Abstract
The successful treatment of nonunions represents a major challenge for orthopaedic surgeons. Lately, ongoing advances made in the field of molecular medicine and molecular biology have increased our understanding of the pathways and involvement of mediators surrounding the bone healing process. As a result, the surgeon’s armamentarium has been increased in terms of options for intervention. This article aims to provide an overview of minimally invasive techniques applicable in the treatment of nonunions of fractures.
Introduction
The incidence of impaired healing of long bone fractures has been reported to range between 5 and 10% [25, 88, 94, 118, 119]. It requires complex and expensive treatment and a variable degree of morbidity is often a common finding. Nonunion represents a challenging clinical problem for both the patient and the treating physician [10]. The implementation of treatment protocols can be time consuming. Not infrequently, nonunion is associated with chronic pain, functional and psychosocial disability, to say nothing of the socio-economic burden [27, 116].
Implementation of a treatment strategy for aseptic nonunions depends on an accurate assessment and classification of the nonunion [52, 73]. Apart from the physiology of the host (patient) both the presence of mechanical stability and the state of the surrounding soft tissue envelope are of paramount importance.
Autologous cancellous bone grafting (ABG) remains the gold standard biological method used to promote bone healing by stimulating the local micro-environment at the nonunion site [7, 9, 34, 35, 87, 101]. However, its limited availability, as well as the donor site morbidity and complications such as chronic pain, neurovascular injury and infection have dictated the need for the development of alternative methods of biological stimulation [3, 15, 17, 24, 34, 35, 39, 49, 96, 105, 112]. Other methods of biological stimulation, used either alone or in combination, include allo-grafting, the use of electrical, ultrasound, and shockwave stimulation, a variety of bone graft substitutes, with either osteoconductive or both osteoconductive and osteoinductive properties and bone marrow injections [26, 52, 73, 84, 87, 90, 122].
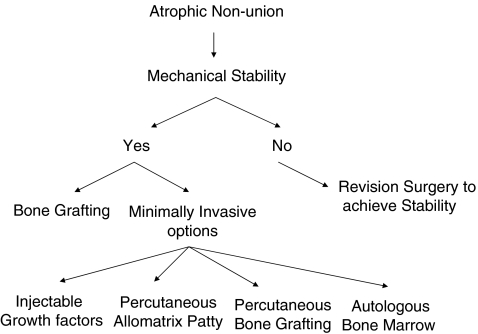
Assuming that the mechanical stability factor is sound, each treatment option of biological stimulation may require a degree of surgical dissection and exposure for the delivery of bone stimulating substances or autologous bone graft. Extensile surgical exposures ideally should be avoided as they could negatively affect the already compromised local biological environment of the nonunion site. In this context, where appropriate, minimally invasive techniques of biological stimulation are desirable (Fig. 1).
Fig. 1.
Considerations for the treatment of atrophic nonunions
The purpose of this study therefore was to investigate the current advances in the enhancement of the fracture healing response and treatment of nonunions with minimally invasive techniques.
Materials and methods
We searched MEDLINE for general keywords such as ‘nonunion’, ‘autologous bone marrow’, and ‘minimally invasive techniques’, both isolated or in combination with specific words including ‘growth factors’, ‘bone marrow injection’ and ‘percutaneous patty’, from 1970 until the present. For paper selection, the initial inclusion criteria were studies reporting results on minimally invasive techniques applied in the treatment of fresh fractures and nonunions both in humans and animal models. We focussed our research methodology retrieving studies on four potential options on biological stimulation using non-invasive techniques: (1) injection of growth factors, (2) percutaneous bone grafting, (3) percutaneous patty and (4) autologous bone marrow injections. Such parameters as the type of nonunion, number of treated fractures, time to union, and technique used were extracted and analysed from the studies. The reference list of studies which met the inclusion criteria were further screened for inclusion of manuscripts which could have been omitted from the initial screening process.
Exclusion criteria included publications in the non-English language or studies having incomplete documentation.
Results
A total of 99 articles met the inclusion criteria [1–6, 11–13, 16–22, 25, 28–32, 36, 37, 39–51, 53–72, 74–83, 85, 86, 88, 89, 91–93, 95–100, 102–113, 115–117, 119–121, 123].
Studies selected were grouped as experimental or clinical as described in this paper.
Experimental studies
Injection of growth factors for enhancement of fracture healing
The usefulness of this technique is still under intense study by several investigators using different animal models [4, 67, 77, 95, 97, 100]. Simple injection of osteoinductive growth factors in subcutaneous tissue has showed that it can promote bone formation [97, 100]. A simple local percutaneous injection in fractures has been shown to enhance bone healing [23]. Similarly, injection of BMP-2 in a rat segmental defect model showed a dose-dependent effect, with a low dose resulting in large amounts of fibrous tissue and cartilage while larger doses resulted in trabecular bone and small amounts of cartilage [4]. Such a protein-based approach though poses potential disadvantages derived from the short protein half-life and the poor retention in the fracture, nonunion or defect site. Gene transfer could overcome these limitations. Several viral vectors carrying BMP-2 cDNA have been studied on animal models over the years [4, 5, 104]. All showed promising results for future clinical application.
The potential of other molecules have been studied alongside BMPs in recent years. Basic fibroblast growth factor (bFGF) injection was found to contribute to the formation of a larger cartilaginous callus but without any accelerating effect in fracture consolidation [77]. Rozen et al. studied the effect of enhancement of each stage of fracture healing by injecting IL-6 during the inflammatory stage, PTH 1-34 during the granulation stage and PTH 28-48 during the callus formatting stage [95]. Their findings revealed a 300% increase in mechanical resistance in the treated rat tibiae [95].
Percutaneous patty administration
The inspiration for the development of an injectable osteoinductive graft for the treatment of bone defects and nonunions came with the use of demineralised bone matrix (DBM). So far, the available animal studies prove that this technique can be used in such pathologies [16, 109, 110]. Comparison with autogenous bone grafting in animals has shown that injectable allomatrix patty was as effective as autograft bone, and was also excellent in handling [107]. DBM, when applied in nonunions, tend to remodel from the periphery, which may explain a lower volume of bone but without any difference in bone mineral density and signs of radiographic or histological healing [54]. In a canine nonunion model, DBM stimulated defect healing, but its combination with bone marrow exerted a greater healing potential [108].
