Abstract
We retrospectively studied the functional and oncological results of 15 patients after reconstruction of the distal radius with osteoarticular allograft or non-vascularised fibular graft following wide excision of an aggressive benign or malignant tumour. Eight patients underwent osteoarticular allograft and seven patients had a non-vascularised autogenous fibular graft reconstruction. The average time for incorporation of the graft was 6 and 5 months in each reconstruction respectively. There was no tumour recurrence after follow up over 41.5–95.5 (average 60.5) months. All patients had good and excellent functional results. Three patients in the group reconstructed with osteoarticular allograft had plate loosening and graft fractures which were successfully treated subsequently.
Résumé
Nous avons étudié de façon rétrospective les résultats fonctionnels et carcinologiques de 15 patients ayant bénéficié d’une reconstruction de l’extrémité distale du radius par allogreffes ostéo-articulaires ou par greffe du péroné après excision large pour une tumeur bénigne agressive ou pour une tumeur maligne. 8 patients ont bénéficié d’une allogreffe et 7 patients d’une autogreffe par péroné non vascularisé. Le temps moyen d’incorporation des greffons a été respectivement de 6 et 5 mois. Il n’y a pas eu de récidive de la tumeur au recul moyen de 60,5 mois (de 41,5 à 95,5). Tous les patients ont eu de bons ou d’excellents résultats. 3 patients du groupe allogreffes ont présenté un démontage du matériel avec une fracture de la greffe qui a été reprise et traitée avec succès.
Introduction
The distal radius is a common location for bone tumour and it is the third most common site (after the distal femur and proximal tibia) of giant cell tumour [3]. There is a high rate of local recurrence after most methods of local treatment by intralesional curettage and/or cauterisation with phenol [3, 10, 19]. Therefore complete removal of the tumour and reconstruction following tumour resection in this location might be necessary in aggressive cases which exhibit extraosseous extension or recurrence after previous treatment [12, 19, 21]. Reconstruction of the distal radius is a challenge with regard to regaining normal functioning of the hand with minimal complications. Various techniques, including resection arthroplasty [4], use of a non-vascularised [1, 6, 18] or vascularised autogenous fibular graft [9, 15, 22] and allograft replacement [14], prosthetic replacement [11], ulnar translocation [23] and arthrodesis [5, 20, 25] have been used for reconstruction. The purpose of this study was to assess the long-term outcome of the functional results, complications and oncological outcomes of patients after reconstruction of the distal radius with osteoarticular allografts and non-vascularised autogenous fibular grafts.
Material and methods
Between 1997 and 2003, 15 patients underwent wide excision and reconstruction of tumours of the distal radius. Eight patients with giant cell tumour underwent osteoarticular allograft, six were done primarily and two after tumour recurrence. All patients were categorised as grade 3 using Enneking’s classification [8]. Seven patients underwent non-vascularized autogenous fibular graft reconstruction. Four patients in this particular reconstruction had grade-3 giant cell tumour. One had grade-3 simple bone cyst, stage IB well-differentiated osteosarcoma and stage IIB malignant fibrous histiocytoma. The reason for using non-vascularised autogenous fibular graft in the last seven patients was the lack of allograft from the bone bank network at the time.
Preoperative investigation of each patient included: radiographs of the forearm, wrist and chest, magnetic resonance imaging of the lesion, bone scan and open biopsy. The tumour excision and reconstruction was performed through a standard dorsal longitudinal incision overlying the radius. The soft tissue envelope excised with the distal radius was dependent on the extent of the soft tissue component of the tumour. All tumours were removed by an intra-articular wide excision and the radius was transected proximally. An osteoarticular allograft or a non-vascularised autogenous fibular graft was then implanted in the forearm. The reconstruction was as anatomical as possible and maintained the height and rotation of the distal radial segment. All grafts were fixed to the proximal radius by a small dynamic compression plate and screws, and the preserved fibrous capsule was reinserted to the condyle. The additional stability of the radiocarpal joint was achieved by passage of 2 K-wires through the distal radius and into the proximal carpal row. The patient’s forearm was immobilised in a long arm cast and the K-wires were removed after 4 weeks. A coaptation splint was continued for immobilisation until radiographic proximal union was demonstrated (8–12 weeks). In patients with non-vascularised autogenous fibular graft reconstruction, the lateral collateral ligament was detached from the harvested graft and reattached to the lateral tibial plateau with a staple. Patients were immobilised with a knee brace for 4 weeks, then an active range of motion exercise of the knee was allowed.
