Abstract
The objective of this study was to determine whether BMP-2 and -14, noggin, and chordin could be detected in human fractures and to assess their regional and cellular distribution. The expression of these proteins was detected by immunohistochemistry in an archive of human fractures. BMP-2 and BMP-14 expression was strongest in areas of cartilage formation and, to a lesser extent, in areas of bone formation. Within areas of cartilage formation, both BMP-2 and BMP-14 were expressed more strongly by the non-hypertrophic chondrocytes. The BMP inhibitors noggin and chordin were also expressed most intensely in areas of cartilage formation and there was no difference in their expression between the non-hypertrophic and hypertrophic chondrocytes. Our study demonstrates the expression of BMP-14 and the BMP inhibitors in human fractures for the first time, and our findings will contribute to an improved understanding of the physiological processes in bone repair.
Résumé
Le but de cette étude est de déterminer si la BMP-2 et la BMP-14 et leurs inhibiteurs noggin et chordin peuvent être détectées dans les fractures chez l’homme, avec une évaluation de leur distribution cellulaire et régionale. Matériels et méthode: l’expression de ces protéines est détectée par immunohistochimie. Résultats: la BMP-2 et la BMP-14 s’expriment de façon importante au niveau de la formation du cartilage et au niveau de la restauration osseuse. Au niveau du cartilage, la BMP-2 et la BMP-14 s’expriment surtout au niveau des chondrocytes non hypertrophiques. Les inhibiteurs de la BMP s’expriment également au niveau de la formation cartilagineuse, il n’y a pas de différence dans leur expression pour les chondrocytes non hyperthrophiques ou hyperthrophiques. En conclusion: notre étude démontre que l’expression de la BMP-14 et de ses inhibiteurs peuvent être détectées dans les fractures chez l’homme. Nos constatations doivent contribuer à une meilleure compréhension du processus de réparation osseuse.
Introduction
Fracture repair is a complex phenomenon, involving various cellular and molecular processes, under the influence of growth factors, biomechanical forces, and other systemic factors [4, 19]. A critical family of growth factors involved are the bone morphogenetic proteins (BMPs), which have received considerable attention because of their potential clinical applications. Cellular responses to BMPs are initiated by their binding to transmembrane receptors, whose cytoplasmic domains become phosphorylated at specific serine and threonine residues, thereby triggering Smad intracellular signalling pathways [24]. BMPs have been studied extensively in animal models of fracture repair, and BMP-2 and BMP-7 are currently in clinical use [3]. BMP-2 has been shown to be among the most osteoinductive members of the family, with biological activity throughout most of the stages of fracture repair [6].
A lesser known member of the BMP family is BMP-14, also known as growth and differentiation factor-5 and cartilage-derived morphogenetic-protein-1. BMP-14 influences endochondral bone growth [22] and its ectopic implantation intramuscularly induces the formation of cartilage and bone [9]. BMP-14 deficiency inhibits long bone fracture healing, secondary to a delay in the cellular recruitment and chondrocyte differentiation [7]. It is not known at what stage of fracture repair BMP-14 is expressed and which cells express the protein.
Although BMPs have shown some promise in improving fracture repair, there are still limitations associated with its widespread clinical use. Recombinant BMP-2 and BMP-7 are used clinically at supra-physiological concentrations and are very expensive. There is a distinct paucity of human data to match the impressive regenerative capacities shown by BMPs in animal models of fracture repair [14]. There is therefore a need for an improved understanding of the in vivo physiological activity of BMPs in human fractures.
The activity of BMPs can be limited by a number of extracellular physiological antagonists, which bind to them and interfere with their ability to induce receptor activation. These extracellular BMP inhibitors share structural similarities with their ligands, and when they bind to BMPs they prevent the subsequent binding of the BMPs to the BMP receptors. Two BMP inhibitors which have been studied in developmental systems are noggin and chordin.
Noggin is known to bind and antagonise BMP-2, 4, and 7, with a higher affinity for BMP-2 and 4 [27]. Noggin inhibits BMP-4 activity in a competitive manner by binding to BMP-4 and consequently interfering with the ability of BMP-4 to bind to cell-surface receptors [27]. Additionally, noggin has been shown to interact directly with BMP-14 in vitro and influences its effects on the skeletogenesis of the embryonic chick [17]. Chordin also binds BMP-2, 4, and 7, in a similar fashion to noggin [20]. To date, the expression of BMP inhibitors has not been demonstrated in human fractures.