Bone marrow injection for fresh fractures and nonunions
The use of BM to facilitate the healing of bone defects and nonunions is extensively covered by several animal models. The findings reported have been favourable [21, 83]. In segmental bone defects of canine ulna the animals treated with autologous bone marrow united in contrast with the untreated control animals which developed nonunions [42]. Percutaneous BM injections were also able to heal bone defects in rabbits [61]. In dogs suffering from tibial shaft nonunions the injection of BM or demineralised bone matrix in the site of nonunions revealed that both were able to stimulate the healing of the defect [110]. The combination though appeared to be more potent compared to each of them alone. BM combined with platelet-rich plasma and freeze-dried bone allografts could serve as an alternative treatment modality [21].
Ma et al. reported a new method of promoting fracture healing by the administration of multiple cryopreserved injections of BM. In a rabbit model such an approach increased the incidence of union, the radiological bone volume and the bone mineral density [63].
Clinical studies
Injection of growth factors for treatment of nonunions
The injection of growth factors at the nonunion site could be used as an alternative, less invasive technique. The rationale behind this method may be associated with signal transduction upregulating the activity of host mesenchymal stem cells, with the increase of vascularity but also with the enhancement of the low levels of expression of regulatory molecules encountered at the cells at the nonunion site [78]. Currently, BMP-2 and BMP-7 are commercially available and the results obtained by open implantation of these molecules at the nonunion site appears to be promising [2, 11, 28, 29, 36, 37, 40, 52, 54, 98, 111, 121]. However, as far as we know, there are no studies on the outcome of this technique in humans. This is mainly due to formulation problems and the lack of a perfect delivery vehicle [97].
Percutaneous bone grafting
Minimally invasive bone grafting for tibial nonunions could serve as an alternative to open reduction with favourable results. The technique consists of a cylindrical cut through the nonunion site, replaced by a similar piece obtained from the iliac crest. Kettumen et al. evaluated this technique in 41 patients, achieving union in 37 (90%) by a period of 13 weeks (range 10–48). Similarly, Bhan and Mehara [5] and Maghsudi et al. [64] reported union rates in tibial nonunions of 86% (18 out of 21 patients) and 88% (seven out of eight patients), respectively. Maneerit et al., in a randomised prospective trial, compared the results of open reduction and percutaneous bone grafting for tibial fractures. They reported equal rates of union by both techniques, but with definite superiority of the percutaneous technique in terms of blood loss and reduction of operative time [68]. Alternatively, the technique of arthroscopy could be applied for the visualisation, exposure and application of bone grafts at the nonunion site. Johnson et al. successfully treated eight patients suffering from nonunions by arthroscopic delivery of cancellous bone grafts [51].
Percutaneous patty
Wilkins et al. used injectable allomatrix patty for the treatment of 35 patients suffering from long bone nonunions [116]. Their results revealed an 85.1% union rate. On the other hand, a recent study by Ziran et al., which evaluated an open application of a new calcium sulphate-demineralised bone matrix/allomatrix in the treatment of nonunions, presented an unacceptably high rate of complications which included postoperative drainage (54%), deep infection (34%) and an overall failure rate of 46% [123].
Bone marrow injection
The osteogenic properties of BM were first reported by Gourjon et al. in 1869 after the observation that injections of bone marrow to heterotopic sites resulted in the formation of new bone. Since then several reports have documented the osteogenic potential of BM which was attributed to primitive wandering cells capable of osteogenic differentiation. Our understanding of these observations was improved in 1971 with the discovery of “bone marrow derived osteogenic precursor cells” or the so-called BM-derived mesenchymal stem cells.
Mesenchymal stem cells (MSCs) are osteoprogenitor cells, readily available to differentiate towards chondrocytes or osteoblasts and support endochondral and intramembranous ossification. The frequency of MSCs obtained from BM aspirates is about 0.01% or lower [20]. Hernigou et al. concentrated BM by centrifugation, preparing injections containing an average of 2,579 ± 1,121 progenitors/ml compared to the 612 ± 134 progenitors/ml in simple bone marrow aspirates [47]. However, it should be clear that bone itself, as well as many other tissues like periosteum, fat and muscle host mesenchymal stem cells capable of producing bone [91]. Furthermore, it was suggested that genetic reprogramming or transdifferentiation can occur, so fully differentiated cells of one type could switch into another fully functional cell type [20]. These issues though, are poorly understood, and currently there is no indication for the existence of the “ideal progenitor cell” responsible for fracture healing.
In any case, the fate of MSCs is influenced by the local microenvironment, chemotaxis and interactions with the extracellular matrix [70, 82]. During fracture healing they can migrate outside BM and differentiate into osteoblasts to form callus [106]. It was also shown that they could also contribute to fracture healing after systemic injection, being localized at the fracture site [22].
The concept of the delivery of fresh autologous bone marrow as an autograft has developed over the last two decades. It is an inexpensive method that requires minimal hospitalisation and patient care. Patients undergoing BM harvesting can return to their daily activities within 12 hours [80]. Several animal models together have proven the feasibility of this technique both in enhancement of the outcome in fresh fractures and in the treatment of delayed unions or nonunions.
For fresh fractures The feasibility of BM injection in fresh fractures with high risk for nonunions is currently under investigation. Khanal et al., in a prospective randomised study of tibial fresh closed fractures, compared the results obtained by conventional treatment and injection of 15 ml of bone marrow [57]. In the group that received BM all fractures united, while in the control group the union rate was 95% (19 out of 20). Patients who received BM injection experienced a lower time to union. Another application of BM was in a case of non-ossifying fibroma of a radius of a ten-year-old girl with a pathological fracture, whereby the curettage and the concomitant filling of the void with autologous bone marrow resulted in complete union in 12 months [44].
For nonunions The first application of injectable bone marrow for un-united fractures appeared in the 1980s [45, 50]. Connolly et al., using relatively large amounts of bone marrow of 100–150 ml, successfully treated eight out of ten tibial nonunions [18]. Healey et al. evaluated the feasibility of this technique in eight patients suffering from nonunion after reconstruction for primary sarcoma. Five of the eight patients received chemotherapy. The authors reported union in five cases (three out of five of whom received chemotherapy) [45]. Thereafter, bone marrow injections were widely used for tibial [18, 19, 32, 99, 103, 111, 113], femoral [69, 103, 115], humeral [32, 102, 103, 115], and clavicular nonunions [107], and also for nonunions of radius and ulna [32, 101, 103]. Various amounts of bone marrow were used, from 10 ml to 150 ml, without showing any difference in the success rates (Table 1). However, a definite relationship was apparent between the number of osteoprogenitors injected and the rate of union [47]. Hernigou et al. compared the number of mesenchymal stem cells injected in 53 cases of successful union to seven cases where this technique failed [47]. The united cases received 54,962 ± 17,431 MSCs while the unsuccessful ones received 19,324 ± 6843 MSCs [47]. In total, 336 cases of nonunions have been described. From those, 249 (72.1%) were tibial nonunions, 40 (11.6%) were femoral nonunions, 20 (5.8%) were humeral and the rest (36; 10.4%) were nonunions of the radius and ulna (Table 1). The mean age was 38.1 years. The union rate after treatment with percutaneous injection of autologous bone marrow was 88.8% (296 out of 336 nonunions; range 57%–94%). The mean time to union was 4.8 months (range 2.5–8.1).In addition to pure bone marrow grafting, composites of allogenic demineralised bone matrix impregnated with aspirated bone marrow were evaluated for their efficacy in nonunions of long bones. This approach resulted in an overall success rate of 88% [117]. Similarly, Kocialkowski et al. reported the successful treatment of 11 cases (100% success rate) of nonunions with the use of composites of 1:1 collagen and ceramic enriched with bone marrow [59].
