Abstract
In this study, we performed a mechanical analysis of the effect of fibroblast growth factor-2 (FGF-2) on autologous osteochondral transplantation in a rabbit model. A full-thickness cartilage defect (diameter: 5 mm; depth: 5 mm) made in the right femoral condyle was treated with osteochondral transplantation using an osteochondral plug (diameter: 6 mm; depth: 5 mm) taken from the left femoral condyle. The animals were divided into three groups: Group I, the defect was filled with 0.1 ml of gelatin hydrogel containing 1 μg of FGF-2; Group II, the defect was filled with 0.1 ml of gelatin hydrogel only; Group III, the defect was left untreated. Thereafter, osteochondral plugs were transplanted and the transplanted osteochondral grafts were evaluated mechanically and histologically at postoperative weeks 1, 3, 8 and 12. The structural property of the osteochondral graft was significantly greater in Group I than in Groups II and III at postoperative week 3. Histological analysis at 3 weeks revealed a tendency towards increased subchondral bone trabeculae in Group I compared with the other groups. Autologous osteochondral grafts transplanted with gelatin hydrogel containing FGF-2 acquired adequate stiffness at an early postoperative phase.
Résumé
Le but de ce travail est de mettre en évidence les effets du « fibroblast growth factor-2 » (FGF-2) sur les greffes autologues ostéo cartilagineuses chez le lapin. Une lésion cartilagineuse de 5 mm de diamètre et de 5 mm de profondeur a été réalisée sur l’extrémité inférieure du fémur droit du lapin. Le traitement a été réalisé par une greffe ostéo cartilagineuse, utilisant un implant ostéochondral de type bouchon de 6 mm de diamètre et de 5 mm d’épaisseur prélevé sur le condyle fémoral gauche. Les animaux ont été divisés en 3 groupes : groupe 1 le défect étant traité par mise en place d’un hydrogel de gélatine de 0.1ml et contenant 1 fEg FGF-2. Le groupe 2 est traité seulement avec un gel de gélatine hydrogel isolé et le groupe 3 n’a pas été traité. Par la suite, les transplants ont été prélevés, et évalués mécaniquement, histologiquement à 1, 3, 8 et 12 semaines post-opératoires. En trois semaines, l’aspect structurel de la greffe est bien meilleur dans le groupe 1 que dans les groupes 2 et 3. Néanmoins, en ce qui concerne l’analyse histologique, toujours à 3 semaines, il existe une tendance à l’amélioration de l’ossification sous chondrale avec ossification trobéculaire. En conclusion, la transplantation de greffes ostéo autologues, de greffes ostéo cartilagineuses en association avec un hydrogel de gélatine contenant du FGF-2, permet d’avoir une meilleure résistance du transplant dans la phase post-opératoire précoce.
Introduction
An articular cartilage lesion caused by injury or degenerative joint disease does not spontaneously repair, as the articular cartilage has a limited capacity for regeneration. Abrasion arthroplasty, subchondral drilling, autologous osteochondral grafts, periosteal–perichondral grafts, chondrocyte transplantation and gene therapy have been developed for the treatment of focal chondral lesions with acceptable results [1, 7, 12, 19, 22]. Successful long-term clinical results are obtained with the use of an autologous osteochondral cylindrical graft for the repair of osteochondral defects [5, 11, 18].
Both histological and mechanical analyses are important for evaluating the effects of autologous osteochondral transplantation. Histological analysis of transplanted osteochondral grafts indicates that, in an optimal autologous osteochondral transplantation, osteochondral grafts are incorporated into the defect, thereby, retaining the integrity of hyaline cartilage and cancellous bone, and maintaining the congruity of the articular surface in weight-bearing areas [5, 13]. In contrast, changes in the mechanical properties of the transplanted osteochondral graft have been analysed in various autologous osteochondral transplantation models and evaluated at various periods after surgery [9, 10, 15, 28]. We previously demonstrated that the structural properties of an osteochondral cylinder-graft-recipient construct immediately after surgery are not significantly different from those of normal cartilage and that the structural properties of an osteochondral cylinder-graft-recipient reconstruction are significantly worse at postoperative weeks 1 through 8 than in normal cartilage [14]. We speculated that changes in the structural properties of the transplanted osteochondral graft are influenced by the healing process at the subchondral bone level.
