Abstract
Osteoid osteoma is the third most common type of bone tumour. Radiofrequency ablation and other percutaneous procedures are the treatment of choice. However, in some sites these methods are difficult or dangerous. Our objective of this study was to evaluate whether open resection and intraoperative nidus detection with a hand-held gamma probe is an efficient method for treating this type of tumour. Fifty-three patients with osteoid osteomas were submitted to surgical treatment. The first group (gamma group) consisted of 34 patients submitted to open nidus resection guided by a hand-held gamma probe. The control group consisted of 19 patients operated on by conventional technique. In the postoperative period, histopathology, imaging studies, and clinical outcome were evaluated. The gamma group patients were followed up for an average 26.2 months; the control group patients were followed up for an average 38 months. There was no difference with regard to pain relief and histopathology findings between the two groups. However, in the postoperative imaging studies, there was significantly less nidus present in the gamma group (p = 0.01).The gamma probe helped to locate the osteoid osteoma nidus more precisely, as demonstrated by the postoperative imaging studies.
Résumé
L’ostéome ostéoïde est la troisième tumeur osseuse par ordre de fréquence. L’ablation par radio fréquence et les autres procédés d’éxérèse percutanée, représentent le traitement de choix de ces tumeurs, cependant, pour quelques sites, ces méthodes sont difficiles voire dangereuses. Notre objectif est d’évaluer la possibilité d’une résection à foyer ouvert avec détection per-opératoire du nidus à l’aide d’une sonde à rayonnements gamma. Matériel et méthode: 53 patients présentant une ostéome ostéoïde ont bénéficié de ce traitement chirurgical. Le premier groupe (gamma groupe) représentait 34 patients avec une résection à foyer ouvert guidé par sonde manuelle gamma. Le groupe contrôle, 19 patients étaient opérés selon la technique conventionnelle avec, dans la période post-opératoire, examen histopathologique et évaluation clinique. Le groupe par détection sonde gamma a été suivi, en moyenne, 26,2 mois et le groupe contrôle 38 mois. Résultats: il n’y a pas de différences concernant la récidive des douleurs et les constatations histopathologiques parmi les deux groupes. Cependant, il y a de façon significative moins de nidus présents dans le groupe gamma en post-opératoire. En conclusion: la sonde avec rayonnement gamma permet de localiser le nidus de l’ostéome ostéoïde de façon plus précise comme l’a démontré notre évaluation post-opératoire.
Introduction
Osteoid osteoma is the third most common benign bone tumour. Its incidence is 11% among benign bone tumours and 3% among all primary bone tumours [6]. Osteoid osteoma was initially described by Bergstrand in 1930 but only in 1935 was it recognised as a distinct entity [5]. Osteoid osteoma is prevalent among children and young adults but can be found in older individuals. It is more common in men. Osteoid osteomas have been described in virtually all bones, with the exception of the sternum, and more frequently in the long bones, especially the proximal femur.
The diagnosis of osteoid osteoma is based on clinical, radiographic, and scintingraphic findings. Frequently, patients have pain for months or years until the lesion is discovered. The classic radiographic presentation is of a small geographic lesion no greater than 2 cm in diameter in the cortical layer of the long bone. The lesion is surrounded by intense reactive sclerosis. Bone scintigraphy is the best method for localisation. The classic scintigraphic double density appearance is very specific for osteoid osteoma and is used as a guide for a dedicated computerised tomography (CT) study [11]. There are descriptions of false negative osteoid osteomas on scintigraphic images; however, these are rare situations with no definite explanation. This may occur when the nidus is smaller than 5 mm with minimal reactive sclerosis [5] or in a patient previously submitted to bone manipulation.
Osteoid osteomas can be treated with medication because after 2 or 3 years the pain may subside with oral nonsteroidal antiinflammatory drugs [12]. However, surgical treatment is very attractive because the pain disappears immediately after surgery and the patient may then resume their normal life. The most frequently performed surgical technique is an en-bloc resection and nidus curettage with or without local adjuvant [2, 23]. Some institutions prefer percutaneous CT scan-guided thermal ablation of the nidus with a radio frequency probe. However, this method should not be used in the vicinity of neurovascular structures [22] nor in bone extremities. In these situations, open resection is the procedure of choice.
The major concern during an open osteoid osteoma operation is the precise localisation and resection of the nidus with minimal disruption of the surrounding tissues to avoid complications [22]. The most important complication is a bone fracture.