Table 1.
The effectiveness of bone marrow (BM) injections for nonunions
| First author/year | Number of nonunions | Type | Mean age (y), n (range) | United, n (%) | Time to union (months), n (range) | Comments |
|---|---|---|---|---|---|---|
| Healey/1990 [45] | 8 | Femur 8 | 27 (6–60) | 5 (62.5) | 4.95 (1–9) | Nonunion occurred after reconstruction for lower limb cancer resection |
| 50 ml percutaneous injection | ||||||
| Connolly/1991 [18] | 10 | Tibia 10 | 30 (18–82) | 8 (80) | 6.7 (5–10) | 100–150 ml of BM aspirated and injected |
| Fractures were immobilised by cast | ||||||
| Garg/1993 [32] | 20 | Tibia 15 | 35 (18–65) | 17 (85) | 5 (3–7) | Percutaneous injection of 15–20 ml of BM |
| Humerus 3 | ||||||
| Ulna 2 | ||||||
| Sim/1993 [102] | 11 | Tibia 8 | 38 (19–62) | 9 (81) | 2.5 (1–5.75) | Percutaneous injection of 50–200 ml of BM |
| Femur 1 | ||||||
| Humerus 1 | ||||||
| Ulna 1 | ||||||
| Garg/1995 [33] | Case report | Tibia | 12 | 1 (100) | 18 | ∼20 ml of BM were aspirated and injected in pseudoarthrosis while limb was placed in cast |
| After 3 weeks the procedure was repeated | ||||||
| Matsuda/1998 [69] | 7 | Femur | 53 (24–70) | 4 (57) | na (5–9) | Percutaneous technique with injection of 150 ml of BM |
| Two infections encountered resulting in failed union | ||||||
| Sebecic/1999 [99] | Case report | Tibia 1 | 44 | 1 (100) | 5 | 150 ml injected and immobilisation achieved by external fixation |
| Siwach/2001 [103] | 72 | Tibia 42 | 41.2 (26–56) | 68 (94) | na | A maximum of 30 ml of BM aspirated and injected percutaneously at nonunion site |
| Femur 8 | The procedure was repeated where required after 4–6 weeks after initial injection | |||||
| Humerus 12 | ||||||
| Forearm 10 | ||||||
| Wang/2001 [113] | 56 | Tibia | na (19–72) | 53 (94) | na | BM aspirated and injected percutaneously at nonunion site |
| The procedure was repeated every month 2–3 times | ||||||
| Wilkins/2003 [115] | 69 | Tibia 36 | 42 (15–81) | 61 (88) | 8.1 (2–36) | Percutaneous technique with BM combined with allograft demineralised bone matrix |
| Femur 16 | ||||||
| Humerus 4 | ||||||
| Others 13 | ||||||
| Goel/2005 [38] | 20 | Tibia 20 | 37.5 (24–60) | 15 (75) | 3.5 (1.5–5.5) | A maximum of 15 ml of BM was aspirated and percutaneously injected |
| Hernigou/2005 [47] | 60 | Tibia 60 | 40 (18–78) | 53 (88) | 3 (1–4) | An average of 306 ± 24 ml was aspirated, concentrated to 20 ml and percutaneously injected |
| Tetreault/2007 [107] | Case report | Clavicle | 39 | 1 (100) | na (within 12) | Percutaneous injection of 10 ml of BM |
na not available
Level of Evidence: All presented studies are 2C except case reports which are 1C
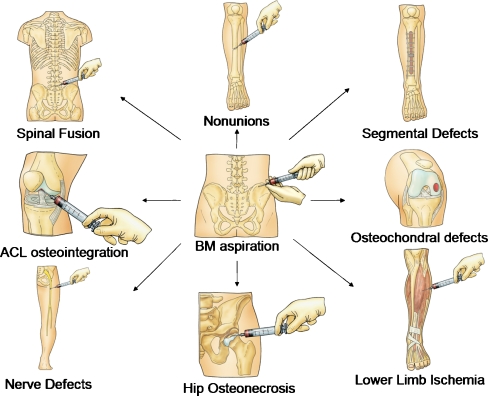
For other orthopaedic applications The value of bone marrow is not only restricted to the enhancement of fracture union and healing of nonunions, but rather has proved important in other orthopaedic pathologies (Fig. 2). The isolation of osteoprogenitors contained in BM, followed by their uploading onto scaffolds and implantation in bone defects and difficult nonunions, is another successful method of treatment [81, 93]. Orozco et al. has proposed a method comprising aspiration of 7–30 ml of BM at 12 days prior to surgery, followed by mesenchymal stem cell isolation and expansion [81]. During surgical fixation the cultured cells were mixed with β-tricalcium phosphate matrix and placed at the nonunion site. Radiological callus formation occurred in five out of six patients at an average of eight weeks (range 6–24 weeks). Additionally, culture-expanded mesenchymal stem cells are used in distraction osteogenesis showing acceleration of regeneration with shortening of the treatment time [58]. Another application of BM consists of BM stem cell auto-transplantation at the ischaemic lower limb, which resulted in an improvement of the peripheral blood flow and percutaneous oxygen partial pressure [80]. Moreover, BM and its osteoprogenitors were used in spinal fusion [22, 73], osteonecrosis of the femoral head [30, 31, 46], bone cysts [14], osteochondral defects [13], tendon osteointegration [62], and in facilitation of nerve repair [12, 120].
Fig. 2.
Bone marrow applications
Discussion
The normal physiological reaction to fracture is a spontaneous sequence of events briefly summarised as initial inflammation, followed by soft and hard callus formation and ultimately bone remodelling [91]. When this process does not occur, as in cases of nonunions or traumatic bone defects, surgical intervention is required and is commonly combined with autologous grafting, which enhances the local environment in terms of osteoprogenitor cells, structural substrate and bone inducing proteins [92].