All patients were assessed clinically and radiographically. The functional outcomes were analysed by using the Musculoskeletal Tumour Society functional classification regarding the levels of pain, emotional acceptance and function [7]. Any complications or further surgery performed were also recorded after the first reconstruction. Patients reconstructed with non-vascularised autogenous fibular graft reconstruction were also questioned about possible donor site morbidity and specifically about weakness of dorsiflexion of the foot and pain. Patients were also examined for cosmesis and deformity of the affected forearm. The range of movements of the forearm and wrist were assessed. The power grip was measured and compared with the contralateral wrist by using a grip strength meter by an occupational therapist. Radiographs were obtained of the operated forearm and wrist. These were studied for radiographic union and for subluxation of the radiocarpal and distal radioulnar joints.
Results
The follow-up time ranged from 41.5 to 95.5 (average 60.5) months. There were seven female patients and eight male patients. The mean age of the patients who had an osteoarticular allograft was 40.3 years (21–61) and the mean age of the patients who had a non-vascularised autogenous fibular graft was 27.7 years (18–43). Surgery involved the right radius in 12 patients and the left in three patients. Surgery involved the dominant hand in ten patients. The length of tumour excision for non-vascularised autogenous fibular graft reconstruction ranged from 7 to 10 (average 7.8) cms and for osteoarticular allograft ranged from 6.5 to 12 (average 8.3) cms. All patients continue to be disease free, except one patient with a recurrent giant cell tumour. At initial presentation, he had developed lung metastasis and is still alive with the disease. This patient was treated under multiple drug chemotherapy without thoracic surgery. His chest radiograph was improved with smaller lung nodules at the last follow-up visit.
There was no recurrence or infection in this series and none of the patients underwent an amputation. All patients were assessed for functional outcomes. The average Musculoskeletal Tumour Society functional analysis was 93% (80–100) in both groups. The range of motion and grip power of the operated wrists of the patients was compared with the normal contralateral side and is shown in Table 1. For the group that had osteoarticular allograft reconstruction, the average range of motion of the wrist was 40° dorsiflexion, 35° flexion, 15° radial deviation, 22° ulnar deviation, 70° supination and 50° pronation. (Fig. 1a–d) The patients in this group retained 72.5% of their range of motion and 72.2% of their grip strength compared with the contralateral side. For the group that had non-vascularised autogenous fibular graft reconstruction, the average range of motion of the wrist was 45° dorsiflexion, 38° flexion, 20° radial deviation, 28° ulnar deviation, 80° supination and 42° pronation (Fig. 2a–d). The patients in this group had 73.7% of their range of motion and 69% of their grip strength.
Table 1.
