Abstract
Computer-assisted surgery (CAS) in total knee arthroplasty (TKA) could be useful in reducing the overall blood loss. A prospective randomised study was performed with two groups of 50 patients each of whom were treated for knee arthritis. Patients of group A were treated by a conventional standard procedure, while for patients of group B a specific CAS procedure was used. We determined the intraoperative blood loss according to the Orthopaedic Surgery Transfusion Haemoglobin European Overview (OSTHEO) study. The average blood loss in patients of group A was 1,974 ml (range: 450–3,930 ml) compared to 1,677 ml of patients of group B (range: 500–2,634 ml). A statistically significant difference was found between the two groups (p = 0.0283). Computer-assisted surgery is highly recommended in TKR to save blood. It creates more possibilities to operate on anaemic patients and subjects who cannot accept blood products by reducing blood loss risk.
Résumé
La chirurgie assistée par ordinateur dans les prothèses totales du genou peut être utile afin de réduire les pertes sanguines. Matériel et méthode: une étude prospective, randomisée, a été réalisée avec deux groupes de 50 patients, chacun, traités pour une arthrose du genou. Les patients du groupe A ont été opérés sur un mode conventionnel alors que l’autre groupe l’a été avec assistance informatique dans le cadre de la navigation. Nous avons déterminé les perte sanguines per-opératoires selon l’évaluation OSTEO. Résultats: le total des pertes sanguines du groupe A a été de 1974 ml en moyenne (de 450 à 3930) en comparaison des 1677 ml des patients du groupe B (de 500 à 2634 ml). La différence est statistiquement significative entre les deux groupes, p = 0,0283). Conclusion: la navigation avec chirurgie assistée par ordinateur est recommandée pour les prothèses de remplacement articulaire afin de réduire les pertes sanguines. Cette technique donne plus de possibilité pour opérer les sujets anémiques qui ne peuvent supporter de grosses pertes sanguines.
Introduction
Total knee arthroplasty (TKA) is one of the main operations associated with a major postoperative blood loss [8]; therefore, transfusion is often required. However, surgeons can minimise the intraoperative bleeding by the use of a tourniquet [21], diathermy coagulation and antifibrinolytic agents [14], as well as by controlling the position of the knee [10], and more importantly, by performing minimally invasive surgical techniques [12, 20].
Computer-assisted surgery (CAS) in TKA has been a very successful modality in the treatment of knee arthritis and has become increasingly common during recent years [3, 18, 19].
Since navigation systems potentially improve accuracy with regard to implant placement and limb alignment without breaching the intramedullary cavities [1, 2, 16], they could also bring about a reduction in blood loss. However, even if CAS does not need to breach the intramedullary cavities, it usually requires the insertion of single bicortical tracker pins into the tibia and femur. The presence of these pins, together with a prolongation of the operating time, as currently observed in CAS, may negate this benefit.
The purpose of this prospective comparative randomised study was to evaluate the blood loss in CAS-TKA compared with the blood loss using a conventional surgical technique with intramedullary alignment guide in mobile bearing TKA.
Materials and methods
Between February 2004 and November 2006, 100 patients with primary gonarthrosis were selected for unilateral mobile bearing TKA. All patients were randomly allocated by draw either to group A (conventional TKA) or to group B (CAS-TKA). Group A comprised 50 patients, 16 men and 34 women, with a mean age of 73.6 (range: 54–85) treated with standard instrumentation (Milestone, DePuy International Ltd, Leeds, UK); group B comprised 50 patients, 16 men and 34 women, with a mean age of 70.4 (range: 53–81) treated with the use of a computed tomography (CT)-free navigation system (Ci ™ navigation system, DePuy I-Orthopaedics, Munich, Germany). The two groups were homogeneous for age and gender.
Patients with history of a bleeding diathesis and in whom non-steroid anti-inflammatory medication was contraindicated were excluded. We recorded the following parameters for all patients: age, gender, weight, height and preoperative blood levels of haemoglobin (Hb) and haematocrit (Hct). Data are reported in Table 1.
Table 1.
Demographics and preoperative blood levels
| Group A | Group B | |
|---|---|---|
| Conventional TKA | CAS-TKA | |
| n | 50 | 50 |
| Age (years) | 73.6 (54–85) | 70.4 (53–81) |
| Female (%) | 68 | 68 |
| BMI | 29 (37.5–20.1) | 30 (41.8–20.1) |
| Preoperative Hb (g/dl) | 12.8 (16.2–10.5) | 12.5 (15.1–9.8) |
| Preoperative Hct (%) | 39 (51–32) | 38 (45–32) |
BMI body mass index, Hb haemoglobin level, Hct haematocrit level
Before surgery, all patients in both groups had predonated two units of autologous blood according to an autologous transfusion programme undertaken following a preordained schedule. All patients fully consented to their inclusion in the study.
