Abstract
Open fractures of the tibial diaphysis are the result of high-energy trauma. They are usually associated with extensive soft tissue loss and represent serious clinical problems. Surgical treatment of these injuries has been associated with substantial complications such as osteomyelitis, delayed bone healing, poor functional outcome, soft-tissue failure, or even amputations. More recently a staged treatment, with initial application of spanning external fixators followed by definitive fixation at secondary phase, has been advocated. Plating of these fractures in the acute setting remains a topic of heated discussion. A systematic review of the literature was carried out in order to investigate the existing evidence concerning the efficacy and safety of this method of osteosynthesis. Eleven papers met the inclusion criteria, accumulating 492 open tibial fractures treated with plating. The overall union rate ranged from 62–95% across all studies, with time to union ranging from 13–42 weeks. The reoperation rate ranged from 8–69% and a pooled estimate of deep infection rate was calculated at 11%. Plate fixation for the treatment of open tibial fractures can be considered under specific conditions which need to be elicited and clarified with future well-designed and conducted clinical trials.
Résumé
Les fractures ouvertes de la diaphyse tibiale sont généralement secondaires à un traumatisme violent. Elles sont généralement associées avec des lésions ou des pertes de substance des tissus mous. Ceci entraîne de sérieux problèmes thérapeutiques. Le traitement chirurgical de ces traumatismes est émaillé de sérieuses complications: infections, retards de consolidation, problèmes fonctionnels, lésions persistantes des tissus mous, ces complications pouvant parfois conduire à une amputation. Récemment, un traitement en deux étapes a été mis au point avec mise en place initiale d’un fixateur externe, suivi secondairement par une fixation définitive. L’ostéosynthèse par plaques de ces fractures, en période aigue reste un domaine très discuté. Une revue systématique de la littérature nous a permis d’évaluer les résultats de cette méthode d’ostéosynthèse. 11 articles regroupant 492 fractures ouvertes traitées par plaque ont pu être recensés. Le taux de consolidation varie de 62 à 95% avec un temps de consolidation de 13 à 42 semaines. Le taux de réintervation varie également de 8 à 69% et le taux d’infection profonde peut être estimé à 11%. L’ostéosynthèse par plaque dans le traitement des fractures ouvertes peut être considérée, sous certaines conditions, comme un traitement dont les résultats peuvent être parfaitement définis ceci nécessitant également quelques études ultérieures.
Introduction
The tibial shaft is more prone to open fractures than any other long bone of the human skeleton. Epidemiological studies have shown that open fractures comprise 23.5% of all tibial shaft fractures [6]. The lack of muscular protection along the anteromedial aspect of the tibia and poor blood supply predispose open tibial fractures (OTSF) to certain complications. They present with a 10–20-fold increased risk of developing infection [25] than open fractures in other anatomical areas, and a non-union rate as high as 28% has been reported in the literature [4, 24].
Administration of intravenous broad-spectrum antibiotics, meticulous wound debridement, operative stabilisation of the skeletal injury and early soft tissue coverage of the open wound are all part of the therapeutic protocols [8, 9, 12]. Despite the general consensus supporting early skeletal stabilisation, the optimal method of achieving osseous stability still remains a topic of controversy [11, 24]. External fixators have been widely used as they offer versatility, ease of application with minimum operative trauma, access to the wound and usually no interference with free joint movement. However, they were also associated with high rates of pin-loosening, mal-union and non-union [7, 14]. Reamed intramedullary nails have few advocates, especially for severe OTSF due to the damage of the endosteal blood supply during the reaming process [11, 16]. The use of unreamed intramedullary nails has been associated with acceptable infection rates, apparently due to less interference with endosteal circulation, but a high rate of hardware failure has been reported in several studies [23, 28]. The use of plates and screws has been discouraged by many authors due to the potential damage to the periosteal blood supply during soft tissue stripping, and the increased risk for septic complications [2, 18]. The development of new biological techniques and implants have revived the interest towards open reduction and plate fixation [12]. Nevertheless, the exact role of plate fixation in the treatment of OTSF remains obscure, as the literature is lacking in randomised control trials (RCTs) comparing plate fixation with the other established methods of treatment.