Patient demographic data
| Patient number | Age (yrs)/gendera | Siteb | Diagnosis | Grade/stage | Procedure | Duration of follow-up (months) | Range of motion: involved/uninvolved (%) | Grip strength: involved/uninvolved (%) | Functional analysisd | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21/F | R | Giant cell tumour | 3 | Osteoarticular allograft | 42.3 | 67% | 64% | Excellent | Revised plate and bone grafting |
| 2 | 56/F | R | Giant cell tumour | 3 | Osteoarticular allograft | 90.9 | 73% | 70% | Good | - |
| 3 | 61/F | R | Giant cell tumour | 3 | Osteoarticular allograft | 50.4 | NAc | NA | Good | - |
| 4 | 41/M | R | Giant cell tumour, recurrent | 3 | Osteoarticular allograft | 54.0 | Fusion | Fusion | Good | Ulnar translocation radiocarpal |
| 5 | 43/M | R | Giant cell tumour | 3 | Osteoarticular allograft | 41.5 | 72% | 77% | Excellent | - |
| 6 | 36/M | L | Giant cell tumour | 3 | Osteoarticular allograft | 42.0 | 65% | 58% | Excellent | Revised plate and bone grafting |
| 7 | 39/F | R | Giant cell tumour | 3 | Osteoarticular allograft | 48.2 | 78% | 80% | Excellent | - |
| 8 | 25/M | R | Giant cell tumour, recurrent | 3 | Osteoarticular allograft | 52.2 | 80% | 84% | Excellent | - |
| 9 | 21/F | R | Giant cell tumour | 3 | Fibular autograft | 94.4 | 64% | 48% | Excellent | - |
| 10 | 34/M | R | Giant cell tumour | 3 | Fibular autograft | 95.5 | NA | NA | Good | - |
| 11 | 27/M | L | Giant cell tumour | 3 | Fibular autograft | 57.2 | 78% | 76% | Excellent | - |
| 12 | 18/F | R | Giant cell tumour | 3 | Fibular autograft | 71.8 | 73% | 58% | Excellent | - |
| 13 | 24/M | R | Malignant fibrous histiocyxoma | IIB | Fibular autograft | 57.9 | 77% | 82% | Excellent | - |
| 14 | 27/F | L | Simple bone cyst | 3 | Fibular autograft | 50.6 | 70% | 64% | Excellent | - |
| 15 | 43/M | R | Well-differentiated osteosarcoma | IB | Fibular autograft | 58.8 | 80% | 86% | Excellent | - |
aF female; M male
bR right; L left
cNA data not applicable
dMusculoskeletal Tumour Society functional classification
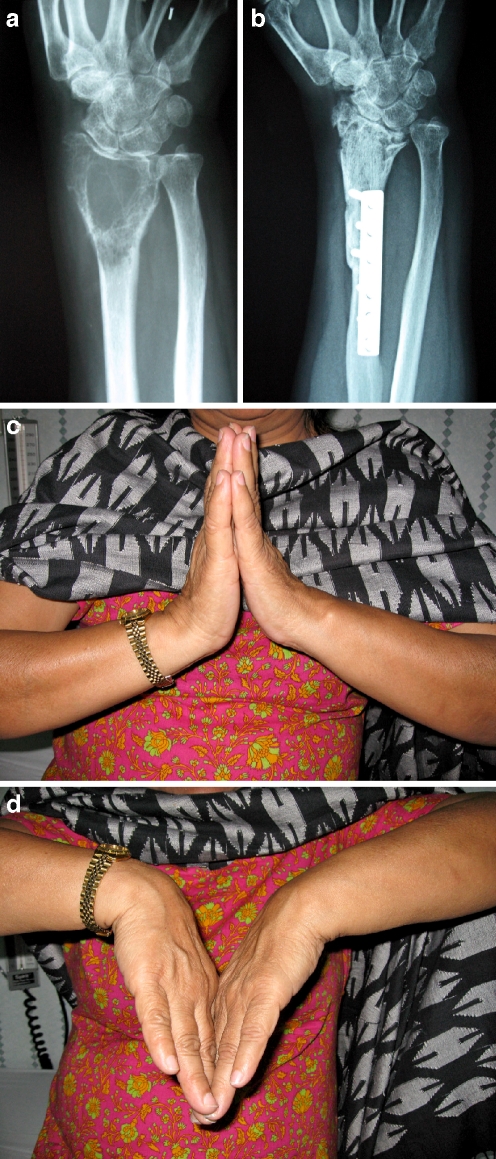
Fig. 1.
