Abstract
Methotrexate (MTX) released from bone cement showed a useful local effect in animal models of bone tumours. However, local toxic reactions such as impaired wound healing were observed in areas surrounding the MTX-loaded implant. Therefore, we hypothesised that MTX released from bone cement would have harmful effects on human mesenchymal stem cells (MSC)—one of the basic components of bone marrow and tissue reparatory processes. Moreover, elution of MTX was calculated from implants prepared either with liquid or powdered MTX. During the 28-day incubation, the cement compounded with liquid MTX showed the highest elution rate of the drug. MTX released from pellets produced a significant decrease in proliferation of MSC as a consequence of a blockade of their cell cycle in the S/G2 phase. These findings indicate impairment of stem cell function in marginal areas surrounding the MTX-loaded cement and may help to explain problems with regeneration of tissues in these locations.
Résumé
Le Methotrexate (MTX) libéré par le ciment acrylique a un effet certain sur les tumeurs osseuses développées sur un modèle animal. Cependant, des réactions toxiques locales telles que défauts de cicatrisation ont été observées à proximité de l’implantation de ce ciment. Nous faisons donc l’hypothèse que le Methotrexate MTX libéré par le ciment a un effet nocif néfaste sur les cellules mésenchyméteuses humaines MSC qui sont l’un des composants de base de la moelle osseuse et des phénomènes de la réparation tissulaire. De plus, la dynamique de pénétration du méthotrexate a été analysée à partir d’implants préparés avec du liquide ou de la poudre de méthotrexate. Au cours des 28 jours d’incubation, le ciment préparé avec du méthotrexate liquide montre un taux important de pénétration de la drogue. Le méthotrexate libéré des billes de ciment entraîne une diminution significative de la prolifération des cellules mésenchyméteuses avec un bloquage cellulaire à la phase S/G2. Ces travaux montrent que la fonction cellulaire est altérée au voisInage du méthotrexate pouvant expliquer les problèmes de régénétration tissulaire.
Introduction
Methotrexate (MTX)-loaded bone cement has been suggested as a promising alternative for local chemotherapy of primary and secondary malignant bone tumors after their surgical excision [1, 2]. The combination retains useful mechanical properties of polymethylmethacrylate alone and offers the advantage of local cytostatic influence of MTX on remaining tumour cells in surrounding tissue [3, 4], as has been shown during a series of in vitro toxicity testing on various cell lines including those from osteosarcoma [1, 3, 5–7]. In addition, in vivo experiments in rats with osteosarcoma demonstrated that implantation of MTX-loaded cement to the centre of a tumour temporally slows tumour growth, attenuates bone destruction, and prolongs survival of animals [1, 4]. Regarding the use of this MTX formulation in humans, little data is available. Heringou et al. [1] reported a group of 14 patients with implanted MTX (100 mg) containing cement. The local chemotherapy was well tolerated and no patient showed general toxicity. Nevertheless, wound healing problems occurred in two patients and one patient needed removal of the cement block. Similar adverse effects were also observed in animal studies. In view of reported long-lasting release of MTX from cement observed during in vitro studies (at least four to six months), and the high local concentration of MTX in vacuum drainage (10,000 times the blood concentration) from the site of implantation, it is obvious that data about local toxicity of released MTX on surrounding healthy structures such as proliferating cells of bone marrow are essential.
Mesenchymal stem cells (MSCs) are multipotent cells that are capable of self-renewal and rapid expansion. They are located primarily in the bone marrow and are characterised by a capacity to differentiate into mesenchymal lineages such as bone, tendon, fat, and cartilage [8–10]. In general, during the bone healing process, MSCs migrate into the impaired bone area, wherein they proliferate, differentiate, and thus may support stabilisation of implanted materials [11–13]. Any failure in mobilisation, proliferation, and differentiation of these progenitor cells may lead to failure in new bone formation. Therefore, the potential toxic effect of the compound incorporated in the cement on these progenitor cells may compromise the bone-healing process [12].