Several methods for localisation of the nidus have been described in the literature. These consist of resection of a bone segment or curettage based on landmarks determined by a preoperative image (radiography or CT) [25], after oral tetracycline with the identification of the golden yellow fluorescent nidus seen under ultraviolet light [1], and preoperative CT-guided needle or Kirschner wire marks made on the bone surface near the nidus [15].
This study is a retrospective cohort study that completes the series of a previous study [4]. We describe the use of an intraoperative hand-held gamma probe for precise localisation of the osteoid osteoma nidus.
The objective of this study was to evaluate the efficiency of resection of the osteoma osteoid nidus guided by a gamma probe.
Materials and methods
Patient characteristics
Fifty-three patients who were submitted to surgical treatment for osteoid osteoma were included in this study. Thirty-nine patients were male and 14 were female with ages ranging from 4 to 57 (mean 18.7) years. Patients were divided into two groups. The first group, the “gamma-probe group”, consisted of 34 patients operated on with the use of a gamma probe as the intraoperative guide for nidus localisation. The “control group” consisted of 19 patients submitted to open resection with, at most, the use of radiography or fluoroscopy as intraoperative guides for nidus localisation. Lesions were more frequently located in the inferior limbs, with the femur being the most involved bone (Table 1).
Table 1.
Sites of distribution of osteoid osteoma in each group
| Site | Group | |
|---|---|---|
| Gamma-probe | Control | |
| Humerus | 1 | 1 |
| Radius | 0 | 1 |
| Metacarpus | 2 | 0 |
| Spine | 2 | 1 |
| Pelvis | 2 | 0 |
| Femur | 15 | 10 |
| Tibia | 10 | 2 |
| Fibula | 1 | 1 |
| Talus | 0 | 1 |
| Calcaneus | 0 | 2 |
| Cuboid | 1 | 0 |
Bone scintigraphy
Gamma-probe group patients were submitted to three-phase bone scintigraphy for initial diagnosis of an osteoid osteoma. A second bone scintigraphy was performed on the day of the operation. Patients received an intravenous injection of 1,110 MBq of 99mTc-MDP (adult dose). The children’s doses were adjusted according to weight. After 2–3 hours, a whole-body scan and static images in the anterior, posterior, and lateral positions were obtained in a camera–computer system equipped with a low-energy, high-resolution collimator.
Surgical procedures
All resections were performed in the operating theatre. Gamma-probe group patients were escorted to the surgical room after completion of bone scintigraphy. Patients received general or spinal anaesthesia. When the lesion was close to the bladder, urinary catheterisation was performed due to the high levels of radioactivity in the urine, which could interfere with detection of lesions. A permanent ink mark was drawn over the skin area with the highest radioactive counts to indicate the surgical approach. The sclerotic bone over the nidus was resected with a high-speed burr or an osteotome. The nidus was identified and curetted. The nidus cavity was widened until its counts decreased to the level of the counts of the sclerotic area.
In the control group, the surgical incision was based on preoperative imaging studies, including radiographs or CT. In some patients, intraoperative radiograph or fluoroscopy was necessary to confirm the site of the nidus.
Patient follow-up
Both groups were compared according to postoperative complications, postoperative imaging, clinical outcome, and histopathology. The complications were classified as minor or major.
Minor complications were those that did not cause functional compromise. Major complications were defined as causing functional compromise or those in which surgical intervention was needed. Postoperative imaging studies performed included radiographs, CT scan, and/or three-phase bone scintigraphy. The studies were performed to investigate the presence or absence of nidus. The clinical outcome was determined by postoperative pain relief. Histopathology studies were directed to identifying nidus in samples.
Statistical analysis
Statistical analysis compared the results between control and gamma-probe groups. For analysis of the follow-up, the unpaired t test was used. Fischer’s exact test was performed for the analysis of complications, pain relief, radiological images, and nidus detection on histopathology analysing a contingency table.
Results
Table 2 displays the results. Mean follow-up was 30.5 (1–98) months. Patients in the control group were followed-up for a mean of 38 months (range, 3–98; median, 37). Patients in the gamma-probe group were followed-up for a mean of 26.2 months (range, 1–75; median, 21). There were no significant differences between the follow-up time intervals between the two groups (p = 0.082).
Table 2.