These observations led us to perform a systematic review of relevant studies on open reduction and plate fixation of open tibial shaft fractures aiming to obtain a combined estimate for solid union, deep infection and re-operation rates.
Materials and methods
The methodology for conducting a systematic review of the literature is well established and should be structured following published guidelines [1, 21]. In order to reduce bias we established a strict protocol for the literature review and introduced a quality score before commencing our search. An electronic search of the Medline database was conducted using the PubMed search machine, entering the following terms and Boolean operators: “open tibial fractures” and “internal fixation”. The query was limited to the period between January 1975 and January 2006, to publications in English or German and to clinical papers dealing exclusively with humans. Articles were considered eligible for this review if they met certain inclusion criteria: (1) samples of at least 20 open fractures, (2) plate fixation was used, (3) at least one of the outcomes of interest was described, and (4) details regarding the severity of the soft tissue injury were available. Our exclusion criteria included animal or experimental studies, papers reporting on paediatric cohorts (age <14 years), dealing exclusively with polytrauma patients, or including periarticular fractures of either the knee or the ankle.
Two reviewers (CP, NKK) reviewed each article. If it could not be excluded unequivocally a decision was reached after evaluation of the whole manuscript and discussion between the two reviewers. Each eligible study was assigned a quality score by each reviewer based on the quality instrument described by Littenberg et al. [20]. The intraclass correlation coefficient for the calculated score was used to evaluate the inter-reviewer agreement.
Certain numerical data and descriptive characteristics were extracted from each eligible report (Table 1). The main outcomes of interest were union, infection, and re-operation rates, and were expressed as a proportion of events (Table 2). Fracture union was assumed when bone healing progressed uneventfully. Deep infection was considered to occur when terms such as fistula, deep abscess, sequestration, osteitis and infected non-union were used in the manuscripts. Reoperation was defined as at least one surgical procedure following the index procedure.
Table 1.
Numerical data and descriptive characteristics
| Reference | Study duration | Study type | Mean age (years) | M/F ratio | F up rate | Mean time of follow-up (mo) | Number of followed frx | Proportion of comminuted frx (pcom) | Proportion of severe open frx (pse) | Prophylactic use of antibiotics | Soft tissue reconstruction | Bone graft rate | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ruedi T, 1976[27] | 1966–72 | R | nd | nd | 94% | >12 | 95 | nd | 0.60 | 50% of cases | nd | nd | 4 |
| Jensen JS, 1977[15] | 1966–75 | R | 34 | 3.4 | 96% | 40.8 | 27 | 0.36 | 0.41 | Y | nd | nd | 4 |
| Kristensen K, 1979 [18] | 1968–76 | R | nd | nd | 82% | 12 | 23 | 0.70 | 0.30 | Y | nd | 8.7% | 5 |
| Langenberg, 1987 [19] | 1975–86 | R | 37 | 1.8 | 100% | Nd | 21 | 0.22 | 0.75 | nd | nd | nd | 5 |
| Clifford, 1988[5] | 4-year period | R | 29.2 | 5.8 | 88% | >12 | 97 | 0.75 | 0.62 | nd | Del. flap: 3 SSG: 30 del. clos.: 2 prim clos.: 27 gran.: 35 | nd | 5 |
| Vara-Thorbeck R, 1989 [31] | 1971–87 | R | nd | 2.2 | 100% | >12 | 50 | 0.52 | 0.35 | Y | nd | nd | 6 |
| Bach, 1989 [2] | 16-month period | P rand | 37 | 2.1 | 95% | >12 | 26 | nd | 1.00 | Y | Delayed closure/flap | 42.3% | 7 |
| Korovessis, 1992 [17] | 1984–86 | R | 35 | 10.5 | 97% | >12 | 44 | 0.59 | 0.14 | Y | 86.4% | 6 | |
| Bilat, 1994 [3] | 1980–84 | R | 36 | 1.8 | 96% | 79.0 | 42 | 0.63 | 0.71 | Y | nd | nd | 6 |
| Singh, 1997 [30] | 1989–90 | R | 35.2 | 5.7 | 99% | >12 | 37 | 0.54 | 0.41 | Y | nd | nd | 7 |
| Gopal, 2000 [12] | 1990–98 | R | 37 | 5.15 | 100% | >12 | 30 | 0.80 | 1.00 | Y | Immed flap: 10 early flap: 13 del. flap: 7 | 36.7% | 7 |
clos closure, del.: delayed, frx : fractures, gran.: granulation, immed.: immediate, M/F: male/female, nd: not documented, prim.: primary, P: prospective, R: retrospective, rand: randomized, SSG: split skin graft, Y: yes
Table 2.