The aim of this project was to describe the acute influence of MTX released in vitro from bone acrylic cement on the viability, proliferation, and structure of the cell cycle of human stem cells isolated from the bone marrow. In addition, we intend to describe in detail elution kinetics of MTX from bone cement with respect to MTX content in the cement and the formulation of MTX used for preparation of the pellet (MTX solution or powder).
Materials and methods
Preparation of MTX-loaded bone cement pellets
Pellets containing MTX were prepared using methotrexate either as powder (a kind gift from PLIVA-Lachema a.s., Czech Republic) or liquid (Methotrexat Lachema 1000 inj. sol., PLIVA-Lachema a.s., Czech Republic) and Palacos R bone cement (Heraeus Medical, Czech Republic), which is composed of 40 g of polymer powder (containing 33.8 g polymethylmethacrylate, 5.9 g zirconium dioxide, and 0.3 g benzoyl peroxide) and 18.8 ml of solvent (0.4 g of polymerisation accelerator N,N-dimethyl-p-toluidine and 18.4 g methylmethacrylate monomer). The pellets from powdered MTX were prepared under sterile conditions by mixing 10 and 100 mg methotrexate powder with 10 g polymer powder and 5 ml monomer solution. The pellets from MTX original solution were prepared at the same concentration of MTX (1 and 10 mg per 1 g polymer powder) but the MTX solution was initially incubated for 15 min with monomer solution and thereafter mixed with polymer powder. This approach was chosen on the basis of previous experiments and allows better dissolution of MTX solution in bone cement. The mixture was poured into moulds before polymerisation. Each pellet was 20 mm in diameter and 3–4 mm thick.
MTX elution from MTX-loaded cement pellets
The hardened pellets were processed as described previously [5], i.e. washed in sterile saline solution for one minute, sterilised with 70% ethanol for 20 min, and again washed twice for one minute. This approach allows removal of rapidly available MTX from pellet and thereafter an estimation of release kinetics of the “slow” component. For comparison with clinical conditions where cement is directly applied to artificial bone cavity, whereby chemical sterilisation is not possible, a separate set of pellets containing 10 mg of MTX per 1 g of polymer powder were only rinsed for one minute in sterile saline solution. All pellets were thereafter placed in six-well plates. All wells contained 6 ml of PBS (phosphate buffered saline) [1]. The well plates were incubated at 37°C in a 5% CO2 atmosphere for 28 days. The PBS was exchanged daily. Samples with eluted MTX were stored at −80°C until analysis. The concentrations of methotrexate were measured after deproteination of samples according to a previously described HPLC method [14].
Mesenchymal stem cells (MSCs)
Bone marrow (BM) was obtained from healthy donors undergoing total hip replacement following informed consent according to guidelines from the University Hospital in Hradec Kralove. Patients suffering from cancer, infectious diseases, and patients undergoing dialysis or immunosuppression were excluded from our study.
Cells from BM were isolated by Ficoll-Paque (Amersham Biosciences, Sweden) gradient centrifugation and were cultured on untreated plastic (Sarstedt culture flasks) at 37°C under aerobic conditions (5% CO2) with 2% FCS-containing alpha-MEM expansion medium containing alpha-MEM (Gibco), FCS (PAA), ascorbic acid 2-phosphate (Sigma), dexamethasone (Sigma), L-glutamine, penicillin, streptomycin (Gibco), EGF and PDGF-BB (Peprotech, USA) [15].
For our experiment, cells of one cytogenetically stable line (passage No. 6) were plated in 6-well multidishes (Corning, USA) with 100.000 cells per well. Following 24 hours of cultivation, Transwell Inserts (24 mm in diameter, pores 0.4 μm, polyester membrane-Corning) were added into each well. Subsequently, sterile control or MTX- (1 or 10 mg of liquid form per 1 g of polymer powder) loaded cement pellets washed only twice in sterile saline solution (to imitate a clinical situation) were added to the cultivation system. Cells were thereafter cultivated for 14 days. The analysis of viability and proliferation was performed after one, three, seven, ten, and 14 days using a Z2 counter or cell viability analyzer Vi-Cell XR 2.03 (both Beckman Coulter, USA) according to manufacturer’s instructions. The cultivation medium was mixed in the culture system twice daily and replaced once daily. Samples of medium for determination of MTX concentrations were stored at −80°C until analysis.