Absolute and relative results of tested parameters
| Parameter | Group | p value | |||
|---|---|---|---|---|---|
| Gamma-probe | Control | ||||
| n | % | n | % | ||
| Complication | 3/34 | 8.8 | 3/19 | 7.6 | 0.650 |
| Pain relief | 32/34 | 94.1 | 14/19 | 73.6 | 0.083 |
| Absence of nidus in postoperative images | 28/30 | 93.3 | 10/16 | 62.5 | 0.014 |
| Nidus detected on histopathology | 28/34 | 82.3 | 13/19 | 68.4 | 0.317 |
| Total patients | 34 | 100 | 19 | 100 | |
Six complications were found in five patients. Complications in the gamma-probe group were slight limitation of extension of the metacarpophalangeal joint, a postoperative thigh haematoma, and a femoral shaft fracture. Three complications were found in the control group: patellofemoral pain in one patient and femoral neck fracture followed by avascular necrosis of the femoral head in one patient. No significant correlation was found in the complication rates between the groups (p = 0.65).
Pain relief was more frequent in the gamma-probe group; however, no significant difference was found between groups (p = 0.083).
Postoperative imaging was performed in 46 of the 53 patients. In the control group, 16 patients had postoperative imaging and 30 patients in the gamma-probe group. Significant differences between these two groups were found comparing the postoperative images (p = 0.014). Nidus identification on histopathology was most commonly seen in the gamma-probe group when compared to the control group; however, this difference was not significant (p = 0.3175).
Discussion
Several articles and many case reports studying osteoid osteomas have been published. The majority of these studies are case series limited to the description of the results of a specific surgical technique. The studies using open surgical procedures are case series and fail to compare nidus detection methods. The open procedure is still the most frequently performed for resection of osteoid osteoma by the general orthopaedic surgeon. Even with the use of percutaneous procedures, open surgery will still be necessary in some cases, for example, when the nidus is located near vital structures or in bones of the extremities (Fig. 1). This study is a retrospective cohort study that completes the series of a previous study [4], comparing two different methods using the open procedure. The parameters considered were the incidence of complications, pain relief, and postoperative images.
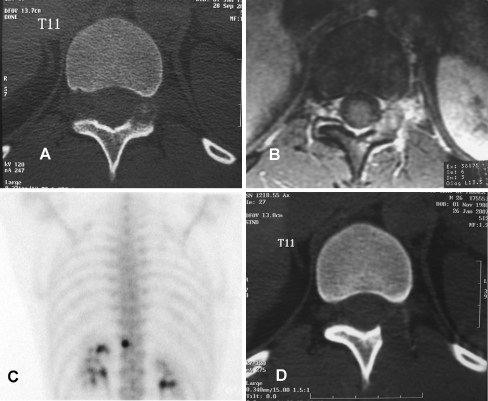
Fig. 1.
Athlete with chronic back pain radiating to the abdomen, relieved by antiinflammatory medication. a Transaxial CT scan images demonstrate a lesion in the left posterior arch of T11 occupying the left foramen. b Transaxial MRI images show oedema surrounding the lesion and the posterior arch of T11. c Bone scintigraphy shows a focal area of increased radiotracer uptake in the left posterior arch of T11, consistent with an osteoid osteoma. Percutaneous treatment could not be performed in this particular case due to proximity with neural structures. The patient was submitted to resection of the nidus with the use of the gamma probe. d Transaxial CT images showing complete removal of the nidus. The patient showed complete remission of pain in the first few days post surgery
Some authors claim that there is no need for ancillary methods to locate the nidus during open procedures [2, 24, 25]. However, one of these studies recommends en-bloc resection of femoral neck and talar osteoid osteomas due to difficulties in finding the nidus [25]. Hence, in open procedures, intraoperative guides would be most often indicated in these locations in order to perform economic resection and avoid complications [1, 3, 8–10, 14–16, 18].
Bone scintigraphy followed by intraoperative nidus detection with a hand-held gamma probe is the method that combines at least three advantages—the procedure can be performed in the vicinity of vital structures, with a lower dosimetry in comparison to patients treated with CT scan-guided procedures, and it can be done in the bones of the extremities. The disadvantages are related to soft tissue and bone damage.
In this study the operative procedure was performed using a hand-held gamma probe, a method that makes the intraoperative nidus detection easier. This approach provides surgical precision with the advantage of less aggressive bone resection, preserving more bone tissue. In this study, postoperative complications occurred in the first few months of follow-up in both groups, and there were no differences between the two groups regarding complication rates. The major complication in the gamma-probe group occurred in the first cases operated on and can probably be attributed to the learning curve.