Many growth factors affected chondrocyte metabolism and chondrogenesis [1, 8, 20]. Basic fibroblastic growth factor (FGF-2) promotes the repair response in full-thickness articular cartilage defects and bone formation [3, 4, 6, 17, 25]. As a carrier material system, the gelatin-hydrogel-incorporated growth factor leads to regeneration for cell differentiation and proliferation [24]. Therefore, we hypothesised that osteochondral grafts treated with FGF-2-impregnated gelatin hydrogel will develop adequate structural properties earlier after surgery. We therefore analysed the effect of FGF-2 on autologous osteochondral transplantation in a rabbit model.
Methods
This investigation was approved by the Animal Research Committee of the Kobe University Graduate School of Medicine according to the guidelines for animal experimentation. Skeletally mature female Japanese white rabbits (n = 60; Kitayama Labs, Nagano, Japan) were used in the study. The mean weight was approximately 3.2 kg (range, 2.7–3.5 kg). Human recombinant basic FGF and gelatin hydrogel with a water content of 98% were provided by Kaken Pharmaceutical Co., Ltd. (Tokyo, Japan).
Preparation of gelatin hydrogel
Gelatin was crosslinked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide, hydrochloride salt (EDC) to prepare the biodegradable hydrogel. Following the crosslinking reaction for 24 h at 4°C, the resulting hydrogel was immersed for 1 h in HCl aqueous solution (pH 3.0), washed with water to remove the unreacted EDC and dried. Then, 100 μl of distilled water containing 1 μg of FGF-2 was impregnated into the dried hydrogel to prepare the FGF-2-impregnated hydrogel. Similarly, FGF-2-free gelatin hydrogel was prepared using distilled water without FGF-2.
Surgical procedures
Intravenous pentobarbital sodium solution (30 mg/kg body weight) was used to induce and maintain general anaesthesia. The rabbits were placed in a supine position and the surgery was performed on the right knee. In each rabbit, the lower limbs were disinfected and 3 ml of 1% lidocaine was injected subcutaneously into the parapatellar region. A medial parapatellar approach was used to expose the knee joint and the patella was laterally dislocated. The region of the femoral groove, which contacted the patella when the knee was flexed at 90°, was selected as the site for the osteochondral defect. To produce the cartilage defect, a full-thickness cylindrical osteochondral fragment (diameter: 5 mm; depth: 5 mm), which included the articular cartilage and subchondral bone, was removed using the Osteochondral Autograft Transfer System (OATS: Arthrex, Naples, FL).
The animals were divided into three groups: in Group I, the defect was filled with 0.1 ml of gelatin hydrogel containing 1 μg of FGF-2 before osteochondral transplantation; in Group II, the defect was filled with 0.1 ml of gelatin hydrogel without FGF-2 before osteochondral transplantation; in Group III, the defect was repaired with an osteochondral plug using neither hydrogel nor FGF-2. To repair the defect, an osteochondral plug (diameter: 6 mm; depth: 5 mm) taken from the left femoral condyle using the OATS was transplanted. The patella was repositioned and the capsule was repaired with 4-0 nylon sutures. The rabbits were allowed to feed freely in their cage immediately after the operation without a cast.
The implanted osteochondral grafts were then evaluated mechanically at postoperative weeks 1, 3, 8 and 12. The specimen was examined histologically after mechanical analysis at each time point and five rabbits were sacrificed at each time point; there were 20 animals in each group.
Mechanical analysis
The structural properties were assessed using a tactile sensor system (Biosensor system: AXIOM Co. Ltd., Fukushima, Japan). This system functions on the basis of changes in the resonance frequency under non-touching and touching conditions, which depend on the stiffness or hardness of the object [16, 26, 29]. When the tactile sensor, vibrating at its own frequency, touches the articular cartilage of the osteochondral graft, the resulting shift in resonance frequency is recorded using a computer. The resonance frequency was transformed to stiffness using the calibration formula (N/m2)=233.2×Δf (Hz)+104948.0 (where R2 = 0.91) [26]. The stiffness data were analysed by one-way analysis of variance (one-way ANOVA). A p value of less than 0.05 was considered to be statistically significant.
Histological evaluation
For histological examination, the resected tissue samples were fixed in 4% paraformaldehyde phosphate buffer solution, decalcified with 0.25 mol/L of ethylenediaminetetraacetic acid in phosphate buffered saline at pH 7.5, dehydrated in graded alcohol solutions and embedded in paraffin wax. Sagittal sections (7-μm thick) were cut and stained with haematoxylin and eosin. Serial sections were examined microscopically to assess the osteochondral graft.