The objectives of this study were therefore to determine whether BMP-2 and -14, noggin, and chordin could be detected in human fractures and to assess their regional and cellular distribution in healing fractures. Although the expression of BMP-2 has been demonstrated in human fractures previously [12], its relative expression compared to BMP inhibitors is not known. In in vitro models of osteogenesis, BMP inhibitors, such as noggin and chordin, are produced by osteoprogenitor cells and reduce the rate of osteogenic differentiation by exerting a paracrine/autocrine effect on physiologically-produced BMP-2 [1, 13]. The primary hypothesis driving this study was therefore that the physiological production of BMP inhibitors would occur in areas of bone formation during fracture repair in man. A sub-hypothesis was that the expression of the BMP inhibitors would be strongest in osteoprogenitor cells. Owing to the importance of BMP-14 in endochondral bone growth, it was also hypothesised that BMP-14 would be expressed locally in areas of cartilage formation within healing fractures.
Materials and methods
Human fracture specimens
The availability of fracture calluses from humans is limited. For sound ethical and clinical reasons, it is not possible to biopsy human fractures which are healing normally, as this would interfere with the healing process. Instead, to investigate healing fractures, our group and others have obtained biopsies from patients undergoing surgery and who were subsequently found to have fracture union on follow-up [2, 12].
All tissue samples used in this study were obtained with the approval of the Central Manchester and Manchester Children’s University Hospitals Local Research Ethics committees (in accordance with the Helsinki declaration) and informed consent was obtained from the patients. Biopsies of fracture calluses were obtained from patients who were being surgically treated for failure of conservative management, or failure of the original surgical fixation to maintain alignment of their fracture. These operations were all performed between one and four weeks after the initial injury. The biopsies were taken from 15 patients, aged between 18 and 87 years, who were otherwise fit. The sites of the fracture biopsies were extra-articular sites of the humerus, clavicle, femur, tibia, fibula, and acetabulum. All fractures were found to have united on subsequent follow-up.
The biopsy specimens were fixed in 10% neutral buffered formalin and decalcified in 20% EDTA (pH 7.2) until radiologically complete (two to four weeks). The specimens were then embedded in paraffin wax and sectioned at 5 μm. One slide per patient was stained with haematoxylin and eosin for routine evaluation of the stage of fracture repair and other slides used for immunohistochemical analysis.
Immunohistochemical analysis
All chemicals used were from Sigma-Aldrich UK, unless stated otherwise. Goat and rabbit (noggin only) polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, California, USA) were used for the immunohistochemical detection of the four proteins under investigation.
The sections were prepared for the immunohistochemistry by first dewaxing and rehydrating. After washing in deionised water, sections were exposed to their respective antigen retrieval system at 37°C. BMP-2 antigen retrieval consisted of exposure to 0.4% pepsin in 0.01 M hydrochloric acid for twenty minutes. BMP-14 antigen retrieval was performed by twenty-minute treatment with 0.01% chymotrypsin 0.1% CaCl 2 dissolved in Tris buffered saline (TBS). Noggin and chordin antigen retrieval was performed with exposure to 1 mg/ml of trypsin for fifteen minutes. The endogenous peroxidase enzyme activity was blocked using methanol containing 3% hydrogen peroxide for thirty minutes at room temperature. Following washing, non-specific binding was inhibited by the following incubation for thirty minutes at room temperature in a humid chamber: 10% swine serum/ 1% BSA (bovine serum albumin) for noggin, 10% donkey serum/2% BSA for chordin, 10% donkey serum/1% BSA for BMP-2, and 10% donkey serum/1% BSA for BMP-14.
Immunostaining was then carried out by applying the appropriate concentration of antibody to each sample (BMP-2: 1:10; BMP-14: 1:100; noggin 1:200; chordin 1:100) and incubating overnight at 4°C. As a negative control, normal IgG at the equivalent concentration was used in place of the primary antibodies. Following incubation, the samples were washed in TBS and incubated with the biotinylated secondary antibody (Santa Cruz Biotechnology) for one hour at room temperature. The avidin–biotin–peroxidase complex (Elite ABC Reagent, Vector Laboratories, Burlingame, CA, USA) was then added for thirty minutes at room temperature. The colour reaction was developed with use of 3, 3-diaminobenzidine tetrahydrochloride solution. Counterstaining was performed with use of Mayer’s Haematoxylin (Raymond A Lamb, East Sussex, UK), dehydrated, and a coverslip applied with Pertex (Histolab, Gothenburg, Sweden).