Flow cytometry
The influence of methotrexate on the cell cycle was assessed ten days from the start of co-cultivation by flow cytometric DNA analysis using a Coulter DNA Prep Reagents Kit (based on propidium iodide staining) and flow cytometer Cell Lab Quanta (Beckman Coulter, USA). Data were analysed by Multi Cycle software (Phoenix Flow Systems, USA).
Data analysis
All experiments were carried out in duplicates, and the results were presented as means and SEMs (standard error of the mean). The kinetics of MTX release from pellet were evaluated by special pharmacokinetic software (Kinetica, Thermoscientific, USA). The elimination rate constant (Kel) was estimated by subjecting the concentrations in the terminal phase to linear regression analysis. The terminal elimination phase half-life (t1/2) was calculated by dividing ln2 by Kel.
Results
In vitro release of MTX from cement pellets
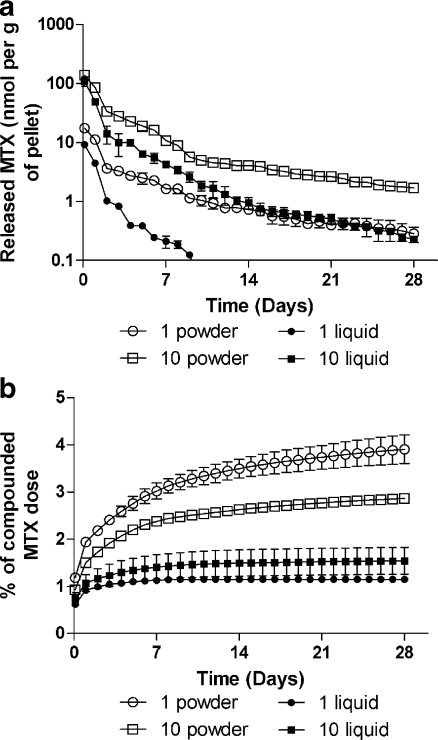
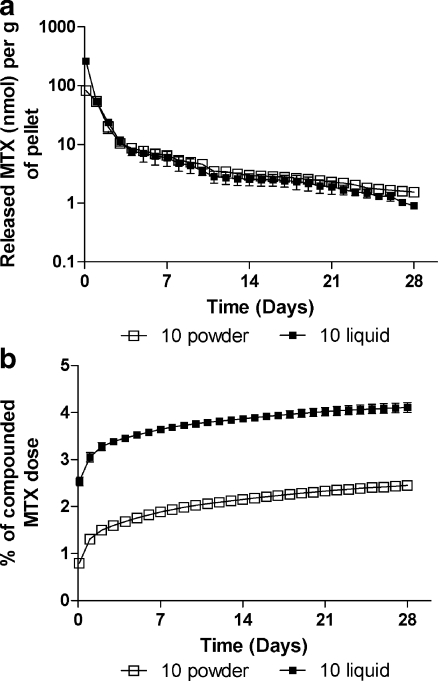
Initially, the experiments with different concentrations of MTX showed that no more than 10 mg MTX as the original solution could reliably be mixed per 1 g monomer powder. Therefore further experiments were performed with concentrations of MTX (either powdered or liquid form) 1 or 10 mg per 1 g of monomer powder. The results of the release test from chemically sterilised pellets are shown as the eluted amount per gram of pellets over time (Fig. 1a). The eluted quantity of MTX was greatest during the first day, and then the amount decreased rapidly until the end of the first week, reaching a plateau in the third week. One exception was the elution of MTX from pellets containing 1 mg MTX in liquid form, where the concentrations in medium drop below the detection limits on day nine of incubation. The initial amount of released MTX was generally lower in pellets prepared from original MTX solution. Processing the data with specialised kinetic software yielded the terminal half-lives (measured since day 14 of incubation) for MTX elution as follows: 12.4 ± 1.9 days, 11.8 ± 0.3 days, and 8.1 ± 1.8 days for 1 mg/g of powdered, 10 mg/g of powdered, and 10 mg/g of liquid MTX, respectively. Figure 1b presents the cumulative release of the drug. The amount of MTX released during 28-day incubation ranged from 1.1–4.2% of MTX compounded in pellets. To evaluate the influence of preincubation handling with pellets, MTX release was also monitored in “unwashed” pellets as depicted in Fig. 2a, b. Time profiles of MTX release from pellets containing 10 mg/g of powdered MTX were identical to the data from ethanol-sterilised pellets. In contrast, direct incubation of pellets prepared with original MTX solution without a prior washing procedure yielded raised amounts of released MTX, which was higher, especially during the initial period, than the values observed for powdered MTX. The half-lives for MTX elution from these pellets were 11.4 ± 1.2 days and 9.6 ± 1.4 days for powdered and liquid MTX (10 mg/g), respectively.