The surgical technique usually implies extended incisions for the revision of fixation of the affected bone [3, 17, 39, 49, 65, 96, 105, 112]. The use of judiciously applied orthopaedic implants through limited incisions to treat fractures and nonunions is a recently developed alternative to the traditional techniques [92]. Similarly, the introduction of osteoprogenitor cells or osteoinductive materials has gained more support lately with satisfactory results [20, 45, 47, 71].
Injection of growth factors is based on the tremendous effect that these molecules exert to induce upregulation of bone healing [91]. Currently, a variety of molecules including BMP-2, BMP-7, platelet-derived growth factor, fibroblast derived growth factor and PTH have been used for the treatment of nonunions in experimental studies (animal models) [4, 7, 8, 50, 67, 71, 77, 95, 97, 100, 114]. The results have shown that the molecules are capable of upregulating the healing process, thus accelerating the fracture consolidation and mechanical properties [50, 71, 77, 89, 95, 100, 114]. However, there are no clinical data involving injectable formulations. This can be attributed to the slow development of appropriate delivery vehicles (liquid formulations), the lack of identification of the ideal concentration and the lack of safety data.
Percutaneous bone grafting could be the alternative to local application of osteoinductive molecules. In animal models, it has been well established that DBM is capable of stimulating new bone formation, but also that its combination with osteoprogenitor cells produces better results [16, 109, 110]. In humans this approach proved controversial as the use of injectable demineralised bone resulted in a high rate of complications [123]. Percutaneous bone grafting on the other hand has been proven successful in the safe treatment of nonunions [5, 54, 56]. This could be attributed to the fact that autologous bone chips contain the required structural osteoconductive substrate combined with the osteoinductive properties of the autologous growth factors and osteoproductive cells. Although this has been proven to be true, donor site morbidity, potential unavailability and restrictions due to the size of the defect are limiting factors to be taken into account.
Percutaneous bone marrow implantation for the treatment of nonunions is a low risk inexpensive procedure with high biological activity [79]. The results from published papers indicate that the success rate of treating established nonunions with percutaneous injection of autologous bone marrow can be as high as 88.8% (Table 1). Literature describing conventional approaches using autologous bone grafts have reported a variable success rate. Tibial nonunions heal at a rate of 90–95% [25, 88, 119], whereas in femoral nonunions the rate of healing is slightly lower, ranging from 78.3% to 92.3% [43, 85, 86, 117]. Similarly, in humeral nonunions the healing rates vary between 76% and 100% [1, 41, 48, 72]. Therefore, it can be clearly concluded that percutaneous bone marrow injection could be as effective as open autologous grafting. This efficacy can be attributed to the implantation of viable osteoprogenitor cells. However, the concentration of MSCs may vary significantly between individuals, aspiration sites, and aspiration technique [66, 74, 75].
Over the past several decades, minimally invasive surgery has revolutionised many fields of medicine. In orthopaedics and especially in challenging situations, it has decreased the damage to soft tissues, minimised scaring, reduced postoperative pain and improved recovery time [51, 68]. All of these have lead to accelerated return to work, sports and normal daily living. Among these techniques the choice of the most appropriate approach can be difficult. The injection of growth factors can enhance the local biology and optimise the potential of osteoprogenitors. This technique though is not fully developed for human use yet and will not benefit poor local environment [97]. Percutaneous bone grafting seems to be an excellent alternative to extensive open bone grafting. It enhances the local environment with fresh bone and cells required to facilitate healing [5, 56, 64]. The disadvantages of this technique can be the limited extent of grafted bone, the potentially small number of grafted osteoprogenitors, and the small but present donor site morbidity. Autologous bone marrow injection on the other hand, augments cellular numbers and has extremely low donor site morbidity but stimulates the grafted cells to produce a structural osteoconductive substrate [79]. Our view is that all these techniques can be used in the treatment of simple or challenging cases with favourable results. However, simple cost-effective techniques such as autologous bone marrow injection have been proven to have results comparable to those of open techniques. By standardised nomenclature, enhanced training, and rigorous evidence-based research, these emerging techniques will continue to improve the surgical outcomes for hundreds of thousands of patients.
Conclusion
Minimally invasive techniques for the treatment of nonunions and enhancement of fracture healing appear to be effective as a single treatment modality. Current literature, although limited, supports the idea that a simple injection of bone marrow can have the same results as those obtained from open techniques. However, more studies and randomised trials are needed for definitive conclusions.
Footnotes
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. No funds were received in support of this study.
References
- 1.Mukaimi A, Mokhtar A, Abo El Nass M. The use of locked nails in the treatment of humeral shaft: delayed union and nonunion. Med Princ Pract. 2005;14:245–249. doi: 10.1159/000085743. [DOI] [PubMed] [Google Scholar]
- 2.Axelrad TW, Kakar S, Einhorn TA. New technologies for the enhancement of skeletal repair. Injury. 2007;38:S49–S62. doi: 10.1016/j.injury.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Babhulkar SS, Pande KC, Babhulkar S. Ankle instability after fibular resection. J Bone Joint Surg Br. 1995;77:258–261. [PubMed] [Google Scholar]