Operative technique and prosthesis All patients in both groups were treated by a single surgeon (FC) in one hospital using a consistent surgical approach. The operations were carried out in a bloodless field using a pneumatic tourniquet at a pressure of 450 mmHg after exsanguination. We used a medial parapatellar approach and medial parapatellar arthrotomy. The length of incision was approximately the same in all cases (9–12 cm) with no significant differences between CAS and standard procedure. Ligament balancing was always done in the same sequence: at the beginning of the procedure, when required and during flexion-extension gap measurements after the proximal tibial cut. The wounds were closed with the same suture. Single intra-articular drainage with a recovery blood system was used. A crepe bandage dressing was applied from the ankle to the proximal portion of the thigh about 30 min after the tourniquet was released. Knee reconstruction with the standard technique was performed with the Milestone instruments, while minimally invasive guides were used in CAS. For this reason during surgery we noted a less invasive approach to the joint. A minor release of soft tissues was required, especially in the medial compartment and lateral retinaculum. An important difference between the two groups was the use of intramedullary femoral alignment jigs in group A, while in group B three bicortical tracker pins were inserted, one into the femur and two into the tibia. In group A a bone plug was used to seal the defect created by the intramedullary rod in the femur before wound closure. Lateral release was performed as required in both groups.The prosthesis used was always the Low Contact Stress (LCS) Total Knee System with rotating platform (DePuy International Ltd., Leeds, UK) and uncemented technique. None of the patients had the patella resurfaced.
Anaesthesia and thromboprophylaxis In both groups all patients received spinal anaesthesia. Thromboprophylaxis was always done with enoxaparin sodium, 4,000 IU per day, starting the day of surgery; a passive foot pump was used in both groups immediately after surgery.
Rehabilitation protocol A continuous passive motion machine was not used in either group. After operation, patients rested with the knee in full extension. From the second day after surgery passive and active range of motion (ROM) were started and patients were allowed to walk fully weight-bearing with crutches.
Computation of the blood loss For all patients one or two units were transfused as necessary according to the volume in the drains (> 100 ml), the clinical status and the haemodynamic parameters (Hb < 7.0 g/dl). The volume in the drains was recorded and the drains were removed on the second day after surgery.The blood loss was determined according to the Orthopaedic Surgery Transfusion Haemoglobin European Overview (OSTHEO) formula [13], which can be considered the most suitable for this purpose. This method calculates the blood loss by a formula that takes into account several parameters such as sex, weight, height, pre- and postoperative haematocrit, homologous and/or autologous transfusions, avoiding the difficulties of calculating the actual bleeding during surgery and postoperative period (Table 2).
Table 2.
OSTHEO algorithm for blood loss
| Total blood loss (ml) = [total RBC loss (ml)]/0.35 |
| Total RBC loss (ml) = [uncompensated RBC loss (ml)] + [compensated RBC loss (ml)] |
| Uncompensated RBC loss (ml) = [initial RBC (ml)] – [final RBC (ml)] |
| Compensated RBC loss = sum of RBCs received from the various sources of transfusion |
| Initial RBC (ml) = [estimated blood volume (ml)] × [initial Hct level (%)] at day −1 |
| Final RBC (ml) = [estimated blood volume (ml)] × [final Hct level (%)] at day +3 |
| Estimated blood volume (ml) = women: [body surface area (m2)] × 2,430; men: [body surface area (m2)] × 2,530 |
| Body surface area (m2) = 0.0235 × [height (cm)]0.42246 × [weight (kg)]0.52456 |
Statistical analysis The comparisons between means of the variable of interest were performed using the unpaired t-test, after checking the normality assumption through the Kolmogorov-Smirnov test.In order to assess the significance of the test statistics, a significant level for the p values was assumed equal to 0.05. Summary statistics as mean, standard deviation (SD) and 95% confidence intervals (CI) were also provided. SAS software (8.1 version) was used for computations.
Results
The mean tourniquet time was 75 min (60–85) in group A and 90 min (80–110) in group B (p < 0.001). This increase of 15 min was due to the different procedure concerning the CAS technique (computer investigation time, setting up of equipment, etc.) compared with the traditional technique.