Fig. 1.
In vitro release (absolute (a), cumulative (b)) of MTX from loaded bone cement into PBS according to the amount of loaded MTX. Note: 1/10 powder/liquid means pellets containing 1 or 10 mg of powdered or liquid MTX per gram of polymer powder
Fig. 2.
In vitro release (absolute (a), cumulative (b)) of MTX from loaded bone cement without previous chemical sterilisation with 70% ethanol solution. Note: 10 powder/liquid means pellets containing 10 mg of powdered or liquid MTX per gram of polymer powder
MTX toxicity on stem cells
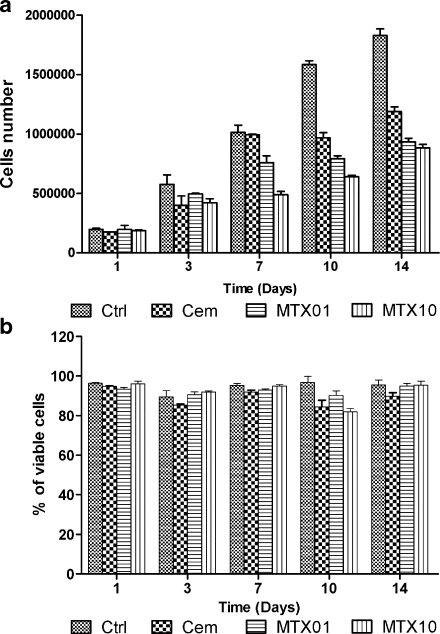
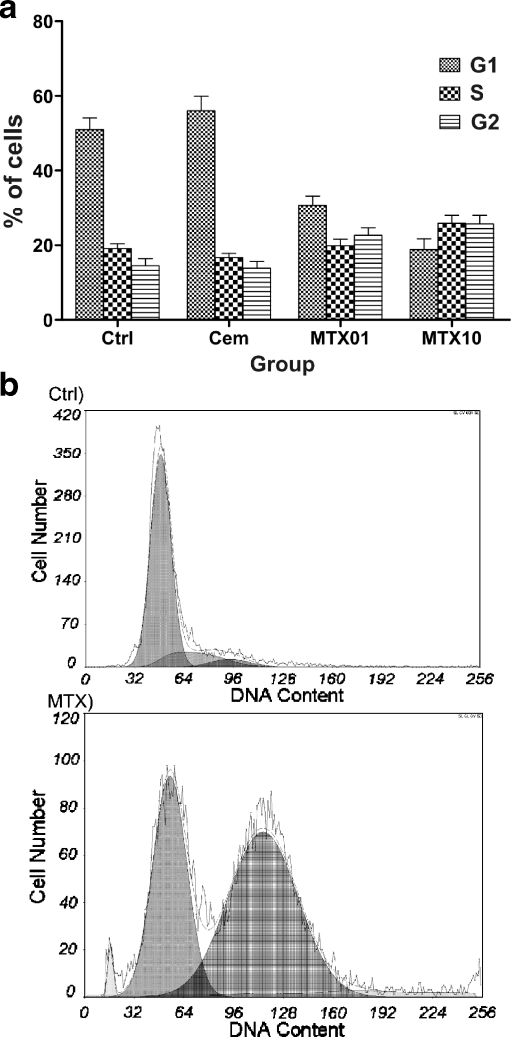
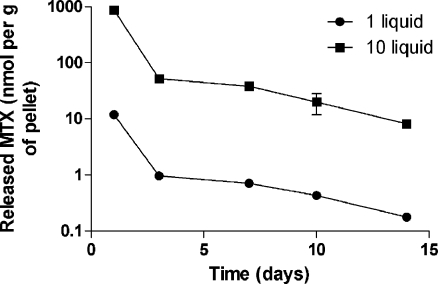
The influence of MTX loaded (1 and 10 mg per gram of polymer powder) cement on human bone marrow stem cells was evaluated one, three, seven, ten, and 14 days after addition of pellets to cells. The observed MTX-induced changes in stem cell proliferation, viability, and cell cycle are plotted in Figs. 3 and 4. MTX produced a decrease in stem cell proliferation starting from day seven of co-cultivation. The viability was not influenced by MTX, and it was rather impaired by the presence of the pellet. Importantly, the most significant effect of MTX was the influence on cell cycle with MTX-induced accumulation of stem cells in S and G2 phases (Fig. 4). To verify elution of MTX into medium, its concentrations were measured in a medium of harvested cells on days one, three, seven, ten and 14 (Fig. 5). The parameters were identical to those reported in Fig. 2a.
Fig. 3.
Proliferation (a) and viability (b) of mesenchymal stem cells in the presence of MTX-loaded bone cement. Abbreviations: Ctrl control incubation without any pellet, Cem incubation with pellet without MTX, MTX01 incubation with pellet containing 1 mg of liquid MTX per gram of polymer powder, MTX10 incubation with pellet containing 1 mg of liquid MTX per gram of polymer powder
Fig. 4.
Influence of MTX released from bone cement on the cell cycle of mesenchymal stem cells (a) with representative DNA analysis and cell cycle distribution in control (Ctrl) and methotrexate treated cells (MTX) with G2 blockage (b). Analysis was performed on the tenth day of incubation. Abbreviations: Ctrl control incubation without any pellet, Cem incubation with pellet without MTX, MTX01 incubation with pellet containing 1 mg of liquid MTX per gram of polymer powder, MTX10 incubation with pellet containing 1 mg of liquid MTX per gram of polymer powder
