Abstract
A full thickness defect was made in the central portion of the patellar tendon of 48 New Zealand white rabbits. Platelet-rich plasma (PRP) gel was then applied and filled the tendon defect. The same procedure was performed in the control group, without the application of PRP. Animals were sacrificed after one, two, three, and four weeks. Histological and immunohistochemical analyses using a monoclonal antibody against CD31 were performed. The histological examination showed a superior healing process in the PRP group compared with the control group. Especially in the third week, the tissue formed in the PRP group was more mature and dense with less elastic fibres remaining. Neovascularisation was significantly higher in the PRP group during the first two weeks and significantly lower in the third and fourth weeks (p < 0.0001). Histological examination and study of angiogenesis showed that the application of PRP enhances and accelerates the tendon healing process.
Résumé
Etude immuno histochimique de l’angiogénèse après administration locale d’un plasma enrichi en plaquettes sur le tendon rotulien de lapins après résection de la portion centrale du tendon. Matériel et méthode: une lésion portant sur toute l’épaisseur du tendon a été réalisée sur la partie centrale du tendon rotulien de 48 lapins blancs de Nouvelle Zélande. Un gel plasma riche en plaquettes (PRP) a été appliqué comblant la lésion tendineuse. La même technique a été réalisée sur un groupe contrôle sans application de PRP. Les animaux ont été sacrifiés après 1, 2, 3 et 4 semaines. Une étude histologique et immuno histochimique a été réalisée utilisant un anticorps monoclonal anti CD31. Résultat: l’examen histologique a montré un meilleur processus de cicatrisation dans le groupe PRP que dans le groupe contrôle. Après trois semaines, le tissu néformé dans le groupe PRP est plus mature et plus dense et contient moins de fibres élastiques. La néovasclarisation est significativement plus haute dans le groupe PRP au cours des deux premières semaines et significativement abaissée à la troisième et quatrième semaine (p < 0,0001). Discussion: l’examen histologique et l’étude de l’angiogénèse montre que l’application de PRP améliore et accèlère le processus de cicatrisation tendineuse.
Introduction
A common substitute for ligament reconstruction of the knee is the central one-third portion of the patellar tendon. However, there are many problems associated with the harvest of the tendon, including decreased strength of the quadriceps [24], patello-femoral problems [25, 27], and rupture of the remaining patellar tendon [17, 19]. There have been various attempts in the past to identify modulating agents aiming to enhance tendon healing. Growth factors, a major group of candidates for modulating agents, defined by their primary growth-stimulating functions, have been shown to be involved in almost every stage of the healing process [6, 7, 12].
Platelet-rich plasma (PRP) is an autologous concentration of platelets in a small volume of plasma, which contains several growth factors including platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), fibroblastic growth factor (FGF), vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), and epidermal growth factor (EGF). PRP is a new application of tissue engineering. Recently, it has been investigated for the regeneration of bone [16, 18, 22], cartilage [2, 21], and ligaments [26], and for the treatment of open fractures [15].
Considering that the low-healing capacity of tendon is associated with a reduced blood supply compared with other tissues and that tendon healing and remodelling require angiogenesis, a process that is tightly controlled by growth factors [16], we postulated that an application of PRP might significantly enhance angiogenesis in the patellar tendon after resecting its central third. The aim of this study was to test this hypothesis.
Materials and methods
Animals
Forty-eight skeletally mature New Zealand white rabbits, weighing an average of 3.5 kg each, were used following approval from the regional ethical board. Institutional guidelines for the care and treatment of laboratory animals were adhered to. The rabbits were housed one per cage with food and water available ad libidum. Although there were both male and female rabbits, randomisation was done without stratification for sex. Four groups were assigned, and animals were sacrificed after one, two, three, and four weeks (n = 12 for each group). At each time point, six animals (12 limbs) received the PRP, and six animals (12 limbs) served as an untreated control group. PRPFast (Bioteck) was used for the preparation of growth factors.
PRP preparation
Eight millilitres of blood from an ear vein was collected in a tube immediately after general anaesthesia. The blood was allowed to stand for 15 min in order to reduce platelet activation during centrifugation. Once centrifugation was complete, the upper half was considered platelet poor plasma (PPP) and was removed using sterile pipettes. The lower half, the PRP proper, was retrieved using a pipette by aspirating up to the interphase zone (consisting of blood cells) and then placed into another glass tube. Two millilitres of PRP was collected for every 8 ml of blood. The PRP was applied in a gel form, manufactured by adding 0.5 ml of procoagulant solution in the tube with the liquid PRP and allowing approximately 15 minutes for the solution to become a gel.