- 4.Betz OB, Betz VM, Nazarian A, Pilapil CG, Vrahas MS, Bouxsein ML, Gerstenfeld LC, Einhorn TA, Evans CH. Direct percutaneous gene delivery to enhance healing of segmental bone defects. J Bone Joint Surg Am. 2006;88:355–365. doi: 10.2106/JBJS.E.00464. [DOI] [PubMed] [Google Scholar]
- 5.Betz VM, Betz OB, Glatt V, Gerstenfeld LC, Einhorn TA, Bouxsein ML, Vrahas MS, Evans CH. Healing of segmental bone defects by direct percutaneous gene delivery: effect of vector dose. Hum Gene Ther. 2007;18:907–915. doi: 10.1089/hum.2007.077. [DOI] [PubMed] [Google Scholar]
- 6.Bhan S, Mehara AK. Percutaneous bone grafting for nonunion and delayed union of fractures of the tibial shaft. Int Orthop. 1993;17:310–312. doi: 10.1007/BF00181707. [DOI] [PubMed] [Google Scholar]
- 7.Bilic R, Simic P, Jelic M, Stern-Padovan R, Dodig D, Meerdervoort HP, Martinovic S, Ivankovic D, Pecina M, Vukicevic S. Osteogenic protein-1 (BMP-7) accelerates healing of scaphoid non-union with proximal pole sclerosis. Int Orthop. 2006;30:128–134. doi: 10.1007/s00264-005-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31:721–727. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burwell RG. Studies in the transplantation of bone. VII. The fresh composite homograft-autograft of cancellous bone; an analysis of factors leading to osteonecrosis in marrow transplants and in marrow-containing bone grafts. J Bone Joint Surg Br. 1964;46:110–140. [PubMed] [Google Scholar]
- 10.Calori GM, Albisetti W, Agus A, Iori S, Tagliabue S. Risk factors contributing to fracture non-unions. Injury. 2007;38:S11–S18. doi: 10.1016/s0020-1383(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 11.Calori GM, D'Avino M, Tagliabue L, Albisetti W, d'Imporzano M, Peretti G. An ongoing research for evaluation of treatment with BMPs or AGFs in long bone non-union: protocol description and preliminary results. Injury. 2006;37:S43–S50. doi: 10.1016/j.injury.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Caylan R, Bektas D, Dikmen T, Bektas O, Omay SB, Ovali E. Mesenchymal stem cells in iatrogenic facial nerve paralysis: a possible role in the future. Eur Arch Otorhinolaryngol. 2006;263:963–967. doi: 10.1007/s00405-006-0093-z. [DOI] [PubMed] [Google Scholar]
- 13.Centeno CJ, Kisiday J, Freeman M, Schultz JR. Partial regeneration of the human hip via autologous bone marrow nucleated cell transfer: a case study. Pain Physician. 2006;9:253–256. [PubMed] [Google Scholar]
- 14.Chang CH, Stanton RP, Glutting J. Unicameral bone cysts treated by injection of bone marrow or methylprednisolone. J Bone Joint Surg Br. 2002;84:407–412. doi: 10.1302/0301-620x.84b3.12115. [DOI] [PubMed] [Google Scholar]
- 15.Chapman MW, Bucholz R, Cornell C. Treatment of acute fractures with a collagen-calcium phosphate graft material. A randomized clinical trial. J Bone Joint Surg Am. 1997;79:495–502. doi: 10.2106/00004623-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Clokie CM, Moghadam H, Jackson MT, Sandor GK. Closure of critical sized defects with allogenic and alloplastic bone substitutes. J Craniofac Surg. 2002;13:111–121. doi: 10.1097/00001665-200201000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Colterjohn NR, Bednar DA. Procurement of bone graft from the iliac crest. An operative approach with decreased morbidity. J Bone Joint Surg Am. 1997;79:756–759. doi: 10.2106/00004623-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Connolly JF, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991;266:259–270. [PubMed] [Google Scholar]
- 19.Connolly JF, Shindell R. Percutaneous marrow injection for an ununited tibia. Nebr Med J. 1986;71:105–107. [PubMed] [Google Scholar]
- 20.Connolly JF. Injectable bone marrow preparations to stimulate osteogenic repair. Clin Orthop. 1995;313:8–18. [PubMed] [Google Scholar]
- 21.Dallari D, Fini M, Stagni C, Torricelli P, Nicoli Aldini N, Giavaresi G, Cenni E, Baldini N, Cenacchi A, Bassi A, Giardino R, Fornasari PM, Giunti A. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet rich plasma and freeze-dried bone allografts, alone and in combination. J Orthop Res. 2006;24:877–888. doi: 10.1002/jor.20112. [DOI] [PubMed] [Google Scholar]
- 22.Devine MJ, Mierisch CM, Jang E, Anderson PC, Balian G. Transplanted bone marrow cells localize to fracture callus in a mouse model. J Orthop Res. 2002;20:1232–1239. doi: 10.1016/S0736-0266(02)00051-7. [DOI] [PubMed] [Google Scholar]
- 23.Einhorn TA, Majeska RJ, Mohaideen A, Kagel EM, Bouxsein ML, Turek TJ, Wozney JM. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003;85-A:1425–1435. doi: 10.2106/00004623-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Faundez AA, Taylor S, Kaelin AJ. Instrumented fusion of thoracolumbar fracture with type I mineralized collagen matrix combined with autogenous bone marrow as a bone graft substitute: a four-case report. Eur Spine J. 2006;15:630–635. doi: 10.1007/s00586-006-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldman DS, Shin SS, Madan S, Koval KJ. Correction of tibial malunion and nonunion with six-axis analysis deformity correction using the Taylor Spatial Frame. J Orthop Trauma. 2003;17:549–554. doi: 10.1097/00005131-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A:454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Frangakis EK. Intracapsular fractures of the neck of the femur. Factors influencing non-union and ischaemic necrosis. J Bone Joint Surg Br. 1966;48:17–30. [PubMed] [Google Scholar]
- 28.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A:S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 29.Friedlaender GE. Osteogenic protein-1 in treatment of tibial nonunions: current status. Surg Technol Int. 2004;13:249–252. [PubMed] [Google Scholar]
- 30.Gangji V, Hauzeur JP, Matos C, Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A(6):1153–1160. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am. 2005;87:106–112. doi: 10.2106/JBJS.D.02662. [DOI] [PubMed] [Google Scholar]
- 32.Garg NK, Gaur S, Sharma S. Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop Scand. 1993;64:671–672. doi: 10.3109/17453679308994595. [DOI] [PubMed] [Google Scholar]
- 33.Garg NK, Gaur S. Percutaneous autogenous bone-marrow grafting in congenital tibial pseudarthrosis. J Bone Joint Surg Br. 1995;77:830–831. [PubMed] [Google Scholar]