Fig. 5.
In vitro release of MTX from loaded bone cement into medium during incubation with mesenchymal stem cells. Abbreviations: 1/10 liquid means pellets containing 1 or 10 mg of liquid MTX per gram of polymer powder
Discussion
In this study, we have described in detail the kinetics of MTX release from bone cement with respect to initial MTX formulation (powder or liquid) and two clinically relevant doses. The release of MTX from bone cement as a potential local therapy applied after excision of primary or secondary bone malignancies has been documented by several works. Hernigou et al. [1] reported for the first time that cement implants containing MTX were able to release the drug for a period of months. Subsequent detailed in vitro works evaluated elution kinetics of MTX from the cement pellets loaded with different doses of the drug over the range 1.8–1000 mg MTX per 1 g of cement with the duration ranging from 15 to 30 days [2, 4, 5]. All authors reported the greatest elution during the first day for all concentrations, with rapid decrease during the following days, and stabilisation to a constant rate of release after two weeks of incubation. In our study, using the precise HPLC method and advanced software data processing, we showed similar results. Moreover, the calculated half-life for elution of around ten days means that this phase of MTX release lasted approximately 50 days. However, Wang et al. [3] showed that MTX was still released after 12 weeks of incubation, which suggests another phase of very slow release of MTX from the cement. This finding was supported by absolute amounts of methotrexate, which was released from pellet over time. Kim at al. [5] showed that the mean amount of MTX eluted from pellet over a 28-day period ranged from 7.2% to 11.7%. Our data were similar and suggested that the majority of the MTX remained in the bone cement. Thus, most of the dose was available for slow release from this porous material.