Surgical procedure
The rabbits were anaesthetised with an intramuscular injection of xylazine (Rompun Injectable, Bayer) at a dosage of 5–7 mg/kg and 0.15 mg of atropine (DEMO S.A.). After 10–15 min, ketamine (Imalgene, Rhone Merieux, France) at a dosage of 12–15 mg/kg was injected intramuscularly. During surgery, supplemental sedation was given and administered as required. Local anaesthesia of 1 ml of a 2% lidocaine-adrenaline solution (AstraZeneca, UK) was applied at regular intervals at the site of incisions.
The surgical procedure was performed according to the animal model described by Anaguchi et al. [3]. After the creation of the defect, four markers were placed at the corners of the defect with 5–0 nylon sutures to identify the location of the resected portion. The PRP gel was then applied and filled the tendon defect. The overlying fascia was closed with a running suture of 4–0 nylon so that PRP gel applied into the resected portion would not flow out. Skin was closed with clips. The same procedure was performed in the opposite limb. The same procedure was also performed in both limbs in the control group, without the application of PRP into the patellar tendon defect. No immobilisation was applied after surgery, and the rabbits were allowed unrestricted daily activities in their cages.
Histology
After one, two, three, and four weeks the animals were sacrificed with an overdose of intracardiac injection of 10% KCl solution under general anaesthesia. The entire patellar tendon was then removed and dissected free from other tissues. The specimens were then fixed in a buffered 10% formalin solution and cast in paraffin blocks. The sections were made transversely on the longitudinal axis of the tendon. From each tendon, six paraffin sections were made. Of them, three sections were stained with haematoxylin-eosine and subjected to microscopic examination, while the other three were immunostained with a monoclonal antibody against CD31 (DAKO Co., Carpinteria, CA, USA), which is a marker of vascular endothelial cells. All sections were analysed by a single pathologist who was blinded to the treatment groups.
Image analysis
Image analysis was performed using a light microscope (Nikon Eclipse 80i, Nikon Corp, Tokyo, Japan) equipped with a digital camera (Nikon DS-2MW) with a resolution of 1600×1200 pixels. The digital images were measured semiquantitatively on a PC with the assistance of Image ProPlus software (Image ProPlus v5.1, Media Cybernetics Inc., Maryland, USA).
An evaluation of angiogenesis was then performed using a technique described by Ju et al. [13] (initially established for quantifying tumour angiogenesis [11]). The technique involves identifying the three most vascular areas (hot spots) with the highest number of micro-vessel profiles by scanning each section at low power (x20) and then conducting computer analyses of these areas.
Immunohistochemistry
Immunohistochemistry was applied on 4 μm thick sections. After heating slides at 37°C overnight, slides were deparaffinised in xylene and rehydrated through graded alcohol solutions. Endogenous peroxidase activity was blocked using 0.3% H2O2 in Tris-buffered saline for 30 minutes at room temperature. Antigen retrieval was performed by heating the slides in 10 mM citrate buffer (pH 6.0) at 750 W for 15 minutes (2 cycles, 5 min each) in a microwave oven. After rinsing with Tris-buffered saline, normal horse serum was applied for 30 minutes to block nonspecific antibody binding. Subsequently, sections were incubated overnight at 4°C with the primary antibody (anti-CD31, clone M0823 Dako, Glostrup, Denmark) in a dilution of 1:50.
A two-step technique was used (Envision, Dako, Glostrup, Denmark). For the secondary antibody in this method, anti-mouse enzyme-labelled dextran polymers (DAKO, K4000) were allowed to react for 30 minutes at room temperature. 3,3′-diaminobenzidine tetrahydrochloride was used as the chromogenic substrate, and finally the slides were slightly counterstained with haematoxylin. A positive internal control for immunostaining was used for each section.
Statistical analysis
All results were expressed as mean ± SD. Significant differences among groups were evaluated using the Mann-Whitney U test. A difference of P < 0.05 was considered to be statistically significant (SPSS 11.5.0,SPSS Inc., Chicago, IL, USA).
Results
Histology–immunohistochemistry
Longitudinal sections from specimens were stained with haematoxylin-eosin. In general, the study under light microscopy revealed the formation of a highly vascularised granulomatous tissue in the repair site. Its composition was time dependent, and as it was becoming more mature, the portion of the constituent elements changed. The immunoexpression of CD31 was investigated at the same time points.