Image and statistical analysis
All slides were visualised using a Leica RMDB research microscope (Leica Camera Limited, Knowlhill, Milton Keynes, UK), and images were captured using a digital camera and Delta Pix Viewer Software (Delta Pix, Maalov, Denmark). Following immunohistochemistry, positive and negative controls were examined to check that the intensity of immunoreactivity was similar in all experiments. The slides were examined, using a ×10 objective lens, to determine whether there was positive staining in osteoblasts, osteocytes, fibroblastic cells, osteoclasts, non-hypertrophic chondrocytes, and hypertrophic chondrocytes. The characteristic hypertrophic morphology of chondrocyte rounding up, enlargement and the presence of a light staining cytoplasm, was used to distinguish between the two types of chondrocytes.
The proportion of all the cells staining positively within the cartilaginous area was evaluated using a previously established semi-quantitative scoring system [11]. For this purpose, each sample was scored according to: (a) the percentage of labelled cells (0 = absence of labelling over cells; 1 = less than 30% cells labelled; 2 = 30–60%; and 3 = more than 60%); and (b) the intensity of immunostaining (0 = no staining; 1 = weak; 2 = mild; and 3 = strong staining). Multiplication of both scores allowed the final scoring of samples, ranging from 0 to 9.
Comparison of immunohistochemistry score between the corresponding groups (hypertrophic vs non-hypertrophic chondrocytes) was made by the Student’s t test, using Microsoft Excel Software (Microsoft, Redmond, WA, USA), and the graphs were produced using Graph Pad Prism Software (Graph Pad, San Diego, CA, USA).
Results
Histological findings
Histological examination demonstrated the heterogeneous appearance of healing human fracture calluses (data not shown). There was a mixture of haematoma, fibrous tissue, woven and lamellar bone, and cartilage. Because of this heterogeneous appearance, a previously described classification was applied to group callus specimens into grades 1–3 according to the predominant type of tissue: grade 1, haematoma and granulation tissue; grade 2, definitive matrix formation with formation of cartilage and/or bone; grade 3, matrix remodelling [2].
Immunohistochemical findings
General findings
The expression of all four proteins in healing specimens was greatest in areas of endochondral ossification (stage 2), with some variation in the cell type within that region. There was no significant staining in stages 1 and 3 of the healing fractures. In subsequent experiments, analysis focussed on stage 2 of the fractures.
Expression of BMP inhibitors
Both noggin and chordin were expressed most intensely in areas of cartilage formation (Figs. 1 and 2). Staining was not predominant in any one particular cell type in areas of cartilage formation. There was no significant difference in staining between the hypertrophic chondrocytes and the non-hypertrophic chondrocytes for chordin and noggin. In addition, noggin expression was detected in active osteoblasts in areas of bone formation and its expression was also strong in endothelial cells and pericytes of the newly formed blood vessels of the fracture callus (Fig. 1). There was occasional expression of chordin in osteoblasts in healing human fractures (Fig. 2).
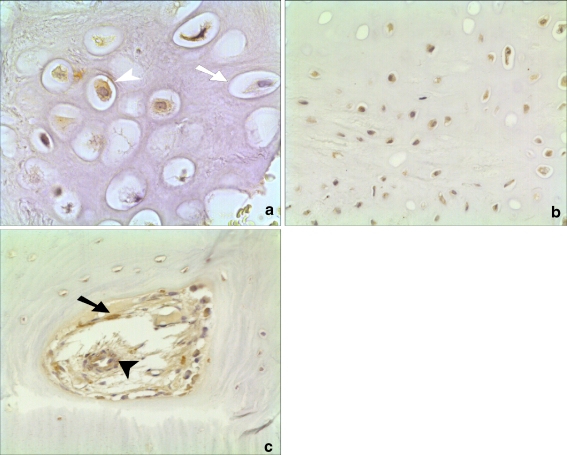
Fig. 1.