Despite no significant difference in pain relief in the two groups, all results were more favourable in the gamma-probe group. These data, in conjunction with the significant difference between the two groups comparing postoperative images, may lead to the hypothesis that with a larger patient population, a greater increase in pain relief could occur in the gamma-probe group.
A positive correlation between histopathology and good treatment results has been described [6, 19]. Positive histopathological analysis is definitive proof that the nidus was reached; however, it does not prove that it was completely removed. The opposite is also true, i.e., a negative histopathology does not mean that the nidus was not removed. These situations may occur during sample selection and processing [21].
Positive histopathology was noted in 68% of the control group in comparison to 82% in the gamma-probe group. Both rates obtained in this study are similar to our previous study [4], but lower than other reports of open osteoid osteoma resection in which histology was 100% positive [2, 10, 25]. However, these studies did not specifically mention histological criteria of positivity. In this study, the positive histology (82.3%) was higher than the 70% obtained with CT-guided radiofrequency ablation.
The images performed after surgery defined whether or not the nidus was removed. They had a major impact on the management of patients whose histology was negative, because if the nidus was removed, a better clinical result is expected. In this study, six patients (five gamma-probe patients and one control group patient) had a negative histological analysis, but the postoperative CT scan demonstrated that the nidus had been resected and the patients were pain free. The number of nidi resected in the gamma group was significantly higher than in the control group.
Percutaneous radiofrequency ablation is a major contribution to the treatment of osteoid osteoma that can be performed by surgeons or interventional radiologists and is a minimally invasive procedure. However, this method is still unavailable in the majority of orthopaedic centres around the world. It should also be noted that radiofrequency ablation has a failure rate and in some instances cannot be applied because of tumour location [13, 22] (Fig. 1). The surgeon or radiologist must have experience with needle trephine biopsy procedures [20]. Some complications have been described with this method, such as recurrence of the pain, reflex sympathetic dystrophy, and an unfavourable clinical outcome [7, 17]. Such data explain why the open procedure still has a wide range of indications in osteoma osteoid treatment.
In this study, functional recovery time and incision extension were not evaluated because it was technically impossible to obtain this information in all patients from the control group. The impression is that the incisions were smaller in gamma-probe group patients with osteoid osteoma of the limbs. Smaller incisions may lead to a faster functional recovery.
Bone scintigraphy followed by intraoperative nidus detection with a hand-held gamma probe gives the surgeon precision when resecting osteoid osteomas. It is a good choice when percutaneous radiofrequency ablation is not available or cannot be performed (Fig. 1). The hand-held gamma probe helps locate the osteoma osteoid nidus more precisely than the standard open procedure. However, this technique requires a learning curve just as any other surgical procedure.
References
- 1.Ayala AG, Murray JA, Erling MA, Raymond AK. Osteoid osteoma: intraoperative tetracycline-fluorescence demonstration of the nidus. J Bone Joint Surg Am. 1986;68:747–751. [PubMed] [Google Scholar]
- 2.Campanacci M, Ruggieri P, Gasbarrini A, Ferraro A, Campanacci L. Osteoid osteoma: direct visual identification and intralesional excision of the nidus with minimal removal of bone. J Bone Joint Surg Br. 1999;81:814–820. doi: 10.1302/0301-620X.81B5.9313. [DOI] [PubMed] [Google Scholar]
- 3.D’Errico G, Rosa MA, Soluri A, Scafe R, Galli M, Chiarini S, et al. Radioguided biopsy of osteoid osteoma: usefulness of imaging probe. Tumori. 2002;88:30–32. doi: 10.1177/030089160208800333. [DOI] [PubMed] [Google Scholar]