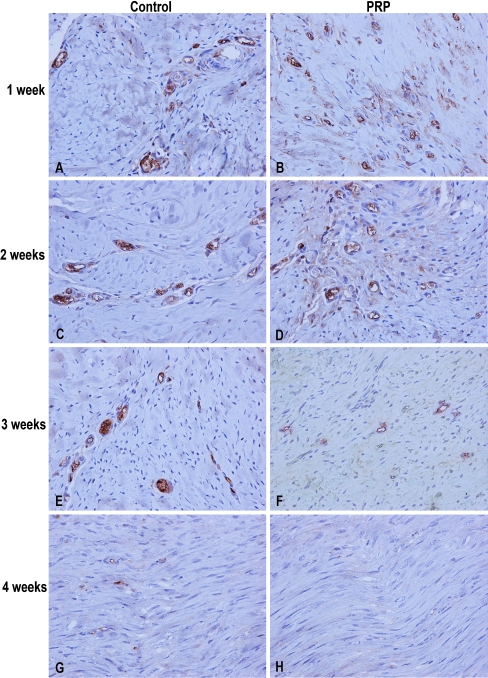
First week: In both groups, histological examination showed the formation of loose, non-orientated fibrovascular tissue which filled the gap, along with some chronic inflammatory elements. The deep layer of the tendon was continuous. High levels of CD31 expression were detected in both groups. The number of vessels was significantly higher in the PRP group (p < 0.0001) (Fig. 1A, B; Table 1).
Second week: Fibroblast-like cells became more obvious in the defect site. There was inconsistent neoformation of blood vessels and rare presence of collagen fibers and fibrosis. The neovascularisation, as it is expressed by CD31 activity, was significantly higher in the PRP group and it reached peak levels (p < 0.0001) (Fig. 1C, D; Table 1).
Third week: The formed tissue remained more immature in the control group, i.e. its synthesis was less compact and there was absence of tenocyte longitudinal orientation. On the other hand, in the PRP group, the tissue was more dense with less elastic fibers remaining (Fig. 2). CD31 immunoreactivity reduced in both groups and the vessel density was significantly lower in the PRP group (p < 0.0001) (Fig. 1E, F; Table 1).
Fourth week: There was no clearly visible border between the healed site and the proximal tendon in the PRP group. In the control group, sparse cellularity was still present. CD31 expression was almost absent in the PRP group, while demonstrating some activity in the control group (p < 0.0001) (Fig. 1G, H; Table 1).
Fig. 1.
Representative high vascularised areas demonstrating CD31 immunoreactivity in 1, 2, 3 and 4 weeks of wound healing (all images were captured under 400x magnification). A,B High CD31 expression seen in the first week in both groups. C,D CD31 expression (and thus vessel formation) increased faster in the PRP group from 1 to 2 weeks than in the control group. E,F As the wound healing progresses with time, CD31 declines faster in the PRP group. G,H A healed tendon in the PRP group, while some CD31 expression still remained in the control group
Table 1.
Immunoexpression of CD31
| Week | Control (vessels/mm2) | PRP (vessels/mm2) | p* |
|---|---|---|---|
| 1 | 1150 ± 23.89 | 1623 ± 14.96 | <0.0001 |
| 2 | 2086 ± 22.9 | 5386 ± 20.7 | <0.0001 |
| 3 | 2089 ± 54.87 | 440 ± 17.65 | <0.0001 |
| 4 | 187 ± 12.29 | 13 ± 5.24 | <0.0001 |
PRP platelet-rich plasma
*P < 0.05 indicates a significant difference between the two groups
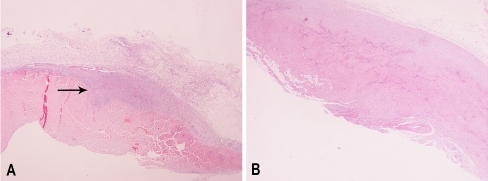
Fig. 2.