The maximum concentration of MTX in implanted cement is limited by potential changes in material strength and the drug toxicity. Wang et al. [3] demonstrated that the breaking strength of cement is not affected by the addition of 12.5 g or less of MTX per 1 g of cement, but is decreased by 13% and 20% when the dose was increased to 25 and 50 mg MTX per 1 g cement, respectively. Accordingly, we selected the MTX dose below this limit. The next question was the MTX formulation. Although most authors are using the powdered MTX [1, 3, 5], for potential use in clinical practice where the bone cement must be prepared under sterile conditions, the sterile form of MTX must be also available. Routinely, MTX is available in hospitals as a sterile infusion solution for anticancer therapy. Therefore, we intend to compare potential changes in release from cement prepared from powdered or liquid MTX. Our data demonstrated for the first time that cement prepared with liquid MTX releases MTX more rapidly, reaching a higher concentration at the start, but the release half-life is shorter. These results accord with the conclusion of Downes [16] who postulated that optimum release is achieved if a crystalline formulation of the drug is used rather than a fine powder. Moreover, Maccauro et al. [2] suggested that at the beginning the drug present on the surface of the cement is likely to be eluted. In support of this is the fact that, intensive initial washing of the pellets in saline and ethanol greatly reduced the amount of released MTX from pellets prepared with the liquid form.
The main limitation for use of MTX-containing cement in humans is its potential toxicity. In all animal studies where authors document a positive therapeutic effect on tumour cells there were also local toxic reactions seen, i.e. impaired wound healing after surgery when the implant was in contact with the skin [1]. This suggests direct toxicity of the drug to the adjacent tissue surrounding the cement insert. Thus we decided to monitor the impact of MTX released from the cement on the basic cellular components of wound and bone healing—stem cells. We detected harmful influence of the drug, for the first time on MSC proliferation through halting the cells in the S/G2 phase of the cell cycle. This complies with the cell cycle specific cytostatic effect of MTX in cancer cells [17, 18]. Importantly, this effect was not induced by acrylic cement alone. That systemic adverse effects on haematopoesis were not seen after implantation of MTX-loaded cement in animals or humans, suggests that the influence on MSCs is limited to areas around the implant.
In conclusion, our results demonstrated differences for the first time in MTX elution kinetics from bone acrylic cement when either powdered or liquid formulation of the drug was used. Composition of cement with liquid formulation of MTX demonstrated faster elution of higher quantity of the drug. Depending on the volume and concentration of MTX solution, only a limited quantity of MTX solution can be incorporated into cement. The most salient information of the study may be the marked decrease in proliferation of bone marrow stem cells produced by MTX released from cement as a consequence of blockade of their cell cycle in the S/G2 phase.
Acknowledgement
This study was supported by grants from the Ministry of Education No. 1P05OC061-COST B25.001 and No. MSM 0021620820.