Results
Mechanical analysis
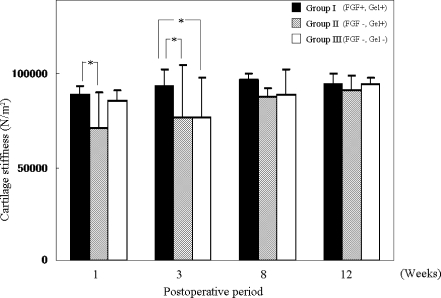
In Group I, at postoperative weeks 1, 3, 8 and 12, the average resonance frequency (Δf) obtained with the tactile sensor was −66.5 ± 18.8 Hz, −48.8 ± 39.0 Hz, −33.3 ± 13.6 Hz and −43.2 ± 25.8 Hz, respectively. In Group II, at weeks 1, 3, 8 and 12, the Δf was −145.1 ± 84.1 Hz, −123.2 ± 122.2 Hz, −73.6 ± 19.1 Hz and −60.2 ± 34.2 Hz, respectively. In Group III, at weeks 1, 3, 8 and 12, the Δf was −80.8 ± 22.3 Hz, −119.0 ± 91.3 Hz, −69.3 ± 58.9 Hz and −44.6 ± 18.1 Hz, respectively. The normal cartilage stiffness at the femoral condyle was −43.7 ± 15.1. Stiffness was calculated using these data and the calibration formula (Fig. 1). In Group I, at postoperative weeks 1, 3, 8 and 12, the average stiffness was 89,430.9 ± 4,388.5, 93,558.5 ± 9,083.5, 97,177.8 ± 3,161.5 and 94,883.1 ± 6,025.3 (N/m2), respectively. In Group II, at weeks 1, 3, 8 and 12, the average stiffness was 71,110.7 ± 19,615.8, 76,217.8 ± 284,96.8, 87,779.8 ± 4,453.9 and 90,918.7 ± 7,975.4 (N/m2), respectively. In Group III, at weeks 1, 3, 8 and 12, the average stiffness was 86,114.8 ± 5,205.6, 77,197.2 ± 21,294.1, 88,777.9 ± 13,730.6 and 94,547.3 ± 4,219.3 (N/m2), respectively. The normal cartilage stiffness was 94,747.9 ± 3,524.1.
Fig. 1.
Stiffness of the articular cartilage based on the calibration formula (N/m2). *indicates significant differences (p < 0.05)
At postoperative week 1, the average stiffness in Group I was significantly greater than that of Group II. At postoperative week 3, the average stiffness in Group I was significantly greater than that in Groups II and III. At postoperative weeks 8 and 12, there were no significant differences in the average stiffness among the three groups.
Histological evaluation
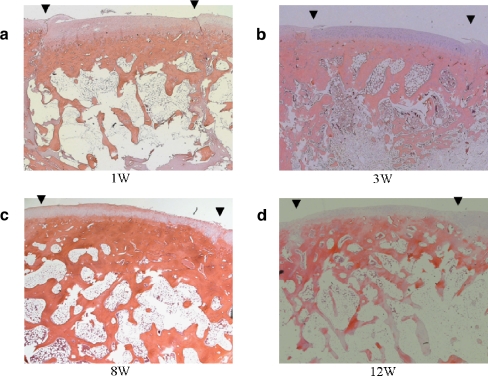
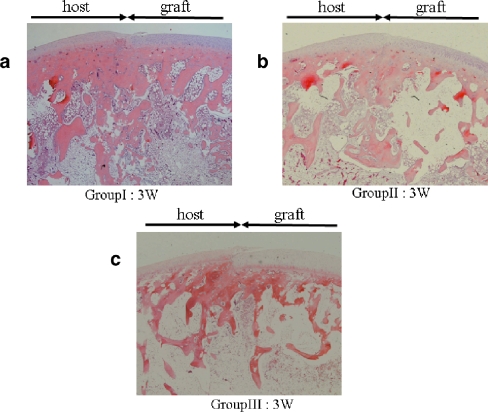
At postoperative week 1, there was a slight gap between the transplanted graft and the recipient site in Group I (Fig. 2a). At 3 weeks, the gap had disappeared and structural integrity of the subchondral bone was observed in all groups (Figs. 2b and 3a–c). The subchondral bone trabeculae of Group I had increased and developed more than that of the other groups. At 8 weeks, the subchondral bone trabeculae had increased even further and progressed to remodelling in Group I (Fig. 2c). The findings in Groups II and III were similar to those in Group I. At 12 weeks, the subchondral bone trabeculae of Group I had decreased and the transplanted grafts were united. The articular surface regularity was smooth (Fig. 2d). In Group I, in which FGF-2 was used, neither hypertrophic nor arthritic change was observed at the graft and the host cartilage during the entire postoperative period.