Histological appearance of noggin expression in a healing human fracture. a High-powered view (×40 magnification) showing brown staining in the nucleus of a hypertrophic chondrocyte (open arrowhead) compared to a non-stained cell (open arrow). b Staining in both hypertrophic and non-hypertrophic chondrocytes (×20). c Staining in new blood vessel forming in human fracture callus (closed arrowhead) and in osteoblast (closed arrow) (×20)
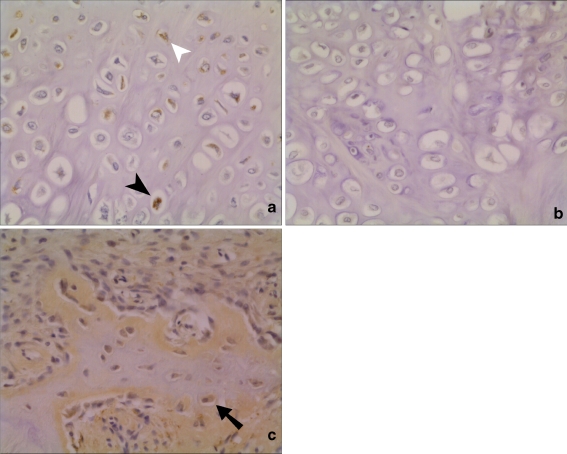
Fig. 2.
Histological appearance of chordin expression in human fractures (×20 view). a and b Serial sections of an area of cartilage formation. Staining in both hypertrophic (closed arrowhead) and non-hypertrophic chondrocytes (open arrowhead), with chordin antibody (a). No staining in when negative control IgG antibody was used as primary antibody during immunohistochemistry (b). c Expression of chordin in osteoblasts in area of active bone formation
Expression of BMP-2 and -14
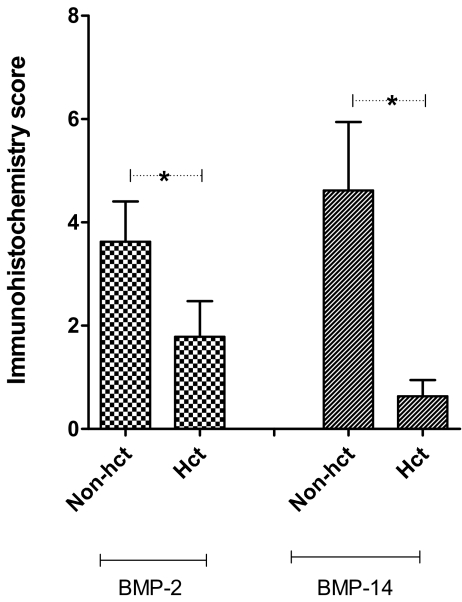
Of the four proteins under investigation, BMP-14 demonstrated the strongest staining in human fractures (however, different antigen presentation methods were used for the four proteins). A larger proportion of non-hypertrophic chondrocytes were immunopositive for BMP-14 and the intensity of staining was greater than the hypertrophic chondrocytes (Fig. 3). There was a statistically significant difference in the immunohistochemistry scores for BMP-14 of non-hypertrophic vs hypertrophic chondrocytes (p = 0.04) (Fig. 5). Expression of BMP-14 in osteoblasts and fibroblastic cells was also detected, although expression of BMP-14 was strongest in areas of cartilage formation.
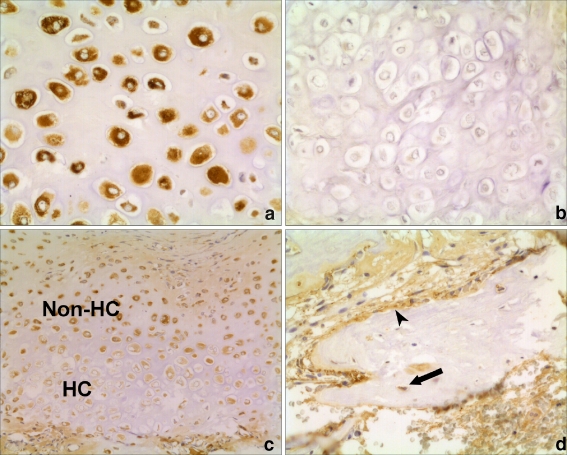
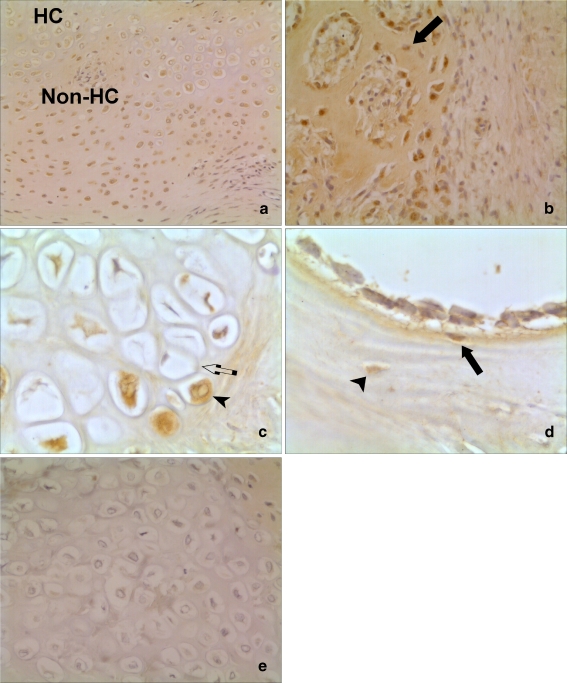
Fig. 3.