The main outcomes
| Author | Number of fractures | Union rate (95 CI) | Mean time to union (weeks) | Infection rate (95 CI) | Reoperation rate (95 CI) |
|---|---|---|---|---|---|
| *Ruedi T, 1976 [27] | 95 | 87% (79–93) | nd | 12% (6–20) | 23% (15–33) |
| †Jensen JS, 1977 [15] | 27 | 93% (76–99) | nd | 11% (2–29) | 15% (4–34) |
| †Kristensen K, 1979 [18] | 23 | 91% (72–99) | nd | 4% (0–22) | 9% (1–28) |
| *Langenberg, 1987 [19] | 21 | 95% (76–100) | nd | 10% (1–30) | 14% (3–36) |
| *Clifford, 1988 [5] | 97 | 87% (78–93) | nd | 10% (5–18) | 13% (7–22) |
| †Vara-Thorbeck R, 1989 [31] | 50 | 62% (47–75) | nd | 8% (2–19) | 50% (36–64) |
| *Bach, 1989 [2] | 26 | 62% (41–80) | 24 | 35% (17–56) | 69% (48–86) |
| †Korovessis, 1992 [17] | 44 | 82% (67–92) | 24 | 16% (7–30) | 25% (13–40) |
| *Bilat, 1994 [3] | 42 | 74% (58–86) | 42 | 7% (1–19) | 21% (10–37) |
| †Singh, 1997 [30] | 37 | 95% (82–99) | 13 | 16% (6–32) | 8% (2–22) |
| *Gopal, 2000 [12] | 30 | 90% (73–98) | 27 | 17% (6–35) | 43% (25–63) |
95 CI: 95% confidence interval
nd: non-documented
*Severe open fractures
†Mild open fractures
Statistical heterogeneity across the component studies was detected using the Cochran’s chi-square test (Q test) and I-square test. In the absence of significant statistical heterogeneity we produced a combined estimate of effect size using the inverse variance as normalising weight.
Statistical analysis was performed on a personal computer using NCSS Statistical Software (Kaysville, UT). Comparison of numerical parameters of interest across groups was performed using the two-tailed, unpaired t test. Spearman correlation was used to test any non-parametric relationship between factors of clinical and methodological diversity across included studies and respective outcomes of interest. Differences were considered significant at p<0.05.
Results
The electronic search yielded 417 citations, but only 11 met the inclusion criteria and were considered eligible for the study [2, 3, 5, 12, 15, 17–19, 27, 30, 31]. One study was a prospective quasi-randomised trial [2], and the rest were retrospective case-series. [3, 5, 12, 15, 17–19, 27, 30, 31]. Eight studies provided demographic data and follow-up details for the whole study population and not for the specific group of OTSF treated with plating [2, 3, 15, 17–19, 30, 31]. In these cases we used the data of the whole population of the studies to extrapolate parameters such as mean age, male/female ratio and follow-up rate and we assumed that these data were representative for the “treatment group” of interest.
Various classification systems of the grade of open injury were used among the component studies. The classification of Matter and Rittman was used in three papers [5, 15, 17], the AO classification in two [3, 27], Tscherne’s classification in two [19, 31], while the classical grading system of Gustilo and Anderson in three studies [2, 12, 30]. Finally, one study used a self-made classification system, grading the open injury as “skin perforation” and “skin and muscle laceration” [18]. In order to ensure consistency we used two broad categories: the category of “mild open fractures” comprising all grade I fractures according to the above grading systems and the “severe open fractures” category including all grades II and III open fractures. Thus the proportion of severe open fractures (pse) and of comminuted fractures (pcom) in each OTSF cohort was calculated.
