra- and interobserver error varied between 0 and 6.45% depending on region (Table 1).
Discussion
A few early studies [5, 17, 24] followed the periprosthetic bone mass after cemented THA. They did not exclude the cement mantel from their analysis. The density of the cement mantle will hardly change with time. Therefore, the BMD changes occurring postoperatively should reflect changes of the periprosthetic bone density [14]. The cement itself and especially its content of radiopaque contrast will add a constant to the value of the bone mineral content (BMC), which might imply an underestimation of the recorded changes when evaluated as change in percent.
To obtain baseline values for comparison, we used the postoperative values, measured within a week after the index surgery [2, 14, 18]. With this method, the bone loss due to operation, estimated at 13–19% in the calcar area, was excluded [14].
We found the greatest bone loss around femoral stems during the first year in the calcar region. This early bone loss is probably a consequence of several factors such as stress shielding, patient inactivity during the postoperative period, local vascular injury from surgical trauma, thermal injury from polymerisation of cement and cytotoxicity of the methyl methacrylate monomer. Based on previous studies this loss of bone could be supposed to be most pronounced during the first postoperative months and might have reached its maximum at six months [18, 24]. During the second year the periprosthetic bone tends to recover. It seems that the response of the periprosthetic bone reaches a plateau at two years. After the second year there was a slow decrease of BMD in some regions probably due to ageing. According to Hannan et al. [9] the reduction of BMD amounts to about 1% per year during normal ageing.
In our study the greatest bone loss during the follow-up period was recorded in the calcar area (−12.9%). Prospective studies of cementless components have shown pronounced bone loss in this region up to a level of 40% during a follow-up period of up to seven years [2, 23, 27]. On the other hand, Cohen and Rushton [5] reported only 6.7% reduction of BMD in the calcar region after one year for cemented stems, whereas Venesmaa et al. [24] observed greatest loss of bone mineral in the calcar region (26%) at five years when cemented stems were evaluated. In the latter study the patients were ten years older than in our study and different stems were used. Theoretically, cemented femoral components may produce more uniform transmission of loads in the proximal femur and should thereby be expected to cause less early bone loss due to stress shielding of the calcar region. This effect may, however, vary between stems due to design variations not least in terms of surface finish.
Bone loss increased with age probably due to several factors. In our study age influenced loss of BMD in the calcar region perhaps because thicker stems were used due to a wider femoral canal or because these patients are more sensitive to stress shielding. Differences in remodelling activity between younger and older patients have been reported, both at the cellular level [6] and at the macroscopic level [4]. Our findings suggest that younger patients may have a different remodelling pattern after insertion of orthopaedic implants.
In our study, body weight also affected periprosthetic BMD. The lower the body weight, the higher the percentage of bone loss at five years in the calcar region. According to Ohta et al. [19] who evaluated 21 hips with fully porous-coated stems over three years the lower the body weight the higher the percent loss of BMD at six months. Kiratli et al. [13] examined BMD with DEXA in 89 THA patients in a two-year follow-up period. Body weight was the only variable that affected the rate of remodelling. Hannan et al. [9] evaluated risk factors for bone loss in 800 elderly women and men. According to their finding high body weight appeared to protect against low BMD.
Leptin, the product of the obese (ob) gene, is secreted from adipocytes and its plasma levels are known to be positively correlated with % fat (total fat body mass divided by total body weight). There is recent evidence suggesting that leptin directly stimulates osteoblastic differentiation. Thus, it is possible that the anabolic action of this hormone on bone may participate in the positive correlation between weight and bone mass [26].
Our finding of more bone loss in the calcar region after insertion of a cemented stem in the right hip compared with the same operation performed on the left side was unexpected. Some reports in the literature indicate different BMD between the right and left hip [3, 8]. Few studies have reported inverse relationship between dexterity and the excitability of motoneurons innervating the postural soleus muscle in right- and left-handed subjects. These results suggests that the motoneuronal excitability of the left soleus muscle is greater than that of the right soleus muscles in right-handers [7]. Thus in right-handers the left soleus muscle maintains equilibrium of the body in the upright position while the patient is performing relatively fine movements with the right foot during daily activity. Therefore, according to these studies, it could be expected that the left femur is exposed to the weight-bearing effect of the body more than the right femur. This may also be true for the right femur in left-handed patients. It may be that this difference in the loading effect applied in both hips during daily activities can explain the higher bone loss for the right hips. To our knowledge no other DEXA study has shown different bone remodelling between the right and left hips after THR.