Histological appearance of BMP-14 expression in human fractures. a and b Serial sections of an area of cartilage formation. Intense staining in chondrocytes with BMP-14 antibody (a). No staining when negative control IgG antibody used as primary antibody during immunohistochemistry (×20 magnification) (b). c Increased expression of BMP-14 in the non-hypertrophic chondrocytes (non-HC), compared to the hypertrophic chondrocytes (HC) (×10). d Expression of BMP-14 by osteoblasts (arrowhead) and osteocytes (arrow) in areas of bone formation (×20)
Fig. 5.
Semi-quantitative expression of BMP-2 and -14 in cartilaginous areas of fractures. Chondrocytes were divided into the hypertrophic chondrocytes (Hct) and the rest were divided into the non-hypertrophic chondrocytes (non-hct). A paired Student’s t test was used to evaluate the difference in the scores between the two types of chondrocytes. *p ≤ 0.05
BMP-2 expression was strongest in areas of endochondral ossification, with the non-hypertrophic chondrocytes staining more intensely than the hypertrophic chondrocytes (Fig. 4). The immunohistochemistry scores for BMP-2 between the two types of chondrocytes were also statistically different (p = 0.05) (Fig. 5). BMP-2 was also expressed in osteoblasts, osteocytes, and osteoclasts to a lesser extent.
Fig. 4.
Histological appearance of BMP-2 expression in human fracture calluses. a and c Pattern of expression in area of cartilage formation. Staining predominant in non-hypertrophic chondrocytes (non-HC) compared to hypertrophic chondrocytes (HC) seen in a (×10). c Large power view (×40) of cytoplasmic staining in chondrocyte (arrowhead) compared to a non-staining cell adjacent (arrow). b (×10) and d (×40) Expression of BMP-2 in area of bone formation. Osteoblasts demonstrating BMP-2 expression are demonstrated with an arrow. There was weak expression of BMP-2 in osteocytes (arrowhead). e (×20) No staining when negative control IgG antibody used as primary antibody during immunohistochemistry. This section is a serial section of the one in (a)
Discussion
The initial focus of this study was to demonstrate the expression of the BMP inhibitors, noggin and chordin in human fractures. We chose to focus on these two BMP inhibitors because it has been shown that noggin blockade increases the osteogenic differentiation of murine MSCs [23], and chordin inhibition leads to an increase in the rate of osteogenic differentiation of human MSCs [13]. These studies suggest that it may be possible to increase the rate of fracture repair in vivo by downregulating the activity of physiologically expressed BMP inhibitors. The cellular expression of noggin was previously demonstrated in rodent fractures [25], while, to our knowledge, there are no published studies of the cellular expression of chordin in fracture repair.
This study shows, for the first time, that BMP inhibitors are expressed in human fractures. BMP inhibitor expression was present in areas of bone formation, as hypothesised. However, their expression was strongest in areas of cartilage formation, suggesting that BMP inhibitors have an important role in the cartilaginous bone formation route of fracture repair. Noggin was also expressed strongly within the cells of the vasculature, implying that these cells might have a role in fracture repair. It has been recently reported that endothelial cells co-express BMP-4 and noggin under some pathological conditions [5].
There was no detectable expression of BMPs nor of their inhibitors in stages 1 and 3 of human fractures. This implies that there is no endogenous expression of BMP-2 and -14, noggin and chordin during the haematoma and bone matrix remodelling stage of fracture repair. BMP-2 and BMP-14 were consistently expressed by osteoblasts and occasionally by osteocytes too. Noggin and, to a lesser extent, chordin were also expressed by osteoblasts, while there was no detectable expression of BMP inhibitors in osteocytes.