Fig. 2a–d.
Histological evaluation of the transplanted graft in Group I (defect filled with gelatin hydrogel including 1 μg of FGF-2). a At week 1, there was a slight gap between the graft and the recipient site. b At week 3, the gap had disappeared and the articular layer and subchondral bone increased and developed. c At week 8, the subchondral bone trabeculae had increased. d At week 12, the subchondral bone trabeculae had decreased and the transplanted graft was almost the same as in the other groups. The arrows indicate the margins of the graft (original magnification ×25)
Fig. 3a–c.
Histological evaluation of the junction between the graft and the host (Group I: defect filled with gelatin hydrogel including FGF-2, Group II: defect filled with gelatin hydrogel only, Group III: defect in which neither hydrogel nor FGF was used). At week 3, the structural integrity of the subchondral bone was observed in all groups. Especially, the subchondral bone trabeculae in Group I (a) were increased and more developed than that in Groups II (b) and Group III (c). The arrows indicate the area of the graft and host (original magnification ×25)
Discussion
The use of gelatin hydrogel containing FGF-2 in osteochondral transplantation significantly increased the stiffness of the osteochondral plug at postoperative week 3. The mechanical properties of the transplanted grafts in Group I, in which FGF-2 was used, recovered to a normal level by postoperative week 3, which is earlier than that in Groups II and III. Histological analysis in Group I confirmed the developmental structural integrity of the subchondral bone at postoperative week 3 and there were neither hypertrophic nor arthritic changes of the cartilage layer.
FGF-2 has a mitogenic factor that stimulates chondrocyte differentiation and supports articular cartilage defect repair in vitro [6, 27]. Weisser et al. [27] reported that the treatment of chondrocyte cultures with FGF-2 had a stabilising effect on the differentiated state of the cells in vitro. Otsuka et al. [17] reported that the articular cartilage and subchondral bone regenerated within 8 weeks when FGF-2 is directly administered by osmotic pump in a full-thickness articular defect in a rabbit model. Fujimoto et al. [3] documented cartilage repair following the administration of a collagen sponge containing 700 ng of FGF-2 in a rabbit model. On the other hand, an overdose of FGF-2 induces hypertrophic arthritis [21, 25]. Shida et al. [21], using various doses of FGF-2, reported that the injection of more than 5 μg in normal rat knees resulted in an approximately 3- to 4.5-fold increase in articular cartilage compared to the same area of the control knee. As the half-life period of FGF-2 in vivo is too short to effectively exert biological activity when administered in free form, the biological effects of the growth factor are not reliable [24]. In our study, gelatin hydrogel was used as the drug delivery system because FGF-2-impregnated gelatin hydrogel efficiently induces neovascularisation, tissue granulation and bone regeneration [23]. The biologically active FGF-2 was released as a result of in vivo degradation of the gelatin hydrogel [24]. Therefore, we attempted to use FGF-2-impregnated gelatin hydrogel with osteochondral transplantation to accelerate cartilage repair and subchondral bone remodelling.
In the transplanted osteochondral graft, stiffness might be temporarily decreased in the postoperative course [15, 28]. We previously reported decreased stiffness at postoperative weeks 1, 3 and 8 compared to normal cartilage stiffness. The stiffness at postoperative week 3 was the lowest, suggesting that subchondral bone formation and remodelling influences the changes of cartilage stiffness [14] and similar results were found in this study; that is, the stiffness of the transplanted grafts decreased the most at postoperative week 3 in Group III. At postoperative week 3, the average stiffness in Group I was significantly greater than that in Group III. At postoperative weeks 8 and 12, there were no significant differences in the average stiffness among the three groups. We speculate that FGF-2 accelerates the regeneration of the subchondral bone in articular cartilage defects and promotes the recovery of structural property on the osteochondral plug.
The histological evaluation of grafts transplanted with FGF-2 gelatin hydrogel at postoperative week 3 indicated that the gap between the graft and the recipient site had disappeared and that the subchondral bone trabeculae tended to increase. These findings are consistent with the idea that the articular cartilage and subchondral bone were regenerated by FGF-2 [17]. Thus, the histological findings are compatible with those of the mechanical analysis, indicating that the grafts transplanted with FGF-2 hydrogel were more stable than those in the control group.