A number of methodological and clinical diversities were present among the component studies relating to the study design (retrospective, prospective), male/female ratio, proportion of comminuted fractures (pcom), or severe open fractures (pse), the use of bone graft, etc. No correlation between the above factors of clinical heterogeneity and the outcomes of interest was proven.
Quality score
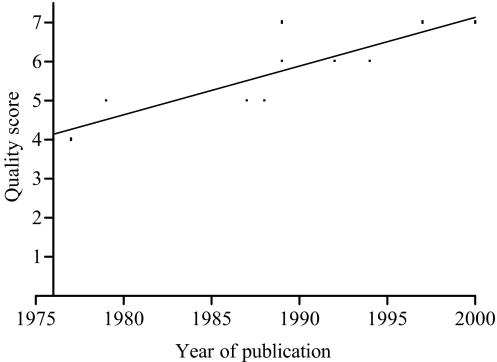
The quality score [20] ranged from 4 to 7 points with a mean value of 5.6 points and a median value of 6 points. The correlation coefficient for interrater agreement was 0.78, (p<0.05). The intraclass correlation coefficient was 0.79 (95% CI, 0.59–0.90). Study quality improved by about 0.12 points per year, as indicated by the linear regression analysis (p=0.004) (Fig. 1).
Fig. 1.
Study quality improvement, as indicated by the linear regression analysis
Union rate
The 11 eligible studies reported on 492 OTSF treated with plate fixation. The union rate ranged from 62–95% across all studies, with a mean time to union ranging from 13–42 weeks (Fig. 2). Mean time to union was documented only in five studies [2, 3, 12, 17, 30]. Significant statistical heterogeneity with respect to the above outcome was detected (Q=34.6; degrees of freedom =10; p<0.001; I2=71) and consequently pooling of the results was avoided.
Fig. 2.
Range of union rate across all studies
We further analysed the above outcome with regards to the severity of open injury (pse) and degree of comminution (pcom). Four subgroups of fractures were created based on whether the values of pse and pcom in each study were equal to or less than 0.50 (pse ≤0.50, pcom ≤0.50) or more than 0.50. (pse >0.50, pcom >0.50).
The subgroup of severely open fractures (pse >50%) was retrieved from six papers [2, 3, 5, 12, 19, 27] reporting on 311 open tibial fractures. The union rate ranged from 62–95%. Significant statistical heterogeneity was detected (Q=13.7; degrees of freedom =5; p<0.05; I2=71) and consequently a pooled estimate of the effect size was not obtained.
Five papers [15, 17, 18, 30, 31] yielded data for the subgroup of mild open fractures (pse ≤0.50), reporting on 181 open tibial fractures. Union rate also ranged from 62–95%, but a combined estimate of effect size was not calculated due to the presence of statistical heterogeneity (Q=20.5; degrees of freedom =4; p<0.001; I2=75.6). No statistically significant difference was detected between the above subgroups with respect to union rate (p=0.80).
Similarly, another two subgroups were created based on to whether pcom >0.50 or pcom ≤0.50. Two papers did not provide details regarding the type of included fractures (simple or comminuted) [2, 27]. The subgroup of comminuted fractures (pcom >0.50) included seven papers reporting on 349 open tibial fractures [3, 5, 12, 17, 18, 30, 31]. Union rate was 62–95% across all studies. Statistical heterogeneity characterised the results (Q=23.4; degrees of freedom =6; p<0.001; I2=70).
The subgroup of pcom ≤0.50 consisted of two papers [15, 19] reporting on 48 fractures. A weighted mean of union rate was calculated in this subgroup at 94% (Q=0.1; degrees of freedom =1; p>0.1; I2=0). There was a statistically significant difference in union rate between these two subgroups (p=0.046).