An extensive grade 3 GCT of the distal radius, patient no. 2 (a) 8-year osteochondral allograft reconstruction radiograph showing good healing of host bone-graft junction and osteoarthritis at radiocarpal joint (b). Range of motion of the reconstructed wrist joint in dorsal and palmar flexion (c–d)
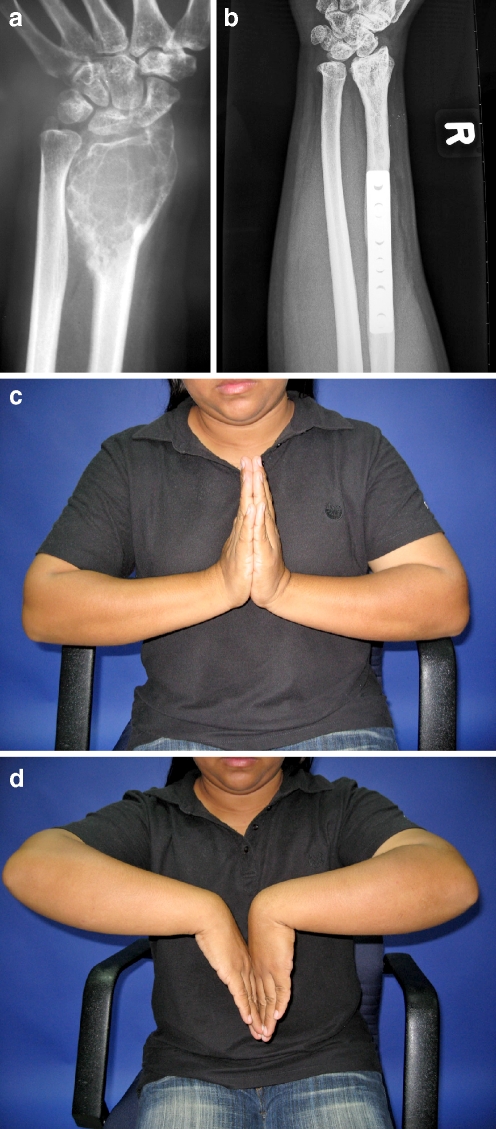
Fig. 2.
A grade 3 GCT of the distal radius, patient no. 12 (a) 6-years after non-vascularised autogenous fibular graft reconstruction radiograph demonstrating good alignment of the graft and radiocarpal osteoarthritis (b). Range of motion of the wrist joint in dorsal and palmar flexion (c–d)
No patients in the non-vascularised autogenous fibular graft reconstruction had any persisting sequelae of common peroneal nerve injury, knee instability or muscular deficiency through fibula harvesting. No patient with this reconstruction has required further surgery due to complications. In the group reconstructed with osteoarticular allografts, there were two plate loosenings from non-union (25%) and one graft fracture (12.5%). Patients with plate loosening were successfully revised with a longer plate and iliac bone grafting at 4 and 7 months from the first surgery respectively. (Fig. 3a–b) The patient with a fractured allograft underwent a centralisation arthrodesis of the carpus over the remaining ulna 9 months from the previous surgery. Most patients including those with complications following the reconstruction could resume their previous professions and normal activities except one patient who suffered restriction of a recreational sporting activity. No patient has needed a further operation up to date.
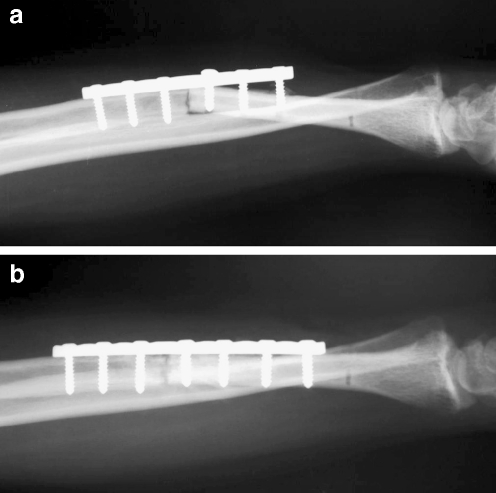
Fig. 3.