Autologous osteochondral transplantation gained popularity for the treatment of articular cartilage injuries following successful experiments and clinical results [2, 5, 11, 18]. Our study suggests that FGF-2 accelerates the integration of the graft and the host in articular cartilage defects in an animal model. A limitation of our study is that the optimal dose of FGF-2 with autologous osteochondral transplantation was not identified and the long-term effects of FGF-2, such as osteoarthritis in humans, is unknown.
In conclusion, the findings of this study suggest that autologous osteochondral grafts treated with gelatin hydrogel containing FGF-2 acquired adequate stiffness in the early postoperative phase in a rabbit model. Further investigations with variable doses of FGF-2 and the long-term follow up of autologous osteochondral transplantation are ongoing for clinical applications of FGF-2.
Contributor Information
Hiroyuki Fujioka, Phone: +81-78-3825985, FAX: +81-78-3516944, Email: hfujioka@med.kobe-u.ac.jp.
Yasuhiko Tabata, Phone: +81-75-7513802, FAX: +81-75-7514646.
References
- 1.Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 2.Chow JCY, Hantes ME, Houle JB, Zalavras CG. Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: a 2- to 5-year follow-up study. Arthroscopy. 2004;20:681–690. doi: 10.1016/j.arthro.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Fujimoto E, Ochi M, Kato Y, Mochizuki Y, Sumen Y, Ikuta Y. Beneficial effect of basic fibroblast growth factor on the repair of full-thickness defects in rabbit articular cartilage. Arch Orthop Trauma Surg. 1999;119:139–145. doi: 10.1007/s004020050377. [DOI] [PubMed] [Google Scholar]
- 4.Fujisato T, Sajiki T, Liu Q, Ikada Y. Effect of basic fibroblast growth factor on cartilage regeneration in chondrocyte-seeded collagen sponge scaffold. Biomaterials. 1996;17:155–162. doi: 10.1016/0142-9612(96)85760-7. [DOI] [PubMed] [Google Scholar]
- 5.Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85:S25–S32. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- 6.Henson FM, Bowe EA, Davies ME. Promotion of the intrinsic damage-repair response in articular cartilage by fibroblastic growth factor-2. Osteoarthritis Cartilage. 2005;13:537–544. doi: 10.1016/j.joca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Jakobsen RB, Engebretsen L, Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005;87:2232–2239. doi: 10.2106/JBJS.D.02904. [DOI] [PubMed] [Google Scholar]
- 8.Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Maticic D, McCartney J, Yin S, Rueger D, Vukicevic S. Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors. 2001;19:101–113. doi: 10.3109/08977190109001079. [DOI] [PubMed] [Google Scholar]
- 9.Kuroki H, Nakagawa Y, Mori K, Ikeuchi K, Nakamura T. Mechanical effects of autogenous osteochondral surgical grafting procedures and instrumentation on grafts of articular cartilage. Am J Sports Med. 2004;32:612–620. doi: 10.1177/0095399703258744. [DOI] [PubMed] [Google Scholar]
- 10.Lane JG, Tontz WL, Jr, Ball ST, Massie JB, Chen AC, Bae WC, Amiel ME, Sah RL, Amiel D. A morphologic, biochemical, and biomechanical assessment of short-term effects of osteochondral autograft plug transfer in an animal model. Arthroscopy. 2001;17:856–863. doi: 10.1016/S0749-8063(01)90010-6. [DOI] [PubMed] [Google Scholar]
- 11.Laprell H, Petersen W. Autologous osteochondral transplantation using the diamond bone-cutting system (DBCS): 6–12 years’ follow-up of 35 patients with osteochondral defects at the knee joint. Arch Orthop Trauma Surg. 2001;121:248–253. doi: 10.1007/s004020000217. [DOI] [PubMed] [Google Scholar]