- 34.Giannoudis P, Psarakis S, Kontakis G. Can we accelerate fracture healing? A critical analysis of the literature. Injury. 2007;38:S81–S89. doi: 10.1016/j.injury.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38:S3–S6. doi: 10.1016/s0020-1383(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 36.Giannoudis PV, Psarakis S, Kanakaris NK, Pape HC. Biological enhancement of bone healing with bone morphogenetic protein-7 at the clinical setting of pelvic girdle non-unions. Injury. 2007;38:S43–S48. doi: 10.1016/s0020-1383(08)70008-1. [DOI] [PubMed] [Google Scholar]
- 37.Giannoudis PV, Tzioupis C. Clinical applications of BMP-7: the UK perspective. Injury. 2005;36:S47–S50. doi: 10.1016/j.injury.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 38.Goel A, Sangwan SS, Siwach RC, Ali AM. Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury. 2005;36:203–206. doi: 10.1016/j.injury.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T, BMP-2 Evaluation in Surgery for Tibial Trauma (BESTT) study group Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Green E, Lubahn JD, Evans J. Risk factors, treatment, and outcomes associated with nonunion of the midshaft humerus fracture. J Surg Orthop Adv. 2005;14:64–72. [PubMed] [Google Scholar]
- 42.Grundel RE, Chapman MW, Yee T, Moore DC. Autogeneic bone marrow and porous biphasic calcium phosphate ceramic for segmental bone defects in the canine ulna. Clin Orthop Relat Res. 1991;266:244–258. [PubMed] [Google Scholar]
- 43.Hak DJ, Lee SS, Goulet JA. Success of exchange reamed intramedullary nailing for femoral shaft nonunion or delayed union. J Orthop Trauma. 2000;14:178–182. doi: 10.1097/00005131-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Hase T, Miki T. Autogenous bone marrow graft to non-ossifying fibroma with a pathologic fracture. Arch Orthop Trauma Surg. 2000;120:458–459. doi: 10.1007/s004029900100. [DOI] [PubMed] [Google Scholar]
- 45.Healey JH, Zimmerman PA, McDonnell JM, Lane JM. Percutaneous bone marrow grafting of delayed union and nonunion in cancer patients. Clin Orthop Relat Res. 1990;256:280–285. [PubMed] [Google Scholar]
- 46.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 48.Hierholzer C, Sama D, Toro JB, Peterson M, Helfet DL. Plate fixation of ununited humeral shaft fractures: effect of type of bone graft on healing. J Bone Joint Surg Am. 2006;88:1442–1447. doi: 10.2106/JBJS.E.00332. [DOI] [PubMed] [Google Scholar]
- 49.Hill NM, Horne JG, Devane PA. Donor site morbidity in the iliac crest bone graft. Aust NZ J Surg. 1999;69:726–728. doi: 10.1046/j.1440-1622.1999.01674.x. [DOI] [PubMed] [Google Scholar]
- 50.Hollinger JO, Hart CE, Hirsch SN, Lynch S, Friedlaender GE. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90:48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- 51.Johnson LL, Morrison KM, Wood DL. The application of arthroscopic principles to bone grafting of delayed union of long bone fractures. Arthroscopy. 2000;16:279–289. doi: 10.1016/S0749-8063(00)90052-5. [DOI] [PubMed] [Google Scholar]
- 52.Jones CB, Mayo KA. Nonunion treatment: iliac crest bone graft techniques. J Orthop Trauma. 2005;19:S11–S13. doi: 10.1097/00005131-200511101-00004. [DOI] [PubMed] [Google Scholar]
- 53.Kanakaris NK, Giannoudis PV. The health economics of the treatment of long-bone non-unions. Injury. 2007;38:S77–S84. doi: 10.1016/s0020-1383(07)80012-x. [DOI] [PubMed] [Google Scholar]
- 54.Kanakaris NK, Paliobeis C, Nlanidakis N, Giannoudis PV. Biological enhancement of tibial diaphyseal aseptic non-unions: the efficacy of autologous bone grafting, BMPs and reaming by-products. Injury. 2007;38:S65–S75. doi: 10.1016/s0020-1383(07)80011-8. [DOI] [PubMed] [Google Scholar]
- 55.Kawcak CE, Trotter GW, Powers BE, Park RD, Turner AS. Comparison of bone healing by demineralized bone matrix and autogenous cancellous bone in horses. Vet Surg. 2000;29:218–226. doi: 10.1053/jvet.2000.5601. [DOI] [PubMed] [Google Scholar]
- 56.Kettunen J, Makela EA, Turunen V, Suomalainen O, Partanen K. Percutaneous bone grafting in the treatment of the delayed union and non-union of tibial fractures. Injury. 2002;33:239–245. doi: 10.1016/s0020-1383(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 57.Khanal GP, Garg M, Singh GK. A prospective randomized trial of percutaneous marrow injection in a series of closed fresh tibial fractures. Int Orthop. 2004;28(3):167–170. doi: 10.1007/s00264-004-0547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitoh H, Kitakoji T, Tsuchiya H, Mitsuyama H, Nakamura H, Katoh M, Ishiguro N. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis—a preliminary result of three cases. Bone. 2004;35:892–898. doi: 10.1016/j.bone.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Kocialkowski A, Wallace WA, Prince HG. Clinical experience with a new artificial bone graft: preliminary results of a prospective study. Injury. 1990;21:142–144. doi: 10.1016/0020-1383(90)90082-6. [DOI] [PubMed] [Google Scholar]
- 60.Koller M, Lorenz W. Study types and study issues in clinical medicine. Forensic Sci Int. 2007;165:98–107. doi: 10.1016/j.forsciint.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Liang YT, Zhang BX, Lu SB. Experimental study and clinical application on osteogenesis of percutaneous autogenous bone marrow grafting in bone defects. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13:148–151. [PubMed] [Google Scholar]
- 62.Lim JK, Hui J, Li L, Thambyah A, Goh J, Lee EH. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899–910. doi: 10.1016/j.arthro.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 63.Ma HL, Chen TH, Hung SC. Development of a new method in promoting fracture healing: multiple cryopreserved bone marrow injections using a rabbit model. Arch Orthop Trauma Surg. 2004;124:448–454. doi: 10.1007/s00402-004-0704-3. [DOI] [PubMed] [Google Scholar]
- 64.Maghsudi M, Neumann C, Hente R, Nerlich M. Percutaneous minimal invasive autologous spongiosa transplantation. Langenbecks Arch Chir Suppl Kongressbd. 1998;115:1218–1220. [PubMed] [Google Scholar]
- 65.Mahendra A, Maclean AD. Available biological treatments for complex non-unions. Injury. 2007;38:S7–S12. doi: 10.1016/s0020-1383(08)70004-4. [DOI] [PubMed] [Google Scholar]