Radiograph showing a plate loosening 4 months after osteochondral allograft reconstruction, patient no. 1 (a). Radiograph made after a revision of plate and autogenous bone grafting (b)
A review of the radiographs showed that nearly all patients had unitied at the host bone-graft junction, except two patients reconstructed with an osteoarticular allograft. The host-graft junction of an osteoarticular allograft united between 5 and 8 (average 6) months and between 4 and 7 (average 5) months for a non-vascularized fibular graft united, and the graft took longer than 2 years to achieve consolidation. There was no subluxation of the radiocarpal or distal radioulnar joints in any of patients at the last follow-up. One patient with osteochondral allograft reconstruction (patient no. 2) and two with non-vascularised autogenous fibular graft reconstruction (patients nos. 12 and 13) had osteoarthritis of the radiocarpal joint. However, these patients had minor pain symptoms and were relieved by periodically using analgesic drugs.
Discussion
The treatment of bone tumours of the distal end radius by wide excision and reconstruction of the bone defect following tumour removal is challenging. This concern is emphasised, especially in patients with a large tumour and extraosseous tumour extension. Most of the patients with tumour at this specific site generally are young and active, demanding normal performance. The objective of treatment is to control local recurrence of the tumour and also preserve the function of the hand, wrist and forearm of the patient. Intralesional curettage and filling the defect of the bone with bone graft or polymethylmethacrylate (PMMA) is the procedure of choice for primary benign lesions without marked bone or joint destruction. However, when a lesion is malignant or an extracompartmental benign lesion extends through the cortex or subchondral bone with poor bone stock, reconstruction of the bone defect following wide or marginal excision of the tumour provides a lower rate of local recurrence. Twelve patients in this series suffered grade-3 giant cell tumours and the others were a huge simple bone cyst and two had malignant bone tumours. Therefore, wide excision with osteoarticular allograft or non-vascularised autogenous fibular graft reconstruction was selectively performed in these patients.
None of the patients in this series had local recurrence, although giant cell tumours in this specific location seem to have a more aggressive behaviour and a greater tendency to local recurrence [3, 6, 13, 24]. McDonald et al. analysed the data of 221 patients with giant cell tumour and did not find any correlation between the rate of recurrence and the size, localisation, the surgical stage of the tumour and the involvement of subchondral bone. The most significant factor is the surgical procedure employed for removal of the tumour i.e., curettage with adjuvant therapy (34% recurrence) versus resection (7% recurrence) [19]. Blackley et al. reported the results of treatment of giant cell tumour in 59 patients. There was no difference in the risk of local recurrence after curettage with a high-speed burr and reconstruction with an autogenous graft with or without allograft bone and after use of PMMA and other adjuvant treatments. It is likely that the adequacy of the removal of the tumour rather than the use of adjuvant modalities is what determines the risk of recurrence [2]. There was no local recurrence in this study which confirmed that the adequacy of the tumour-excised margin is an important factor to determine the risk of local recurrence and the prognosis of the patient with a malignant bone tumour. Recurrence after wide excision is uncommon but does occur. This is attributed to either tumour cells left behind in the soft tissue or seeding of the resected tumour cells [14, 17].
Many techniques have been used for reconstruction after wide margin removal of the tumour in this location [1, 4–6, 9, 11, 14, 15, 18, 20, 22, 23, 25]. Reconstruction of the distal radius with the use of non-vascularised or vascularised fibular grafts and osteoarticular allograft are common reconstruction techniques following removal of the tumour in this area. These biomaterials could be used for both wrist joint preservation and arthrodesis depending on the area of bone and soft tissue removal. Although the outcomes of these reconstructions are promising, complications are not uncommon. Forty-five patients from three series had non-vascularised fibular graft reconstruction [1, 6, 18]. In general, these patients had a limited but functional range of motion of the wrist. There was arthritis of the carpofibular joint, but pain was minimum. Complications included non-union and delayed union, fracture of the graft, subluxation of the wrist and donor-site morbidity. The use of vascularised autogenous fibular graft was described in several series [9, 15, 22]. The advantages of these reconstructions include preservation of motion with earlier incorporation of the graft and hypertrophy. This reconstruction technique is a technically demanding surgery with prolonged operating time, requires extensive radiographic studies of the vascular pattern of the limbs, and two major vessels need to be sacrificed. Complications included delayed union, painful carpal subluxation, painful arthritis of carpofibular joint and donor-site morbidity. Seven patients who had non-vascularised fibular graft reconstruction in this series did not require further surgery due to complications. Postoperative function and wrist-forearm motion could be compared with the previous studies using a vascularised autogenous fibular graft. The mean union time at the junction was a little longer than other studies (6 months and 4.4 months) [15, 22].