- 12.Lind M, Bünger C. Orthopaedic applications of gene therapy. Int Orthop. 2005;29:205–209. doi: 10.1007/s00264-005-0650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makino T, Fujioka H, Terukina M, Yoshiya S, Matsui N, Kurosaka M. The effect of graft sizing on osteochondral transplantation. Arthroscopy. 2004;20:837–840. doi: 10.1016/j.arthro.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 14.Nakaji N, Fujioka H, Nagura I, Kokubu T, Makino T, Sakai H, Kuroda R, Doita M, Kurosaka M. The structural properties of an osteochondral cylinder graft-recipient construct on autologous osteochondral transplantation. Arthroscopy. 2006;22:422–427. doi: 10.1016/j.arthro.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Nam EK, Makhsous M, Koh J, Bowen M, Nuber G, Zhang L-Q. Biomechanical and histological evaluation of osteochondral transplantation in a rabbit model. Am J Sports Med. 2004;32:308–316. doi: 10.1177/0363546503259616. [DOI] [PubMed] [Google Scholar]
- 16.Omata S, Terunuma Y. New tactile sensor like the human hand and its applications. Sens Actuators A. 1992;35:9–15. doi: 10.1016/0924-4247(92)87002-X. [DOI] [Google Scholar]
- 17.Otsuka Y, Mizuta H, Takagi K, Iyama K, Yoshitake Y, Nishikawa K, Suzuki F, Hiraki Y. Requirement of fibroblast growth factor signaling for regeneration of epiphyseal morphology in rabbit full-thickness defects of articular cartilage. Develop Growth Differ. 1997;39:143–156. doi: 10.1046/j.1440-169X.1997.t01-1-00003.x. [DOI] [PubMed] [Google Scholar]
- 18.Oztürk A, Ozdemir MR, Ozkan Y. Osteochondral autografting (mosaicplasty) in grade IV cartilage defects in the knee joint: 2- to 7-year results. Int Orthop. 2006;30:200–204. doi: 10.1007/s00264-005-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pecina M, Jelic M, Ivkovic A, Hudetz D. Gene therapy applications in orthopaedics. Int Orthop. 2006;30:215–216. doi: 10.1007/s00264-005-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pecina M, Jelic M, Martinovic S, Haspl M, Vukicevic S. Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop. 2002;26:131–136. doi: 10.1007/s00264-002-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shida J-I, Jingushi S, Izumi T, Iwaki A, Sugioka Y. Basic fibroblast growth factor stimulates articular cartilage enlargement in young rats in vivo. J Orthop Res. 1996;14:265–272. doi: 10.1002/jor.1100140215. [DOI] [PubMed] [Google Scholar]
- 22.Smith GD, Knutsen G, Richardson JB. A clinical review of cartilage repair techniques. J Bone Joint Surg Br. 2005;87:445–449. doi: 10.1302/0301-620X.87B4.15971. [DOI] [PubMed] [Google Scholar]
- 23.Tabata Y, Hijikata S, Ikada Y. Enhanced vascularization and tissue granulation by basic fibroblast growth factor impregnated in gelatin hydrogels. J Control Release. 1994;31:189–199. doi: 10.1016/0168-3659(94)00035-2. [DOI] [Google Scholar]
- 24.Tabata Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003;9:S5–S15. doi: 10.1089/10763270360696941. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka H, Mizokami H, Shiigi E, Murata H, Ogasa H, Mine T, Kawai S. Effects of basic fibroblast growth factor on the repair of large osteochondral defects of articular cartilage in rabbits: dose–response effects and long-term outcomes. Tissue Eng. 2004;10:633–641. doi: 10.1089/107632704323061988. [DOI] [PubMed] [Google Scholar]
- 26.Uchio Y, Ochi M, Adachi N, Kawasaki K, Iwasa J. Arthroscopic assessment of human cartilage stiffness of the femoral condyles and the patella with a new tactile sensor. Med Eng Phys. 2002;24:431–435. doi: 10.1016/S1350-4533(02)00032-2. [DOI] [PubMed] [Google Scholar]
- 27.Weisser J, Rahfoth B, Timmermann A, Aigner T, Bräuer R, Mark K. Role of growth factors in rabbit articular cartilage repair by chondrocytes in agarose. Osteoarthritis Cartilage. 2001;9:S48–S54. doi: 10.1053/joca.2001.0444. [DOI] [PubMed] [Google Scholar]
- 28.Whiteside RA, Bryant JT, Jakob RP, Mainil-Varlet P, Wyss UP. Short-term load bearing capacity of osteochondral autografts implanted by the mosaicplasty technique: An in vitro porcine model. J Biomech. 2003;36:1203–1208. doi: 10.1016/S0021-9290(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T, Katoh M, Fukushima R, Kurushima T, Ochi M. Effect of glycosaminoglycan production on hardness of cultured cartilage fabricated by the collagen-gel embedding method. Tissue Eng. 2002;8:119–129. doi: 10.1089/107632702753503117. [DOI] [PubMed] [Google Scholar]