We found that after the initial phase of acute bone loss a period of bone recovery follows and thereafter the BMD decreases, which probably reflects the normal ageing of bone after uncomplicated cemented THA. Patient-related factors and especially age, weight and side of operation modify this pattern in the calcar region but only to a limited extent.
Acknowledgements
The institution of the authors has received funding from the Swedish Research Council (Project nr.K2002-73X-0741-16D), the Göteborg Medical Society, Centerpulse, Switzerland and Zimmer, USA.
References
- 1.Aldinger PR, Sabo D, Pritsch M, Thomsen M, Mau H, Ewerbeck V, Breusch SJ. Pattern of periprosthetic bone remodeling around stable uncemented tapered hip stems: a prospective 84-month follow-up study and a median 156-month cross-sectional study with DXA. Calcif Tissue Int. 2003;73:115–121. doi: 10.1007/s00223-002-2036-z. [DOI] [PubMed] [Google Scholar]
- 2.Bodén HS, Sköldenberg OG, Salemyr MO, Lundberg HJ, Adolphson PY. Continuous bone loss around a tapered uncemented femoral stem: a long-term evaluation with DEXA. Acta Orthop. 2006;77:877–885. doi: 10.1080/17453670610013169. [DOI] [PubMed] [Google Scholar]
- 3.Bonnick SL, Nichols DL, Sanborn CF, Payne SG, Moen SM, Heiss CJ. Right and left proximal femur analyses: is there a need to do both? Calcif Tissue Int. 1996;58:307–310. doi: 10.1007/BF02509376. [DOI] [PubMed] [Google Scholar]
- 4.Brockstedt H, Kassem M, Eriksen EF, Mosekilde L, Melsen F. Age- and sex-related changes in iliac cortical bone mass and remodeling. Bone. 1993;14:681–691. doi: 10.1016/8756-3282(93)90092-O. [DOI] [PubMed] [Google Scholar]
- 5.Cohen B, Rushton N. Bone remodeling in the proximal femur after Charnley total hip arthroplasty. J Bone Joint Surg Br. 1995;77:815–819. [PubMed] [Google Scholar]
- 6.Groessner-Schreiber B, Krukowski M, Lyons C, Osdoby P. Osteoclast recruitment in response to human bone matrix is age related. Mech Ageing Dev. 1992;62:143–154. doi: 10.1016/0047-6374(92)90051-E. [DOI] [PubMed] [Google Scholar]
- 7.Gümüştekin K, Akar S, Dane S, Yildirim M, Seven B, Varoglu E. Handedness and bilateral femoral bone densities in men and women. Int J Neurosci. 2004;114:1533–1547. doi: 10.1080/00207450490509186. [DOI] [PubMed] [Google Scholar]
- 8.Hall ML, Heavens J, Ell PJ. Variation between femurs as measured by dual energy X-ray absorptiometry (DEXA) Eur J Nucl Med. 1991;18:38–40. doi: 10.1007/BF00177683. [DOI] [PubMed] [Google Scholar]
- 9.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–720. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 10.Huiskes R. The various stress patterns of press-fit, ingrown, and cemented femoral stems. Clin Orthop Relat Res. 1990;261:27–38. [PubMed] [Google Scholar]
- 11.Kilgus DJ, Shimaoka EE, Tipton JS, Eberle RW. Dual-energy X-ray absorptiometry measurement of bone mineral density around porous-coated cementless femoral implants. Methods and preliminary results. J Bone Joint Surg Br. 1993;75:279–287. doi: 10.1302/0301-620X.75B2.8444950. [DOI] [PubMed] [Google Scholar]
- 12.Kiratli BJ, Heiner JP, McBeath AA, Wilson MA. Determination of bone mineral density by dual x-ray absorptiometry in patients with uncemented total hip arthroplasty. J Orthop Res. 1992;10:836–844. doi: 10.1002/jor.1100100613. [DOI] [PubMed] [Google Scholar]
- 13.Kiratli BJ, Checovich MM, McBeath AA, Wilson MA, Heiner JP. Measurement of bone mineral density by dual-energy x-ray absorptiometry in patients with the Wisconsin hip, an uncemented femoral stem. J Arthroplasty. 1996;11:184–193. doi: 10.1016/S0883-5403(05)80015-4. [DOI] [PubMed] [Google Scholar]