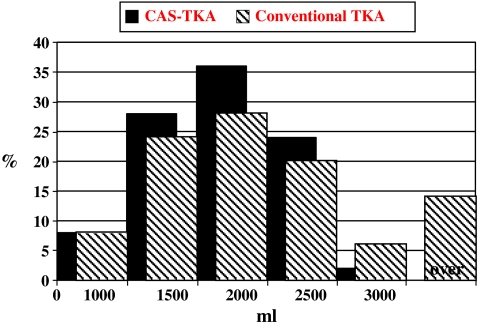
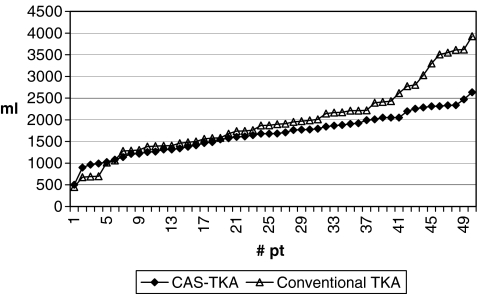
The mean total blood loss was 1,974 ml (range: 450–3,930, SD = 817.6) in group A and 1,677 ml (range: 500–2,634, SD = 463.8) in group B. This difference of 297 ml was statistically significant (p = 0.0283) (Figs. 1 and 2).
Fig. 1.
Histogram showing distribution of blood loss in the two groups (percentage of patients)
Fig. 2.
Graphic showing distribution of blood loss in the two groups (total patients)
The mean Hb loss in group A was 3.34 g/dl (SD = 1.23) versus 3.08 g/dl (SD = 1.03) in group B (p = 0.2667). The mean haematocrit loss in group A was 10.58% (SD = 3.98) versus 9.52% (SD = 2.91) in group B (p = 0.1315).
All patients operated with standard and CAS technique had satisfactory and comparable blood data (variation of Hb and Hct).
The mean total blood loss was 1,974 ml in group A and 1,677 ml in group B. This difference of 297 ml is mainly due to the procedure of blood reinfusion (Table 3).
Table 3.
Blood units reinfused
| Group A | Group B | |
|---|---|---|
| Conventional TKA | CAS-TKA | |
| # pt recovered autologous blood | 23 | 23 |
| # pt recovered homologous blood | 5 | 6 |
| # pt recovered from drainage bottle | 32 | 18 |
| Total # pt that recovered blood | 41 | 35 |
| # autologous blood units reinfused | 30 | 26 |
| # homologous blood units reinfused | 6 | 6 |
| Total # blood units reinfused | 36 | 32 |
| Total ml reinfused from drainage bottle | 11,150 | 4,900 |
| Total ml reinfused (auto. + homo. + drainage) | 25,550 | 17,700 |
| Mean of total blood reinfused | 543.3 | 371.1 |
# number, pt patients
An important figure is the different amount of total blood reinfused (auto. + homo. + drainage) in the two groups, 25,550 ml in group A and 17,700 ml in group B, although the most significant statistic concerns the need for blood reinfusion in the immediate postoperative period (six hours): in group B only 18 of 50 patients had to recover their blood from the drainage; in group A 32 of 50 patients recovered blood after surgery.
Discussion
Blood loss in total knee replacement is an important issue and is commonly underestimated [15]. Patients are usually older than 60 and concomitant pathological conditions are frequently observed, such as hypertension, diabetic disorders, heart disease, etc. The operative and postoperative risks can be increased by blood loss during and after surgery. It is well known from the orthopaedic literature [4] that there are many inherent errors in the estimation of the true blood loss after total knee replacement. The intraoperative blood loss accounted for by various estimates such as counting and weighing sponges and the postoperative blood loss as measured in suction bottles and drains do not reflect the true amount of blood lost by the patient. These measurements are the apparent losses. Quantification of blood loss during surgery is difficult because intraoperative and postoperative bleeding compounds total blood loss. Postoperative haemoglobin and haematocrit levels were not different in the two groups, but both the recovery from drainage bottles and the total reinfusion were statistically different. The mean total reinfusion was 543.3 ml in group A and 371.1 ml in group B. The larger reinfusion in group A compensates the haemoglobin and haematocrit levels. For this reason in order to determine blood loss we used the OSTHEO method [13], which can be considered the most suitable for the purpose of this study.
Orthopaedic surgeons recommend different solutions to minimise intraoperative bleeding, such as the insertion of a bone block to plug the entry hole made by the femoral intramedullary alignment rod [2], use of a tourniquet [21], diathermy coagulation, control of knee position [10], prophylactic administration of antifibrinolytic agents [14] and, more importantly, minimally invasive surgery [12, 20].