Kocher et al. reported 25 patients with osteoarticular allograft reconstruction following tumour excision of the distal radius [14]. After the mean follow-up time of 10.9 years, eight patients (33%) needed an allograft revision or amputation from the complications including four fractures of the allograft, one local recurrence, two painful wrists and one volar dislocation of the carpus. In this study, three patients (37.5%) with the osteoarticular allograft reconstruction had complications from plate loosening in two patients and graft fracture in one patient. Patients with plate loosening were successfully treated by plate-screw revision and bone grafting. The patient with a fractured allograft underwent a centralisation arthrodesis of the carpus over the remaining ulna. Nearly all of these patients could resume all previous activities except the patient who needed an arthrodesis who could not participate in recreational sporting activities. The high-rate of allograft fracture in this series might be the effect of irradiated sterilisation of the allografts with a dose of 25 kGy in our institute. Lietman et al. found a significant difference in the outcomes of the incidence of fractures in bone allografts in cases that received high-dose irradiation and non-irradiated allografts. Irradiated sterilisation with a dose of 10–30 kGy can produce a change in collagen type I cross links of the bone matrix [16].
From the results of this study, the mean range of motion of the wrist and forearm and grip strength compared with the contralateral side in the patients with an osteoarticular allograft reconstruction were 72.5 and 72.2%. Our results are comparable to another study reported using an osteoarticular allograft [14]. Review of the radiographs showed that nearly all patients had unitied at the host bone-graft junction except for two patients who were reconstructed with osteoarticular allografts. The average union time of an osteoarticular allograft was a little longer than a non-vascularised fibular graft (6 and 5 months respectively).
In summary, the results of this study suggest that a good to excellent outcome in hand and wrist function, without compromise of prognosis, can be achieved by using an osteoarticular allograft or a non-vascularised fibular graft reconstruction following aggressive benign or malignant tumour excision of the distal radius. Although more complications were found in the group with osteoarticular allograft, these complications were successfully treated and most patients could resume their previous work and activities.
References
- 1.Aithal VK, Bhaskaranand K. Reconstruction of the distal radius by fibula following excision of giant cell tumor. Int Orthop. 2003;27:110–113. doi: 10.1007/s00264-002-0414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackley HR, Wunder JS, Davis AM, White LM, Kandel R, Bell RS. Treatment of giant-cell tumors of long bones with curettage and bone-grafting. J Bone Joint Surg (Am) 1999;81:811–820. doi: 10.1302/0301-620X.81B1.9001. [DOI] [PubMed] [Google Scholar]
- 3.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg (Am) 1987;69:106–114. [PubMed] [Google Scholar]
- 4.Campanacci M, Laus M, Boriani S. Resection of the distal end of the radius. Italian J Orthop Traumat. 1979;5:142–152. [PubMed] [Google Scholar]
- 5.Campbell CJ, Akbarnia BA. Giant-cell tumor of the radius treated by massive resection and tibial bone graft. J Bone Joint Surg (Am) 1975;57:982–986. [PubMed] [Google Scholar]
- 6.Cheng CY, Shih HN, Hsu KY, Hsu WW. Treatment of giant cell tumor of the distal radius. Clin Orthop. 2001;383:221–228. doi: 10.1097/00003086-200102000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Enneking WF, Dunham WK, Gebhardt MC. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop. 1993;286:241–246. [PubMed] [Google Scholar]