- 66.Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15:546–557. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- 67.Makino T, Kokubu T, Kurosaka M. Effect of recombinant human bone morphogenetic protein on preventing atrophic nonunion. Clin Calcium. 2006;16(5):823–827. [PubMed] [Google Scholar]
- 68.Maneerit J, Meknavin S, Hanpanitkitkan S. Percutaneous versus open bone grafting in the treatment of tibial fractures: a randomized prospective trial. J Med Assoc Thai. 2004;87:1034–1040. [PubMed] [Google Scholar]
- 69.Matsuda Y, Sakayama K, Okumura H, Kawatani Y, Mashima N, Shibata T. Percutaneous autologous bone marrow transplantation for nonunion of the femur. Nippon Geka Hokan. 1998;67:10–17. [PubMed] [Google Scholar]
- 70.Metheny-Barlow LJ, Tian S, Hayes AJ, Li LY. Direct chemotactic action of angiopoietin-1 on mesenchymal cells in the presence of VEGF. Microvasc Res. 2004;68:221–230. doi: 10.1016/j.mvr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Milovancev M, Muir P, Manley PA, Seeherman HJ, Schaefer S. Clinical application of recombinant human bone morphogenetic protein-2 in 4 dogs. Vet Surg. 2007;36:132–140. doi: 10.1111/j.1532-950X.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- 72.Mukaimi A, Mokhtar A, Abo El Nass M. The use of locked nails in the treatment of humeral shaft: delayed union and nonunion. Med Princ Pract. 2005;14:245–249. doi: 10.1159/000085743. [DOI] [PubMed] [Google Scholar]
- 73.Muller ME. Treatment of nonunion by compression. Clin Orthop Relat Res. 1965;43:83–92. [PubMed] [Google Scholar]
- 74.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79:1699–1709. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 75.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–125. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 76.Muschler GF, Nitto H, Matsukura Y, Boehm C, Valdevit A, Kambic H, Davros W, Powell K, Easley K. Spine fusion using cell matrix composites enriched in bone marrow-derived cells. Clin Orthop Relat Res. 2003;407:102–118. doi: 10.1097/00003086-200302000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakajima F, Nakajima A, Ogasawara A, Moriya H, Yamazaki M. Effects of a single percutaneous injection of basic fibroblast growth factor on the healing of a closed femoral shaft fracture in the rat. Calcif Tissue Int. 2007;81:132–138. doi: 10.1007/s00223-007-9048-7. [DOI] [PubMed] [Google Scholar]
- 78.Niikura T, Hak DJ, Reddi AH. Global gene profiling reveals a downregulation of BMP gene expression in experimental atrophic nonunions compared to standard healing fractures. J Orthop Res. 2006;24:1463–1471. doi: 10.1002/jor.20182. [DOI] [PubMed] [Google Scholar]
- 79.Nishimori M, Tateoka A, Tojyo A, Nakao Y, Yamada Y, Hanaoka K. Self-reported recovery time of daily activity after bone marrow harvesting from healthy donors. J Anesth. 2001;15:1–5. doi: 10.1007/s005400170043. [DOI] [PubMed] [Google Scholar]
- 80.Nizankowski R, Petriczek T, Skotnicki A, Szczeklik A. The treatment of advanced chronic lower limb ischaemia with marrow stem cell autotransplantation. Kardiol Pol. 2005;63(4):351–360. [PubMed] [Google Scholar]
- 81.Orozco L, Rodriguez L, Torrico C, Douville J, Hock JM, Armstrong RD, Garcia J, Solano C. Clinical feasibility study: the use of cultured enriched autologous bone marrow cells to treat refractory atrophic and hypotrophic nonunion fractures. http://files.shareholder.com/downloads/ASTM/98210462x0xS950134-05-10343/887359/filing.pdf. Accessed 07 October 2009
- 82.Otto WR, Rao J. Tomorrow's skeleton staff: mesenchymal stem cells and the repair of bone and cartilage. Cell Prolif. 2004;37:97–110. doi: 10.1111/j.1365-2184.2004.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paley D, Young MC, Wiley AM, Fornasier VL, Jackson RW. Percutaneous bone marrow grafting of fractures and bony defects. An experimental study in rabbits. Clin Orthop Relat Res. 1986;208:300–312. [PubMed] [Google Scholar]
- 84.Parikh SN. Bone graft substitutes: past, present, future. J Postgrad Med. 2002;48:142–148. [PubMed] [Google Scholar]
- 85.Patel AA, Ricci WM, McDonald DJ, Borrelli J, Clohisy JC. Treatment of periprosthetic femoral shaft nonunion. J Arthroplast. 2006;21:435–442. doi: 10.1016/j.arth.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 86.Patil S, Montgomery R. Management of complex tibial and femoral nonunion using the Ilizarov technique, and its cost implications. J Bone Joint Surg Br. 2006;88:928–932. doi: 10.1302/0301-620X.88B7.17639. [DOI] [PubMed] [Google Scholar]
- 87.Pecina M, Vukicevic S. Biological aspects of bone, cartilage and tendon regeneration. Int Orthop. 2007;31:719–720. doi: 10.1007/s00264-007-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piriou P, Martin JN, Garreau de Loubresse C, Judet T. Tibia nonunion after intramedullar nailing for fracture: decortication and osteosynthesis by medial plating. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:222–231. doi: 10.1016/s0035-1040(05)84308-9. [DOI] [PubMed] [Google Scholar]
- 89.Pountos I, Corscadden D, Emery P, Giannoudis PV. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury. 2007;38:S23–S33. doi: 10.1016/s0020-1383(08)70006-8. [DOI] [PubMed] [Google Scholar]
- 90.Pountos I, Georgouli T, Blokhuis TJ, Pape HC, Giannoudis PV. Pharmacological agents and impairment of fracture healing: what is the evidence? Injury. 2008;39:384–394. doi: 10.1016/j.injury.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 91.Pountos I, Giannoudis PV. Biology of mesenchymal stem cells. Injury. 2005;3:S8–S12. doi: 10.1016/j.injury.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 92.Pountos I, Jones E, Tzioupis C, McGonagle D, Giannoudis PV. Growing bone and cartilage. The role of mesenchymal stem cells. J Bone Joint Surg Br. 2006;88:421–426. doi: 10.1302/0301-620X.88B4.17060. [DOI] [PubMed] [Google Scholar]
- 93.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 94.Richmond J, Colleran K, Borens O, Kloen P, Helfet DL. Nonunions of the distal tibia treated by reamed intramedullary nailing. J Orthop Trauma. 2004;18:603–610. doi: 10.1097/00005131-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 95.Rozen N, Lewinson D, Bick T, Jacob ZC, Stein H, Soudry M. Fracture repair: modulation of fracture-callus and mechanical properties by sequential application of IL-6 following PTH 1–34 or PTH 28–48. Bone. 2007;41:437–445. doi: 10.1016/j.bone.2007.04.193. [DOI] [PubMed] [Google Scholar]