Longitudinal sections of patellar tendon from the control group (A) and the PRP group (B) at 3 weeks (eosin4hematoxylin stain, x100 original magnification). Fibroelastic tissue is more prominent in the control group, while in the PRP group the wounded site is almost healed, demonstrating better cell orientation and tissue maturation
Discussion
One of the early events of wound healing is angiogenesis, in which neovascularisation prompts delivery of inflammatory cells and fibroblasts to the wound site. An injury such as a traumatic tendon rupture destroys the well-organised peri- and intratendineous network of blood vessels [1]. Wounding and inflammation provoke the release of growth factors and cytokines from platelets, polymorphonuclear leukocytes, macrophages and other inflammatory cells [9]. These growth factors induce neovascularisation and chemotaxis of fibroblasts and tenocytes and stimulate fibroblast and tenocyte proliferation and synthesis of collagen [10, 20]. Considering that the healing process results from the interaction of many factors, we have proposed the use of autologous platelet-rich plasma to simultaneously increase the concentration of several growth factors and subsequently enhance tendon healing. For this purpose we used a patellar tendon defect model in rabbits. The central one-third portion of the patellar tendon is a common substitute for ligament reconstruction of the knee.
In our study, the significant increase of vessel density in the PRP group during the first two weeks of healing in comparison with the controls suggests that PRP enhanced the healing process. On the other hand, our results showed that the angiogenesis decreased in the PRP group significantly in the third and fourth weeks in comparison with the controls. Thus, the decrease of vessel density in combination with the orientation of the fibroblasts indicates that PRP also accelerates the whole healing process.
There are studies in the literature that demonstrate the positive effect of PRP on tendon healing in vitro [4, 8] and in vivo [5, 23]. Furthermore, Anitua et al. showed that the injection of preclotted plasma within the Achilles tendon could increase cellular density and promote neovascularisation [4]. These findings are in accordance with our results.
The only study that considers the effect of PRP on patellar tendon healing is that of Kajikawa et al. [14]. The authors postulated that a delay of time-dependent decrease in circulation-derived cells could improve the healing process of tendons. The results suggested that locally injected PRP into the patellar tendon’s wounds is useful as an activator of circulation-derived cells for enhancement of the initial tendon healing process. The results of the study showed that at seven days after injury the number of vessels in the wounded area decreased. In contrast, in our study the increased number of vessels during the first two weeks was maintained and decreased at day 21. This difference is difficult to explain. First, the patellar tendon wound in the study of Kajikawa et al. was a partial perpendicular laceration. It is possible that the repair process is proportional to the extent of trauma. Second, the effect of PRP may be dose-related. In the study of Kajikawa et al., 20 μl of PRP were injected into the wounded area, whereas in our study an amount of 0.5 ml of PRP in a gel form was used in order to fill the patellar defect.
The limitation of our study is that we have not examined the mechanical properties of the healed tendon. A biomechanical evaluation is necessary to demonstrate the superiority of the healed tendons and consequently to justify the use of PRP in tendon healing. Certainly these results need replication and further research in animal studies, especially in the field of biomechanics. We believe that the high concentration of growth factors in PRP and the benign nature of PRP provide a significant potential for use in human clinical studies.
References
- 1.Ahmed IM, Lagopoulos M, McConnell P, Soames RW, Sefton GK. Blood supply of the Achilles tendon. J Orthop Res. 1998;16(5):591–596. doi: 10.1002/jor.1100160511. [DOI] [PubMed] [Google Scholar]
- 2.Akeda K, An HS, Pichika R, Attawia M, Thonar EJ, Lenz ME, Uchida A, Masuda K. Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine. 2006;31:959–966. doi: 10.1097/01.brs.0000214942.78119.24. [DOI] [PubMed] [Google Scholar]
- 3.Anaguchi Y, Yasuda K, Majima T, Tohyama H, Minami A, Hayashi K. The effect of transforming growth factor-beta on mechanical properties of the fibrous tissue regenerated in the patellar tendon after resecting the central portion. Clin Biomech (Bristol, Avon) 2005;20(9):959–965. doi: 10.1016/j.clinbiomech.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Anitua E, Sánchez M, Nurden AT, Zalduendo M, Fuente M, Orive G, Azofra J, Andia I. Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J Biomed Mater Res A. 2006;77(2):285–293. doi: 10.1002/jbm.a.30585. [DOI] [PubMed] [Google Scholar]