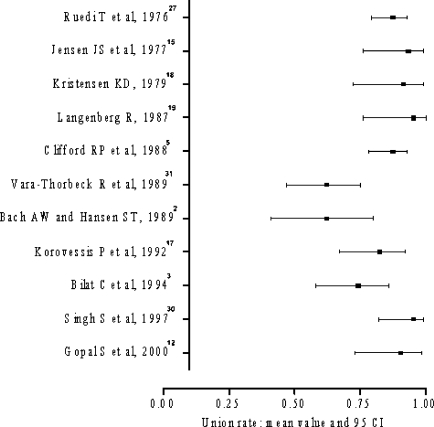
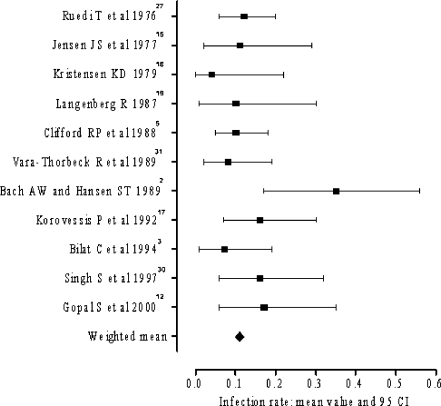
Infection rate
Data regarding the infection rate were available in all included papers. The rate of infection ranged from 4–35% (Fig. 3). Application of both Q and I2 tests did not detect significant statistical heterogeneity of the results and a pooled estimate of infection rate was calculated at 11% (Q=13.56; degrees of freedom =10, p>0.1; I2=26.3).
Fig. 3.
Range of infection rate
In the subgroup of pse >0.50 the infection rate was 5–35%. These results were statistically heterogeneous (Q=9.87; degrees of freedom=5; p<0.10; I2=59.5). The subgroup with pse≤0.50, yielded infection rate ranging from 4–16% (Q=4.97; degrees of freedom =4; p>0.1; I2=0). The weighted mean of infection rate in this subgroup was 10%. No statistically significant difference in infection rate was detected between the above subgroups (p=0.53).
The subgroups of pcom >0.50 yielded infection rates ranging from 4–17%. A combined estimate of effect size was calculated at 10% (Q=6.14; degrees of freedom =6; p>0.1; I2=0). In the subgroup of pcom ≤0.50 a weighted mean of infection rate was calculated at 11% (Q=0.01; degrees of freedom =1; p>0.1; I2=0). No statistically significant difference in infection rate was detected between the two subgroups (p=0.76).
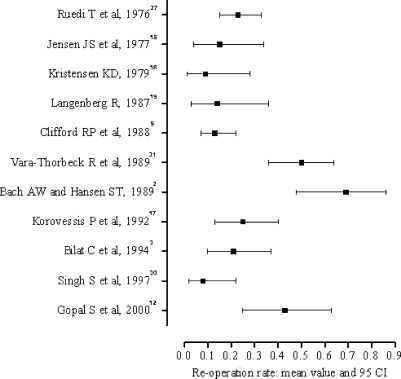
Reoperation rate
A total of 123 fractures underwent at least one repeat procedure. Reoperation rate was recorded in all 11 eligible papers and ranged from 8–69% (Fig. 4). Significant statistical heterogeneity was detected (Q=70.9; degrees of freedom =10; p<0.001; I2=86) and calculation of a pooled estimate of reoperation rate was not performed.
Fig. 4.
Range of reoperation rate
The subgroup of pse ≤0.50 yielded a reoperation rate ranging from 8–50% (Q=29.4, degrees of freedom =4; p<0.001; I2=83). In the subgroup of pse >0.50 the reoperation rate was calculated at 13–69% (Q=40; degrees of freedom =5; p<0.001; I2=90). These two subgroups did not differ significantly (p=0.10).
In the subgroup of pcom ≤0.50 the weighted mean of reoperation rate was 14% (Q=0.01; degrees of freedom =1; p>0.1; I2=0) and the subgroup of pcom >0.50 yielded heterogeneous results (Q=38.9; degrees of freedom =6; p<0.001; I2=82), which ranged from 8–43%. No statistically significant difference was detected between these two groups (p=0.17).