- 4.Etchebehere M, Etchebehere ECSC, Reganin LA, Amstalden EMI, Cliquet A, Jr, Camargo EE. Intraoperative localization of an osteoid-osteoma using a gamma probe. Int Orthop. 2004;28:379–383. doi: 10.1007/s00264-004-0588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fehring TK, Green NE. Negative radionuclide scan in osteoid osteoma. Clin Orthop. 1984;185:245–249. [PubMed] [Google Scholar]
- 6.Frassica FJ, Waltrip RL, Sponseller PD, Ma LD, McCarthy EF. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27:559–574. [PubMed] [Google Scholar]
- 7.Gangi A, Aizadeh H, Wong L, Buy X, Dietemann JL, Roy C. Osteoid osteoma: percutaneous laser ablation an follow-up in 114 patients. Radiology. 2007;242(1):293–301. doi: 10.1148/radiol.2421041404. [DOI] [PubMed] [Google Scholar]
- 8.Ghelman B, Thompsom FM, Arnold WD. Intraoperative radioactive localization of an osteoid-osteoma. J Bone Joint Surg Am. 1981;63:826–827. [PubMed] [Google Scholar]
- 9.Harcke HT, Conway JJ, Tachdjian MO, Dias LS, Noble HB, Macewen GD, et al. Scintigraphic localization of bone lesions during surgery. Skeletal Radiol. 1985;13:211–216. doi: 10.1007/BF00350576. [DOI] [PubMed] [Google Scholar]
- 10.Healey JH, Ghelman B. Osteoid osteoma and osteoblastoma. Current concepts and recent advances. Clin Orthop. 1986;204:76–85. [PubMed] [Google Scholar]
- 11.Helms CA, Hattner RS, Vogler JB. Osteoid osteoma: radionuclide diagnosis. Radiology. 1984;151:779–784. doi: 10.1148/radiology.151.3.6232642. [DOI] [PubMed] [Google Scholar]
- 12.Ilyas I, Younge DA. Medical management of osteoid osteoma. Can J Surg. 2002;45:435–437. [PMC free article] [PubMed] [Google Scholar]
- 13.Ilyas I, Younge DA. Medical management of osteoid osteoma. Can J Surg. 2003;46:60–61. [PMC free article] [PubMed] [Google Scholar]
- 14.Kirchner B, Hillman A, Lottes G, Sciuk J, Bartenstein P, Winkelmann W, et al. Intraoperative, probe-guided curettage of osteoid osteoma. Eur J Nucl Med. 1993;20:609–613. doi: 10.1007/BF00176556. [DOI] [PubMed] [Google Scholar]
- 15.Magre GR, Menendez LR. Technical note. Preoperative CT localization and marking of osteoid osteoma: description of a new technique. Skeletal Radiol. 1996;20:526–529. doi: 10.1097/00004728-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien TM, Murray TE, Malone LA, Derwan P, Walsh M, Mcmanus F, et al. Osteoid osteoma: excision with scintimetric guidance. Radiology. 1984;153:543–544. doi: 10.1148/radiology.153.2.6484185. [DOI] [PubMed] [Google Scholar]
- 17.Peyser A, Applbaum Y, Khoury A, Liebergall M, Atesok K. Osteoid osteoma: CT-guided radiofrequency ablation using a water-cooled probe. Ann Surg Oncol. 2007;14(2):591–596. doi: 10.1245/s10434-006-9293-4. [DOI] [PubMed] [Google Scholar]
- 18.Rinsky LA, Goris M, Bleck EE, Halpern A, Hirshman P. Intraoperative skeletal scintigraphy for localization of osteoid osteoma in the spine. J Bone Joint Surg Am. 1980;62:143–144. [PubMed] [Google Scholar]
- 19.Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80:815–821. doi: 10.2106/00004623-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal DI, Springfield DS, Gebhardt MC, Rosenberg AE, Mankin HJ. Osteoid osteoma: percutaneous radiofrequency ablation. Radiology. 1995;197:451–454. doi: 10.1148/radiology.197.2.7480692. [DOI] [PubMed] [Google Scholar]
- 21.Sim FH, Dahlin CD, Beabout JW. Osteoid osteoma: diagnostic problems. J Bone Joint Surg Am. 1975;57:154–159. [PubMed] [Google Scholar]
- 22.Torriani M, Rosenthal DL. Percutaneous radiofrequency treatment of osteoid osteoma. Pediatr Radiol. 2002;32:615–618. doi: 10.1007/s00247-002-0727-2. [DOI] [PubMed] [Google Scholar]
- 23.Ward WG., Sr . Benign osteoblastic tumors of bone. In: Menendez LR, editor. Orthopaedic knowledge update. Musculoskeletal tumors. Rosemont, IL, USA: American Academy of Orthopaedic Surgeons; 2002. pp. 87–102. [Google Scholar]
- 24.Ward WG, Eckardt JJ, Shayestehfar S, Mirra J, Grogan T, Oppenheim W. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;291:229–235. [PubMed] [Google Scholar]
- 25.Yildiz Y, Bayarakci K, Altay M, Saglik Y. Osteoid osteoma: the results of surgical treatment. Int Orthop. 2001;25:119–122. doi: 10.1007/s002640100231. [DOI] [PMC free article] [PubMed] [Google Scholar]