- 14.Kröger H, Miettinen H, Arnala I, Koski E, Rushton N, Suomalainen O. Evaluation of periprosthetic bone using dual-energy x-ray absorptiometry: precision of the method and effect of operation on bone mineral density. J Bone Miner Res. 1996;11:1526–1530. doi: 10.1002/jbmr.5650111020. [DOI] [PubMed] [Google Scholar]
- 15.Kröger H, Venesmaa P, Jurvelin J, Miettinen H, Suomalainen O, Alhava E. Bone density at the proximal femur after total hip arthroplasty. Clin Orthop Relat Res. 1998;352:66–74. [PubMed] [Google Scholar]
- 16.Kärrholm J, Herberts P, Hultmark P, Malchau H, Nivbrant B, Thanner J. Radiostereometry of hip prostheses. Review of methodology and clinical results. Clin Orthop Relat Res. 1997;344:94–110. [PubMed] [Google Scholar]
- 17.McCarthy CK, Steinberg GG, Agren M, Leahey D, Wyman E, Baran DT. Quantifying bone loss from the proximal femur after total hip arthroplasty. J Bone Joint Surg Br. 1991;73:774–778. doi: 10.1302/0301-620X.73B5.1894664. [DOI] [PubMed] [Google Scholar]
- 18.Nishii T, Sugano N, Masuhara K, Shibuya T, Ochi T, Tamura S. Longitudinal evaluation of time related bone remodeling after cementless total hip arthroplasty. Clin Orthop Relat Res. 1997;339:121–131. doi: 10.1097/00003086-199706000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Ohta H, Kobayashi S, Saito N, Nawata M, Horiuchi H, Takaoka K. Sequential changes in periprosthetic bone mineral density following total hip arthroplasty: a 3-year follow-up. J Bone Miner Metab. 2003;21:229–233. doi: 10.1007/s00774-002-0414-2. [DOI] [PubMed] [Google Scholar]
- 20.Pitto RP, Mueller LA, Reilly K, Schmidt R, Munro J. Quantitative computer-assisted osteodensitometry in total hip arthroplasty. Int Orthop. 2007;31:431–438. doi: 10.1007/s00264-006-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitto RP, Bhargava A, Pandit S, Walker C, Munro JT. Quantitative CT-assisted osteodensitometry of femoral adaptive bone remodelling after uncemented total hip arthroplasty. Int Orthop. 2008;32:589–595. doi: 10.1007/s00264-007-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenthall L, Bobyn JD, Tanzer M. Bone densitometry: influence of prosthetic design and hydroxyapatite coating on regional adaptive bone remodelling. Int Orthop. 1999;23(6):325–329. doi: 10.1007/s002640050383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott DF, Jaffe WL. Host-bone response to porous-coated cobalt-chrome and hydroxyapatite-coated titanium femoral components in hip arthroplasty. Dual-energy x-ray absorptiometry analysis of paired bilateral cases at 5 to 7 years. J Arthroplasty. 1996;11:429–437. doi: 10.1016/S0883-5403(96)80033-7. [DOI] [PubMed] [Google Scholar]
- 24.Venesmaa PK, Kröger HP, Jurvelin JS, Miettinen HJ, Suomalainen OT, Alhava EM. Periprosthetic bone loss after cemented total hip arthroplasty: a prospective 5-year dual energy radiographic absorptiometry study of 15 patients. Acta Orthop Scand. 2003;74:31–36. doi: 10.1080/00016470310013617. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson JM, Peel NF, Elson RA, Stockley I, Eastell R. Measuring bone mineral density of the pelvis and proximal femur after total hip arthroplasty. J Bone Joint Surg Br. 2001;83:283–288. doi: 10.1302/0301-620X.83B2.10562. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, Ozuru R, Sugishita T, Chihara K. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf) 2001;55:341–347. doi: 10.1046/j.1365-2265.2001.01361.x. [DOI] [PubMed] [Google Scholar]
- 27.Zerahn B, Lausten GS, Kanstrup IL. Prospective comparison of differences in bone mineral density adjacent to two biomechanically different types of cementless femoral stems. Int Orthop. 2004;28(3):146–150. doi: 10.1007/s00264-003-0534-x. [DOI] [PMC free article] [PubMed] [Google Scholar]