In 38% of the reoperated fractures the initial plate fixation was revised and, occasionally, bone graft was used; in 42%, operative debridement was performed, due to development of deep infection, and in 14% of the reoperated cases, a secondary bone grafting procedure with preservation of the initial fixation took place, due to delayed healing. Amputation was the reason for reoperation in 6% of the cases. The most common causes for reoperation are listed in Table 3.
Table 3.
The most common causes for reoperation
| Main reasons for reoperation | Number of reoperated fractures | Per cent |
|---|---|---|
| Revision of the initial fixation (occasional use of bone graft) | 47 | 38% |
| Secondary bone grafting (without revision of the initial fixation) | 17 | 14% |
| Operative debridement (for infection) | 52 | 42% |
| Amputation | 7 | 6% |
| Total number of reoperated fractures | 123 | 100% |
Discussion
A consensus has been reached that open tibial fractures should be managed expeditiously with urgent wound debridement, primary skeletal stabilisation and early wound coverage [4, 11, 29]. Open reduction and internal fixation (ORIF) with plates and screws, according to the AO principles, has been generally out of favour due to the unacceptably high rate of complications, particularly infection [2, 13]. However, recent reports on severe OTSF yielded very satisfactory results, despite the fact that several of them had been fixed internally with plates and screws [12]. This fact prompted us to evaluate the role of plate fixation in open tibial fractures.
A systematic review of well-designed, randomised control trials (RCTs) would be the proper method to investigate our review question. Unfortunately, current literature is lacking relevant RCTs and, consequently, we relied on the recruitment of relevant case-series studies. Such studies are characterised by the presence of many uncontrolled parameters, clinical or methodological heterogeneity and consequently are subject to both systematic and random error.
In our study, although we documented several factors of clinical heterogeneity-study design (retrospective, prospective), gender ratio, proportion of comminuted fractures (pcom), proportion of severe open fractures (pse) and frequency of use of bone graft-we did not establish any relationship (Spearman correlation) between any of these factors and the outcomes of interest. Another uncontrolled factor across all studies was the policy with regards to soft tissue cover. Only one study described a clear protocol of early or even immediate cover of open wound with a free, vascularised muscle flap [12]. In the remaining manuscripts, either sufficient information was not documented, or various methods of delayed wound cover were outlined.
The quality of included studies was assessed by a quality instrument, rating four follow-up and one research design parameters [20]. The quality rating of our studies was kept in relatively low levels, ranging from 4–7 points. That was mainly due to the research design of the component studies, being in the majority retrospective or case series. Nevertheless, there was a clear tendency of the quality score to improve with time.
The plating systems used in the studies of our analysis [2, 3, 5, 15, 17–19, 27, 30, 31] included standard 4.5 dynamic compression plates applied by the standard principles of the AO techniques [22]. The study of Gopal et al. [12] refers to the concept of biological plating. The development of biological minimally invasive internal fixation techniques and implants [10, 26, 32] offers additional arguments to the advocates of internal fixation of open fractures, but still lacks sufficient scientific evidence.
Our research aim was to produce a pooled estimate of treatment effect for each of the outcomes of interest. Before pooling of the results, we explored the presence of statistically significant variation of individual estimates of treatment effect of the component studies, commonly referred to as statistical heterogeneity. In the absence of significant statistical heterogeneity we produced a combined estimate of treatment effect.
Significant statistical heterogeneity was detected across the component studies as regards to fracture union and reoperation rates. Wide ranges of union rate (62–95%) and reoperation rate (8–69%) surely represent a high degree of diversity in our material. The results of infection appeared more homogenous and the cumulative estimate of infection rate was 11%. Data synthesis was also feasible in the subgroup including fewer comminuted fractures (pcom ≤0.50). A pooled estimate of reoperation rate was recorded at 13%, of infection rate at 11% and, finally, of union rate at 91%. Pooling of data as regards to infection rate was also done in the subgroup of less severe open fractures (pse ≤0.50). The respective combined estimate of treatment effect was calculated at 10%. The percentage of either severe open or comminuted fractures in the respective subgroups was not found to influence the possibility of infection or reoperation. Union rate, though, was statistically different in the two subgroups of pcom >0.50 and pcom ≤0.50, respectively, implying that a comminuted fracture pattern predisposes to healing complications of open tibial fractures.