The observation that BMP inhibitor expression was strongest in areas of cartilage formation led to the investigation of a member of the BMP family, which is known to be involved in cartilage formation and had not previously been shown to be expressed in human fractures, namely, BMP-14. BMP-14 deficiency inhibits long bone fracture healing, secondary to a delay in the cellular recruitment and chondrocyte differentiation [7]. In a transgenic mouse model over-expressing BMP-14, it was found that BMP-14 promotes the chondrocytes to become hypertrophic [22]. In this study, it was shown that BMP-14 was strongly expressed by the non-hypertrophic chondrocytes and, to a lesser extent, hypertrophic chondrocytes, osteoblasts, and some osteocytes. The distribution of BMP-2 was almost similar to that of BMP-14, although the expression of BMP-2 was not as intense as that of BMP-14.
The pattern of expression of BMP ligands and their inhibitors in cartilaginous areas of fracture repair is interesting and may give insight into the physiological control of the maturation of chondrocytes during the endochondral ossification stage of fracture repair. Previous investigations into the effects of BMP signalling on chondrocyte maturation and hypertrophy have produced conflicting results [15]. Previous in vitro studies also demonstrate that noggin and BMPs modulate the chondrogenic differentiation of mesenchymal stem cells [10, 18]. Chordin attenuates the progression of the growth plate chondrocytes from proliferation to hypertrophy [26]. It is also known that BMP inhibitors are expressed by chondrocytes in human articular cartilage and that an abnormality in the expression of chordin may be involved in the pathogenesis of osteoarthritis [21].
The ratio of BMPs: BMP inhibitors’ expression was higher in the areas occupied predominantly by non-hypertrophic chondrocytes, compared to those in which hypertrophic chondrocytes were predominant. Our data on the local expression of BMPs and their inhibitors in areas of cartilage formation suggest that there is a decrease in the gradient of BMP ligands when the chondrocytes change in morphology from the non-hypertrophic to hypertrophic phenotype. We believe that this has not been reported previously. The ratio of BMPs and their inhibitors may have a role in the duration of the endochondral ossification stage of fracture repair. This would explain why the pharmacological application of BMP-2 increases the rate of callus maturation in a rodent model of fracture repair [8].
To date, biological methods of improving fracture repair have focussed on promoting an osteoinductive environment, via the delivery of BMPs [3, 16]. However, targeting BMP inhibitors, such as chordin and noggin, may provide a novel therapeutic strategy in improving fracture repair [13, 23]. The downregulation of BMP inhibitors in vivo may be achieved with a local injection of short interfering RNA at the site of skeletal injury, an approach yet to be validated in animal models. Alternative approaches include the use of neutralising antibodies, gene transfer, and the design of small molecule antagonists.
In summary, the novel finding of this study is that the expression of the BMP inhibitors, noggin and chordin, was strongest in areas of cartilage formation and, to a lesser extent, areas of bone formation in human fractures. These findings can have useful implications in the treatment of fractures.
Acknowledgement
This study was supported by NIH grant AR 050243.