Kumar et al. [7], using an autologous bone graft to plug the femoral hole, found a significant difference in postoperative suction drainage between plugged and unplugged groups but no difference in the requirement for transfusion. In accordance with these findings, Raut et al. [11] reported the same results using an acrylic cement plug to seal the femoral hole. Ko et al. [6] in a similar study showed that sealing the femoral canal is effective in reducing haemoglobin decrease and transfusion requirement. As a matter of fact, the reason for the smaller amount of blood loss in group B in our study is probably due to the less invasive approach to the intramedullary canal with the CAS technique even if it involves the drilling of multiple bicortical pins. This is very important because using a standard technique the intramedullary femoral hole can be easily plugged with bone, in contrast to the CAS technique in which the smaller incision and the deepest part of the bicortical hole cannot be reached. The bicortical pin approach seems to be a safe procedure even if the risk of haematoma over the thigh cannot be excluded.
We also believe that a minor release of soft tissues observed in CAS, especially in the medial compartment and lateral retinaculum, may contribute to blood loss reduction.
Speck et al. [17] found wound drainage reduced by 30% after 70° flexion for six hours following total knee replacement. However, wound drainage may not represent total blood loss and obstruction of the drain in flexion or the relative elevation of the knee could have caused the reduction in drainage. In a prospective study Ong and Taylor [10] showed a 25% reduction of postoperative blood loss with the elevation of the leg at 35° from the hip with the knee extended for six hours postoperatively. In our groups all legs were extended and level with the bed. However, the study by Ong and Taylor suggests a simple, safe and effective way that may be positively adopted in TKA in association with CAS to further reduce total blood loss.
Lotke et al. [9] studied the effects of tourniquet release and continuous passive motion after total knee replacement. They divided the patients into four groups based on the use of a tourniquet and the immediate postoperative rehabilitation protocol. They found major blood loss in a group where the tourniquet was released intraoperatively, haemostasis was established and the tourniquet was reinflated; a compressive dressing was then applied and continuous passive motion was started immediately in the recovery room. Kalairajah et al. [5] showed less bleeding in CAS compared with a conventional procedure when using an uncemented implant, three suction drains for 48 hours and immediate passive movement.
In a prospective randomised study Vandenbussche et al. [21] studied the effect of tourniquet use in total knee arthroplasty. In one group of patients an arterial tourniquet was applied and in the other group a tourniquet was not used. They found that knee arthroplasties performed without the use of a tourniquet cause greater blood loss and have only small benefits in the early postoperative period.
In our study in all cases the tourniquet was released intraoperatively, haemostasis was achieved by cauterisation and a compressive dressing was applied. Postoperatively no patients were mobilised with continuous passive motion and the rehabilitation protocol was started two days after surgery when the drainage was removed.
We believe that CAS with a minimally invasive approach can reduce blood loss in total knee replacement especially since the intramedullary femoral hole is avoided. The intramedullary femoral hole could be a very crucial issue especially in uncemented mobile bearing knee replacement because avoiding its use can reduce bleeding in the early postoperative period. Probably the reason for the reduced blood loss as reported by Ko et al. [6] could depend on the reduced bleeding from the femoral medullary canal. Future studies should demonstrate that elevating the leg could significantly reduce the postoperative bleeding avoiding the intramedullary rod. The minor invasiveness associated with CAS and new instruments assist the surgeon in better soft tissue management. An increased surgical time in CAS does not seem to increase blood loss. Improved surgical skill and easier CAS procedures could reduce the surgical time in the future and, consequently, total blood loss. In fact, this is our first experience with CAS, with a significant increase of surgical exposure time, especially in the early cases. In our opinion blood loss will further decrease when surgeon and operating room nurse become more familiar with the computer and the new instruments.
We believe that the observed decrease of blood loss is one of the benefits of CAS. Better alignment of the limb and, maybe, a better long-term outcome can also be expected with this innovative procedure.
Acknowledgement
Special thanks to Serena Arima, Maria Brigida Ferraro and Stefania Gubbiotti of Dipartimento di Statistica Probabilità e Statistiche Applicate Università di Roma “La Sapienza”.