Despite the limitations of our study, we believe that our results indicate that plate fixation should not be absolutely precluded as a surgical option for the management of open tibial fractures. New implant designs and the development of less traumatic techniques of plate application along with the concurrent use of elaborate techniques for early and adequate soft tissue cover, preferably with vascularised muscle flaps, could extend the indications of plating in selected cases of severe open fractures, provided that enough experience and close cooperation between orthopaedic and plastic surgical teams are available.
Footnotes
Work attributed to:
Academic Unit, Trauma and Orthopaedic Surgery, Clarendon Wing, Leeds General Infirmary, Great George Street, Leeds, LS1 3EX, UK.
References
- 1.Audige L, Bhandari M, Griffin D, et al (2004) Systematic reviews of nonrandomized clinical studies in the orthopaedic literature. Clin Orthop Relat Res 249–257 [DOI] [PubMed]
- 2.Bach AW, Hansen ST, Jr. (1989) Plates versus external fixation in severe open tibial shaft fractures. A randomized trial. Clin Orthop Relat Res 89–94 [PubMed]
- 3.Bilat C, Leutenegger A, Ruedi T. Osteosynthesis of 245 tibial shaft fractures: early and late complications. Injury. 1994;25:349–358. doi: 10.1016/0020-1383(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 4.Brumback RJ. Open tibial fractures: current orthopaedic management. Instr Course Lect. 1992;41:101–117. [PubMed] [Google Scholar]
- 5.Clifford RP, Beauchamp CG, Kellam JF, et al. Plate fixation of open fractures of the tibia. J Bone Joint Surg Br. 1988;70:644–648. doi: 10.1302/0301-620X.70B4.3403616. [DOI] [PubMed] [Google Scholar]
- 6.Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77:417–421. [PubMed] [Google Scholar]
- 7.Court-Brown CM, Wheelwright EF, Christie J, McQueen MM. External fixation for type III open tibial fractures. J Bone Joint Surg Br. 1990;72:801–804. doi: 10.1302/0301-620X.72B5.2211760. [DOI] [PubMed] [Google Scholar]
- 8.Crowley DJ, Kanakaris NK, Giannoudis PV (2007) Debridement and wound closure of open fractures: The impact of the time factor on infection rates. Injury May 25 [Epub ahead of print] [DOI] [PubMed]
- 9.Crowley DJ, Kanakaris NK, Giannoudis PV. Irrigation of the wounds in open fractures. J Bone Joint Surg Br. 2007;89:580–585. doi: 10.1302/0301-620X.89B5.19286. [DOI] [PubMed] [Google Scholar]
- 10.Farouk O, Krettek C, Miclau T, et al. Minimally invasive plate osteosynthesis and vascularity: preliminary results of a cadaver injection study. Injury. 1997;28(Suppl 1):A7–A12. doi: 10.1016/S0020-1383(97)90110-8. [DOI] [PubMed] [Google Scholar]
- 11.Giannoudis PV, Papakostidis C, Roberts C. A review of the management of open fractures of the tibia and femur. J Bone Joint Surg Br. 2006;88:281–289. doi: 10.1302/0301-620X.88B3.16465. [DOI] [PubMed] [Google Scholar]
- 12.Gopal S, Majumder S, Batchelor AG, et al. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82:959–966. doi: 10.1302/0301-620X.82B7.10482. [DOI] [PubMed] [Google Scholar]
- 13.Gregory P, Sanders R (1995) The management of severe fractures of the lower extremities. Clin Orthop Relat Res 95–105 [PubMed]
- 14.Gustilo RB, Merkow RL, Templeman D. The management of open fractures. J Bone Joint Surg Am. 1990;72:299–304. [PubMed] [Google Scholar]