- 96.Russell JL, Block JE. Surgical harvesting of bone graft from the ilium: point of view. Med Hypotheses. 2000;55:474–479. doi: 10.1054/mehy.2000.1095. [DOI] [PubMed] [Google Scholar]
- 97.Saito N, Okada T, Horiuchi H, Ota H, Takahashi J, Murakami N, Nawata M, Kojima S, Nozaki K, Takaoka K. Local bone formation by injection of recombinant human bone morphogenetic protein-2 contained in polymer carriers. Bone. 2003;32:381–386. doi: 10.1016/s8756-3282(03)00028-0. [DOI] [PubMed] [Google Scholar]
- 98.Schmidmaier G, Schwabe P, Wildemann B, Haas NP. Use of bone morphogenetic proteins for treatment of non-unions and future perspectives. Injury. 2007;38:S35–S41. doi: 10.1016/s0020-1383(08)70007-x. [DOI] [PubMed] [Google Scholar]
- 99.Sebecic B, Gabelica V, Patrlj L, Sosa T. Percutaneous autologous bone marrow grafting on the site of tibial delayed union. Croat Med J. 1999;40:429–432. [PubMed] [Google Scholar]
- 100.Seeherman HJ, Azari K, Bidic S, Rogers L, Li XJ, Hollinger JO, Wozney JM. rhBMP-2 delivered in a calcium phosphate cement accelerates bridging of critical-sized defects in rabbit radii. J Bone Joint Surg Am. 2006;88:1553–1565. doi: 10.2106/JBJS.E.01006. [DOI] [PubMed] [Google Scholar]
- 101.Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38:S75–S80. doi: 10.1016/j.injury.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 102.Sim R, Liang TS, Tay BK. Autologous marrow injection in the treatment of delayed and non-union in long bones. Singapore Med J. 1993;34:412–417. [PubMed] [Google Scholar]
- 103.Siwach RC, Sangwan SS, Singh R, Goel A. Role of percutaneous bone marrow grafting in delayed unions, non-unions and poor regenerates. Indian J Med Sci. 2001;55:326–336. [PubMed] [Google Scholar]
- 104.Sugiyama O, An DS, Kung SP, Feeley BT, Gamradt S, Liu NQ, Chen IS, Lieberman JR. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther. 2005;11:390–398. doi: 10.1016/j.ymthe.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 105.Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 106.Taguchi K, Ogawa R, Migita M, Hanawa H, Ito H, Orimo H. The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem Biophys Res Commun. 2005;331:31–36. doi: 10.1016/j.bbrc.2005.03.119. [DOI] [PubMed] [Google Scholar]
- 107.Tetreault P, Ouellette HA. Healing of a clavicle fracture nonunion with bone marrow injection. J Shoulder Elbow Surg. 2007;16:e23–e24. doi: 10.1016/j.jse.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 108.Tiedeman JJ, Connolly JF, Strates BS, Lippiello L. Treatment of nonunion by percutaneous injection of bone marrow and demineralized bone matrix. An experimental study in dogs. Clin Orthop Relat Res. 1991;268:294–302. [PubMed] [Google Scholar]
- 109.Turner TM, Urban RM, Hall DJ, Cheema N, Lim TH. Restoration of large bone defects using a hard-setting, injectable putty containing demineralized bone particles compared to cancellous autograft bone. Orthopedics. 2003;26:s561–s565. doi: 10.3928/0147-7447-20030502-07. [DOI] [PubMed] [Google Scholar]
- 110.Urban RM, Turner TM, Hall DJ, Infanger SI, Cheema N, Lim TH, Richelsoph K. An injectable calcium sulfate-based bone graft putty using hydroxypropylmethylcellulose as the plasticizer. Orthopedics. 2004;27:s155–s159. doi: 10.3928/0147-7447-20040102-16. [DOI] [PubMed] [Google Scholar]
- 111.Vaibhav B, Nilesh P, Vikram S, Anshul C. Bone morphogenic protein and its application in trauma cases: a current concept update. Injury. 2007;38:1227–1235. doi: 10.1016/j.injury.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 112.Vail TP, Urbaniak JR. Donor-site morbidity with use of vascularized autogenous fibular grafts. J Bone Joint Surg Am. 1996;78:204–211. doi: 10.2106/00004623-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 113.Wang L, Li WS, Zhang QS. Autogenous bone marrow graft for the management of nonunion of tibia. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2001;15:24–25. [PubMed] [Google Scholar]
- 114.White AP, Vaccaro AR, Hall JA, Whang PG, Friel BC, McKee MD. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31:735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilkins RM, Chimenti BT, Rifkin RM. Percutaneous treatment of long bone nonunions: the use of autologous bone marrow and allograft bone matrix. Orthopedics. 2003;26:s549–s554. doi: 10.3928/0147-7447-20030502-04. [DOI] [PubMed] [Google Scholar]
- 116.Wilkins RM, Kelly CM. The effect of allomatrix injectable putty on the outcome of long bone applications. Orthopedics. 2003;26:s567–s570. doi: 10.3928/0147-7447-20030502-08. [DOI] [PubMed] [Google Scholar]
- 117.Yu CW, Wu CC, Chen WJ. Aseptic nonunion of a femoral shaft treated using exchange nailing. Chang Gung Med J. 2002;25:591–598. [PubMed] [Google Scholar]
- 118.Zelle BA, Bhandari M, Espiritu M, Koval KJ, Zlowodzki M, Evidence-based orthopaedic trauma working group Treatment of distal tibia fractures without articular involvement: a systematic review of 1125 fractures. J Orthop Trauma. 2006;20:76–79. doi: 10.1097/01.bot.0000202997.45274.a1. [DOI] [PubMed] [Google Scholar]
- 119.Zelle BA, Gruen GS, Klatt B, Haemmerle MJ, Rosenblum WJ, Prayson MJ. Exchange reamed nailing for aseptic nonunion of the tibia. J Trauma. 2004;57:1053–1059. doi: 10.1097/01.ta.0000100380.50031.dc. [DOI] [PubMed] [Google Scholar]
- 120.Zhang P, He X, Liu K, Zhao F, Fu Z, Zhang D, Zhang Q, Jiang B. Bone marrow stromal cells differentiated into functional Schwann cells in injured rats sciatic nerve. Artif Cells Blood Substit Immobil Biotechnol. 2004;32:509–518. doi: 10.1081/BIO-200039608. [DOI] [PubMed] [Google Scholar]
- 121.Zimmermann G, Moghaddam A, Wagner C, Vock B, Wentzensen A. Clinical experience with bone morphogenetic protein 7 (BMP 7) in nonunions of long bones. Unfallchirurg. 2006;109:528–537. doi: 10.1007/s00113-006-1078-5. [DOI] [PubMed] [Google Scholar]
- 122.Zipfel GJ, Guiot BH, Fessler RG. Bone grafting. Neurosurg Focus. 2003;14:e8. doi: 10.3171/foc.2003.14.2.9. [DOI] [PubMed] [Google Scholar]
- 123.Ziran BH, Smith WR, Morgan SJ. Use of calcium-based demineralized bone matrix/allograft for nonunions and posttraumatic reconstruction of the appendicular skeleton: preliminary results and complications. J Trauma. 2007;63:1324–1328. doi: 10.1097/01.ta.0000240452.64138.b0. [DOI] [PubMed] [Google Scholar]