- 5.Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93–99. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 6.Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. Int Orthop. 2007;31:783–789. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan KM, Liu S, Maffulli N. ACL reconstruction with autogenous patellar tendon graft. Donor site consideration and potential for reharvest. In: Williams and Wilkins, editor. Controversies of orthopaedic sports medicine. Hong Kong: Asia-Pacific Ltd.; 1998. pp. 38–45. [Google Scholar]
- 8.Mos M, Windt AE, Jahr H, Schie HT, Weinans H, Verhaar JA, Osch GJ. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171–1178. doi: 10.1177/0363546508314430. [DOI] [PubMed] [Google Scholar]
- 9.Evans CH. Cytokines and the role they play in the healing of ligaments and tendons. Sports Med. 1999;28(2):71–76. doi: 10.2165/00007256-199928020-00001. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 11.Fox SB, Leek RD, Weekes MP, Whitehouse RM, Gatter KC, Harris AL. Quantitation and prognostic value of breast cancer angiogenesis: comparison of microvessel density, Chalkley count, and computer image analysis. J Pathol. 1995;177:275–283. doi: 10.1002/path.1711770310. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann A, Gross G. Tendon and ligament engineering in the adult organism: mesenchymal stem cells and gene-therapeutic approaches. Int Orthop. 2007;31:791–797. doi: 10.1007/s00264-007-0395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ju YJ, Tohyama H, Kondo E, Yoshikawa T, Muneta T, Shinomiya K, Yasuda K. Effects of local administration of vascular endothelial growth factor on properties of the in situ frozen-thawed anterior cruciate ligament in rabbits. Am J Sports Med. 2006;34(1):84–91. doi: 10.1177/0363546505278700. [DOI] [PubMed] [Google Scholar]
- 14.Kajikawa Y, Morihara T, Sakamoto H, Matsuda K, Oshima Y, Yoshida A, Nagae M, Arai Y, Kawata M, Kubo T. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol. 2008;215(3):837–845. doi: 10.1002/jcp.21368. [DOI] [PubMed] [Google Scholar]
- 15.Kazakos K, Lyras DN, Tilkeridis K, Verettas D, Tryfonidis M (2008) The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury, Epub 12 Aug [DOI] [PubMed]
- 16.Lucarelli E, Fini M, Beccheroni A, Giavaresi G, Bella C, Aldini NN, Guzzardella G, Martini L, Cenacchi A, Maggio N, Sangiorgi L, Fornasari PM, Mercuri M, Giardino R, Donati D. Stromal stem cells and platelet-rich plasma improve bone allograft integration. Clin Orthop Relat Res. 2005;435:62–68. doi: 10.1097/01.blo.0000165736.87628.12. [DOI] [PubMed] [Google Scholar]
- 17.Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24:698–701. doi: 10.1177/036354659602400524. [DOI] [PubMed] [Google Scholar]
- 18.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 19.Miller MD, Nichols T, Butler CA. Patella fracture and proximal patellar tendon rupture following arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 1999;15:640–643. doi: 10.1053/ar.1999.v15.015064. [DOI] [PubMed] [Google Scholar]
- 20.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33(5):381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 21.Nagae M, Ikeda T, Mikami Y, Hase H, Ozawa H, Matsuda KI, Sakamoto H, Tabata Y, Kawata M, Kubo T. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007;13:147–158. doi: 10.1089/ten.2006.0042. [DOI] [PubMed] [Google Scholar]
- 22.Roldan JC, Jepsen S, Miller J, Freitag S, Rueger DC, Acil Y, Terheyden H. Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone. 2004;34:80–90. doi: 10.1016/j.bone.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(20):245–251. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]
- 24.Shelbourne KD, Rubinstein RA, Jr, VanMeter CD, McCarroll JR, Rettig AC. Correlation of remaining patellar tendon width with quadriceps strength after autogenous bone-patellar tendon-bone anterior cruciate ligament reconstruction. Am J Sports Med. 1994;22:774–778. doi: 10.1177/036354659402200608. [DOI] [PubMed] [Google Scholar]
- 25.Shino K, Nakagawa S, Inoue M, Horibe S, Yoneda M. Deterioration of patellofemoral articular surfaces after anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:206–211. doi: 10.1177/036354659302100208. [DOI] [PubMed] [Google Scholar]
- 26.Smith JJ, Ross MW, Smith RK. Anabolic effects of acellular bone marrow, platelet rich plasma, and serum on equine suspensory ligament fibroblasts in vitro. Vet Comp Orthop Traumatol. 2006;19:43–47. [PubMed] [Google Scholar]
- 27.Tria AJ, Jr, Alicea JA, Cody RP. Patella baja in anterior cruciate ligament reconstruction of the knee. Clin Orthop. 1994;299:229–234. [PubMed] [Google Scholar]