- 15.Jensen JS, Hansen FW, Johansen J. Tibial shaft fractures. A comparison of conservative treatment and internal fixation with conventional plates or AO compression plates. Acta Orthop Scand. 1977;48:204–212. doi: 10.3109/17453677708985136. [DOI] [PubMed] [Google Scholar]
- 16.Keating JF, O’Brien PJ, Blachut PA, et al. Locking intramedullary nailing with and without reaming for open fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am. 1997;79:334–341. doi: 10.2106/00004623-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Korovessis P, Milis Z, Christodoulou G, et al. Open tibial shaft fractures: a comparative analysis of different methods of fixation in southwestern Greece. J Trauma. 1992;32:77–81. doi: 10.1097/00005373-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Kristensen KD. Tibial shaft fractures. The frequency of local complications in tibial shaft fractures treated by internal compression osteosynthesis. Acta Orthop Scand. 1979;50:593–598. doi: 10.3109/17453677908989809. [DOI] [PubMed] [Google Scholar]
- 19.Langenberg R. Results of surgical therapy of open tibial shaft fractures. Zentralbl Chir. 1987;112:1508–1514. [PubMed] [Google Scholar]
- 20.Littenberg B, Weinstein LP, McCarren M, et al. Closed fractures of the tibial shaft. A meta-analysis of three methods of treatment. J Bone Joint Surg Am. 1998;80:174–183. doi: 10.2106/00004623-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Montori VM, Swiontkowski MF, Cook DJ (2003) Methodologic issues in systematic reviews and meta-analyses. Clin Orthop Relat Res 43–54 [DOI] [PubMed]
- 22.Muller ME, Allgower M, Schneider R, Willeneger H (1991) Manual of Internal Fixation. Springer-Verlag, Heidelberg
- 23.Oh CW, Park BC, Ihn JC, Park HJ. Primary unreamed intramedullary nailing for open fractures of the tibia. Int Orthop. 2001;24:338–341. doi: 10.1007/s002640000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson SA. Open fractures of the tibial shaft. Instr Course Lect. 1997;46:293–302. [PubMed] [Google Scholar]
- 25.Patzakis MJ, Wilkins J, Moore TM (1983) Considerations in reducing the infection rate in open tibial fractures. Clin Orthop Relat Res 36–41 [PubMed]
- 26.Perren SM. The technology of minimally invasive percutaneous osteosynthesis (MIPO) Injury. 2002;33(Suppl 1):VI–VII. doi: 10.1016/S0020-1383(02)00063-3. [DOI] [PubMed] [Google Scholar]
- 27.Ruedi T, Webb JK, Allgower M. Experience with the dynamic compression plate (DCP) in 418 recent fractures of the tibial shaft. Injury. 1976;7:252–257. doi: 10.1016/S0020-1383(75)80002-7. [DOI] [PubMed] [Google Scholar]
- 28.Sanders R, Jersinovich I, Anglen J, et al. The treatment of open tibial shaft fractures using an interlocked intramedullary nail without reaming. J Orthop Trauma. 1994;8:504–510. [PubMed] [Google Scholar]
- 29.Sanders R, Swiontkowski M, Nunley J, Spiegel P. The management of fractures with soft-tissue disruptions. J Bone Joint Surg Am. 1993;75:778–789. doi: 10.2106/00004623-199305000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Singh S, Ng KC, Chia P. Plating of displaced mid-tibial fractures-a retrospective review of 80 cases. Singapore Med J. 1997;38:58–61. [PubMed] [Google Scholar]
- 31.Vara-Thorbeck R, Ruiz-Morales M, Hernandez-Hernandez MA. Conservative management versus osteosynthesis in the treatment of tibial shaft fractures. Zentralbl Chir. 1989;114:757–765. [PubMed] [Google Scholar]
- 32.Wagner M, Frenk A, Frigg R. New concepts for bone fracture treatment and the Locking Compression Plate. Surg Technol Int. 2004;12:271–277. [PubMed] [Google Scholar]