References
- 1.Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O’Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15:663–673. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 2.Andrew JG, Hoyland JA, Freemont AJ, Marsh DR. Platelet-derived growth factor expression in normally healing human fractures. Bone. 1995;16:455–460. doi: 10.1016/8756-3282(95)90191-4. [DOI] [PubMed] [Google Scholar]
- 3.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31:721–727. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borovecki F, Pecina-Slaus N, Vukicevic S. Biological mechanisms of bone and cartilage remodelling-genomic perspective. Int Orthop. 2007;31:799–805. doi: 10.1007/s00264-007-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- 6.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Chhabra A, Zijerdi D, Zhang J, Kline A, Balian G, Hurwitz S. BMP- 14 deficiency inhibits long bone fracture healing: a biochemical, histologic, and radiographic assessment. J Orthop Trauma. 2005;19:629–634. doi: 10.1097/01.bot.0000177108.38461.9c. [DOI] [PubMed] [Google Scholar]
- 8.Einhorn TA, Majeska RJ, Mohaideen A, Kagel EM, Bouxsein ML, Turek TJ, Wozney JM. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003;85-A:1425–1435. doi: 10.2106/00004623-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hotten GC, Matsumoto T, Kimura M, Bechtold RF, Kron R, Ohara T, Tanaka H, Satoh Y, Okazaki M, Shirai T, Pan H, Kawai S, Pohl JS, Kudo A. Recombinant human growth/differentiation factor 5 stimulates mesenchyme aggregation and chondrogenesis responsible for the skeletal development of limbs. Growth Factors. 1996;13:65–74. doi: 10.3109/08977199609034567. [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Akiyama H, Shigeno C, Nakamura T. Noggin and bone morphogenetic protein-4 coordinately regulate the progression of chondrogenic differentiation in mouse clonal EC cells, ATDC5. Biochem Biophys Res Commun. 1999;260:240–244. doi: 10.1006/bbrc.1999.0882. [DOI] [PubMed] [Google Scholar]
- 11.Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plenat F, Leclere J, Duprez A, Weryha G. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:656–658. doi: 10.1210/jc.86.2.656. [DOI] [PubMed] [Google Scholar]
- 12.Kloen P, Paola M, Borens O, Richmond J, Perino G, Helfet DL, Goumans MJ. BMP signaling components are expressed in human fracture callus. Bone. 2003;33:362–371. doi: 10.1016/S8756-3282(03)00191-1. [DOI] [PubMed] [Google Scholar]
- 13.Kwong FN, Richardson SM, Evans CH. Chordin knockdown enhances the osteogenic differentiation of human mesenchymal stem cells. Arthritis Res Ther. 2008;10:R65. doi: 10.1186/ar2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwong FN, Harris MB (2008) Recent developments in the biology of fracture repair. J Am Acad Orthop Surg (in press) [DOI] [PubMed]
- 15.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 16.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31:729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merino R, Macias D, Ganan Y, Economides AN, Wang X, Wu Q, Stahl N, Sampath KT, Varona P, Hurle JM. Expression and function of Gdf-5 during digit skeletogenesis in the embryonic chick leg bud. Dev Biol. 1999;206:33–45. doi: 10.1006/dbio.1998.9129. [DOI] [PubMed] [Google Scholar]
- 18.Nifuji A, Kellermann O, Noda M. Noggin inhibits chondrogenic but not osteogenic differentiation in mesodermal stem cell line C1 and skeletal cells. Endocrinology. 2004;145:3434–3442. doi: 10.1210/en.2003-0685. [DOI] [PubMed] [Google Scholar]
- 19.Pecina M, Vukicevic S. Biological aspects of bone, cartilage and tendon regeneration. Int Orthop. 2007;31:719–720. doi: 10.1007/s00264-007-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccolo S, Sasai Y, Lu B, Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/S0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tardif G, Hum D, Pelletier JP, Boileau C, Ranger P, Martel-Pelletier J. Differential gene expression and regulation of the bone morphogenetic protein antagonists follistatin and gremlin in normal and osteoarthritic human chondrocytes and synovial fibroblasts. Arthritis Rheum. 2004;50:2521–2530. doi: 10.1002/art.20441. [DOI] [PubMed] [Google Scholar]
- 22.Tsumaki N, Tanaka K, Arikawa-Hirasawa E, Nakase T, Kimura T, Thomas JT, Ochi T, Luyten FP, Yamada Y. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol. 1999;144:161–173. doi: 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan DC, Pomerantz JH, Brunet LJ, Kim JB, Chou YF, Wu BM, Harland R, Blau HM, Longaker MT. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem. 2007;282:26450–26459. doi: 10.1074/jbc.M703282200. [DOI] [PubMed] [Google Scholar]
- 24.Westerhuis RJ, Bezooijen RL, Kloen P. Use of bone morphogenetic proteins in traumatology. Injury. 2005;36:1405–1412. doi: 10.1016/j.injury.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura Y, Nomura S, Kawasaki S, Tsutsumimoto T, Shimizu T, Takaoka K. Colocalization of noggin and bone morphogenetic protein-4 during fracture healing. J Bone Miner Res. 2001;16:876–884. doi: 10.1359/jbmr.2001.16.5.876. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D, Ferguson CM, O’Keefe RJ, Puzas JE, Rosier RN, Reynolds PR. A role for the BMP antagonist chordin in endochondral ossification. J Bone Miner Res. 2002;17:293–300. doi: 10.1359/jbmr.2002.17.2.293. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerman LB, Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/S0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]