References
- 1.Hernigou P, Thiery JP, Benoit J, Voisin MC, Leroux P, Hagege G, Delepine G, Goutallier D. Methotrexate diffusion from acrylic cement. Local chemotherapy for bone tumours. J Bone Joint Surg Br. 1989;71:804–811. doi: 10.1302/0301-620X.71B5.2584251. [DOI] [PubMed] [Google Scholar]
- 2.Maccauro G, Cittadini A, Casarci M, Muratori F, De AD, Piconi C, Rosa MA, Spadoni A, Braden M, Sgambato A. Methotrexate-added acrylic cement: biological and physical properties. J Mater Sci Mater Med. 2007;18:839–844. doi: 10.1007/s10856-006-0036-7. [DOI] [PubMed] [Google Scholar]
- 3.Wang HM, Galasko CSB, Crank S, Oliver G, Ward CA. Methotrexate loaded acrylic cement in the management of skeletal metastases-biomechanical, biological, and systemic effect. Clin Orthop Relat Res. 1995;312:173–186. [PubMed] [Google Scholar]
- 4.Wang HM, Crank S, Oliver G, Galasko CSB. The effect of methotrexate-loaded bone cement on local destruction by the VX(2) tumour. J Bone Joint Surg Br. 1996;78B:14–17. [PubMed] [Google Scholar]
- 5.Kim HS, Park YB, Oh JH, Yoo KH, Lee SH. The cytotoxic effect of methotrexate loaded bone cement on osteosarcoma cell lines. Int Orthop. 2001;25:343–348. doi: 10.1007/s002640100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirchen ME, Menendez LR, Lee JH, Marshall GJ. Methotrexate eluted from bone cement: effect on giant cell tumor of bone in vitro. Clin Orthop Relat Res. 1996;328:294–303. doi: 10.1097/00003086-199607000-00044. [DOI] [PubMed] [Google Scholar]
- 7.Decker S, Winkelmann W, Nies B, van VF. Cytotoxic effect of methotrexate and its solvent on osteosarcoma cells in vitro. J Bone Joint Surg Br. 1999;81:545–551. doi: 10.1302/0301-620X.81B3.9167. [DOI] [PubMed] [Google Scholar]
- 8.Jiang YH, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du JB, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 9.Borovecki F, Pecina-Slaus N, Vukicevic S. Biological mechanisms of bone and cartilage remodelling–genomic perspective. Int Orthop. 2007;31:799–805. doi: 10.1007/s00264-007-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann A, Gross G. Tendon and ligament engineering in the adult organism: mesenchymal stem cells and gene-therapeutic approaches. Int Orthop. 2007;31:791–797. doi: 10.1007/s00264-007-0395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatatarenko-Kozmina TY, Denisov-Nikol’skii YI, Volozhin AI, Doktorov AA, Mal’ginov NN, Krasnov AP. Effect of hydroxyapatite as a component of biostable composites on population and proliferation of mesenchymal stem cells. Bull Exp Biol Med. 2007;143:519–523. doi: 10.1007/s10517-007-0170-3. [DOI] [PubMed] [Google Scholar]
- 12.Chang YH, Goldberg VM, Caplan AI. Toxic effects of gentamicin on marrow-derived human mesenchymal stem cells. Clin Orthop Relat Res. 2006;452:242–249. doi: 10.1097/01.blo.0000229324.75911.c7. [DOI] [PubMed] [Google Scholar]
- 13.Viateau V, Guillemin G, Bousson V, Oudina K, Hannouche D, Sedel L, Logeart-Avramoglou D, Petite H. Long-bone critical-size defects treated with tissue-engineered grafts: A study on sheep. J Orthop Res. 2007;25:741–749. doi: 10.1002/jor.20352. [DOI] [PubMed] [Google Scholar]
- 14.Fuksa L, Brcakova E, Cermanova J, Hroch M, Chladek J, Kolouchova G, Malakova J, Martinkova J, Staud F, Micuda S. Amiodarone modulates pharmacokinetics of low-dose methotrexate in rats. Biopharm Drug Dispos. 2008;29:289–299. doi: 10.1002/bdd.614. [DOI] [PubMed] [Google Scholar]
- 15.Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.V98.9.2615. [DOI] [PubMed] [Google Scholar]
- 16.Downes S. Methods for improving drug release from poly(methyl)methacrylate bone cement. Clin Mater. 1991;7:227–231. doi: 10.1016/0267-6605(91)90063-L. [DOI] [PubMed] [Google Scholar]
- 17.Huang RF, Ho YH, Lin HL, Wei JS, Liu TZ. Folate deficiency induces a cell cycle-specific apoptosis in HepG2 cells. J Nutr. 1999;129:25–31. doi: 10.1093/jn/129.1.25. [DOI] [PubMed] [Google Scholar]
- 18.Taylor IW, Tattersall MH. Methotrexate cytotoxicity in cultured human leukemic cells studied by flow cytometry. Cancer Res. 1981;41:1549–1558. [PubMed] [Google Scholar]