References
- 1.Chauhan SK, Scott RG, Breidahl W, Beaver RJ. Computer-assisted knee arthroplasty versus a conventional jig-based technique. A randomised, prospective trial. J Bone Joint Surg Br. 2004;86:372–377. doi: 10.1302/0301-620X.86B3.14643. [DOI] [PubMed] [Google Scholar]
- 2.Chin PK, Yang KY, Yeo SJ, Lo NN. Randomized control trial comparing radiographic total knee arthroplasty implant placement using computer navigation versus conventional technique. J Arthroplasty. 2005;20(5):618–626. doi: 10.1016/j.arth.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Davies BL, Harris SJ, Lin WJ, Hibberd RD, Middleton R, Cobb JC. Active compliance in robotic surgery—the use of force control as a dynamic constraint. Proc Inst Mech Eng [H] 1997;211:285–292. doi: 10.1243/0954411971534403. [DOI] [PubMed] [Google Scholar]
- 4.Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58:277–280. doi: 10.1097/00000542-198303000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Kalairajah Y, Simpson D, Cossey AJ, Verral GM, Spriggins AJ. Blood loss after total knee replacement: effects of computer-assisted surgery. J Bone Joint Surg Br. 2005;87:1480–1482. doi: 10.1302/0301-620X.87B11.16474. [DOI] [PubMed] [Google Scholar]
- 6.Ko PS, Tio MK, Tang YK, Tsang WL, Lam JJ. Sealing the intramedullary femoral canal with autologous bone plug in total knee arthroplasty. J Arthroplasty. 2003;18:6–9. doi: 10.1054/arth.2003.50001. [DOI] [PubMed] [Google Scholar]
- 7.Kumar N, Saleh J, Gardiner E, Devadoss VG, Howell FR. Plugging the intramedullary canal of the femur in total knee arthroplasty. J Arthroplasty. 2000;15:947–949. doi: 10.1054/arth.2000.8592. [DOI] [PubMed] [Google Scholar]
- 8.Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78:1260–1271. doi: 10.2106/00004623-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Lotke PA, Faralli VJ, Orenstein EM, Ecker ML. Blood loss after total knee replacement. Effects of tourniquet release and continuous passive motion. J Bone Joint Surg Am. 1991;73(7):1037–1040. [PubMed] [Google Scholar]
- 10.Ong SM, Taylor GJ. Can knee position save blood following total knee replacement? Knee. 2003;10(1):81–85. doi: 10.1016/S0968-0160(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 11.Raut VV, Stone MH, Wroblewski BM. Reduction of postoperative blood loss after press-fit condylar knee arthroplasty with use of a femoral intramedullary plug. J Bone Joint Surg Am. 1993;75:1356–1357. doi: 10.2106/00004623-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Repicci JA, Eberle RW. Minimally invasive surgical technique for unicondylar knee arthroplasty. J South Orthop Assoc. 1999;8:20–27. [PubMed] [Google Scholar]
- 13.Rosencher N, Kerkkamp HEM, Macheras G, Munuera LM, Menichella G, Barton DM, Cremers S, Abraham IL. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43:459–469. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 14.Samama CM. A direct antifibrinolytic agent in major orthopedic surgery. Orthopedics. 2004;27(6 Suppl):s675–s680. doi: 10.3928/0147-7447-20040602-09. [DOI] [PubMed] [Google Scholar]
- 15.Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. 2000;7(3):151–155. doi: 10.1016/S0968-0160(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 16.Sparmann M, Wolke B, Czupalla H, Banzer D, Zink A. Positioning of total knee arthroplasty with and without navigation support. A prospective, randomised study. J Bone Joint Surg Br. 2003;85:830–835. [PubMed] [Google Scholar]
- 17.Speck M, Jakob R, et al. Blood loss after total knee arthroplasty in relation to positioning: 70° flexion v extension J Bone Joint Surg Br 199981Suppl 1124510204929 [Google Scholar]
- 18.Stulberg SD, Picard F, Saragaglia D. Computer-assisted total knee replacement arthroplasty. Oper Tech Orthop. 2000;10:25–39. doi: 10.1016/S1048-6666(00)80040-3. [DOI] [Google Scholar]
- 19.Stulberg SD. How accurate is current TKR instrumentation? Clin Orthop Relat Res. 2003;416:177–184. doi: 10.1097/01.blo.0000093029.56370.0f. [DOI] [PubMed] [Google Scholar]
- 20.Tria AJ, Jr, Coon TM. Minimal incision total knee arthroplasty: early experience. Clin Orthop Relat Res. 2003;416:185–190. doi: 10.1097/01.blo.0000093030.56370.d9. [DOI] [PubMed] [Google Scholar]
- 21.Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306–309. doi: 10.1007/s00264-002-0360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]