- 8.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop. 1980;153:106–120. [PubMed] [Google Scholar]
- 9.Ferracini R, Gino G, Battiston B, Linari A, Franz R, Bertolo S. Assessment of vascularized fibular graft one year after reconstruction of the wrist after excision of a giant-cell tumour. J Hand Surg. 1999;24:497–500. doi: 10.1054/jhsb.1999.0165. [DOI] [PubMed] [Google Scholar]
- 10.Gitelis S, Mallin BA, Piasecki P, et al. Intralesional excision compared with en bloc resection for giant-cell tumors of bone. J Bone Joint Surg (Am) 1993;75:1648–1655. doi: 10.2106/00004623-199311000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Gold AM. Use of a prosthesis for the distal portion of the radius following resection of a recurrent giant-cell tumor (follow-up note) J Bone Joint Surg (Am) 1965;47:216–218. [PubMed] [Google Scholar]
- 12.Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone: an analysis of two hundred and eighteen cases. J Bone Joint Surg (Am) 1970;52:619–664. [PubMed] [Google Scholar]
- 13.Harness NG, Mankin HJ. Giant-cell tumor of the distal forearm. J Hand Surg. 2004;29:188–193. doi: 10.1016/j.jhsa.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Kocher MS, Gebhardt MC, Mankin HJ. Reconstruction of the distal aspect of the radius with use of an osteoarticular allograft after excision of a skeletal tumor. J Bone Joint Surg (Am) 1998;80:407–419. doi: 10.2106/00004623-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Koichiro I, Kazuteru D, Kazuhiro S, Manabu Y, Tsukasa K, Shinya K. Vascularized fibular graft after excision of giant cell tumor of the distal radius: a case report. Clin Orthop. 1999;359:189–196. doi: 10.1097/00003086-199902000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Lietman SA, Tomford WW, Gebhardt MC, Springfield DS, Mankin HJ. Complications of irradiated allografts in orthopaedic tumor surgery. Clin Orthop. 2000;375:214–217. doi: 10.1097/00003086-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Marcove RC, Weis LD, Vaghaiwalla MR, Pearson R, Huvos AG. Cryosurgery in the treatment of giant-cell tumors of bone: a report of 52 consecutive cases. Cancer. 1978;41:957–969. doi: 10.1002/1097-0142(197803)41:3<957::AID-CNCR2820410325>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Maruthainar N, Zambakidis C, Harper G, Calder D, Cannon SR, Briggs TWR. Functional outcome following excision of tumours of the distal radius and reconstruction by autologous non-vascularized osteoarticular fibular grafting. J Hand Surg (Br) 2002;27:171–174. doi: 10.1054/jhsb.2001.0707. [DOI] [PubMed] [Google Scholar]
- 19.McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg (Am) 1986;68:235–242. [PubMed] [Google Scholar]
- 20.Murray JA, Schlafly B. Giant-cell tumors in the distal end of the radius. J Bone Joint Surg (Am) 1986;68:687–694. [PubMed] [Google Scholar]
- 21.O’Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg (Am) 1994;76:1827–1833. doi: 10.2106/00004623-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Pho WH. Malignant giant-cell tumor of the distal end of the radius treated by a free vascularized fibular transplant. J Bone Joint Surg (Am) 1981;63:877–884. [PubMed] [Google Scholar]
- 23.Seradge H. Distal ulnar translocation in the treatment of giant-cell tumors of the distal end of the radius. J Bone Joint Surg (Am) 1982;64:67–73. [PubMed] [Google Scholar]
- 24.Sheth DS, Healy JH, Sobel M, Lane JM, Marcove RC. Giant cell tumor of the distal radius. J Hand Surg. 1995;20:432–440. doi: 10.1016/S0363-5023(05)80102-9. [DOI] [PubMed] [Google Scholar]
- 25.Vander Griend RA, Funderburk CH. The treatment of giant-cell tumors of the distal part of the radius. J Bone Joint Surg (Am) 1993;75:899–908. doi: 10.2106/00004623-199306000-00011. [DOI] [PubMed] [Google Scholar]