Abstract
We analysed data from 155 revisions of identical cementless hip prostheses to determine the influence of patient-, implant- and surgery-related factors on the polyethylene wear rate and size of periprosthetic osteolysis (OL). This was calculated by logistic regression analysis. Factors associated with an increased/decreased wear rate included position of the cup relative to Kohler’s line, increase in abduction angle of the cup, traumatic and inflammatory arthritis as a primary diagnosis, and patient height. Severe acetabular bone defects were predicted by an increased wear rate (odds ratio, OR = 5.782 for wear rate above 200 mm3/y), and increased height of the patient (OR = 0.905 per each centimetre). Predictors of severe bone defects in the femur were the increased wear rate (OR = 3.479 for wear rate above 200 mm3/y) and placement of the cup outside of the true acetabulum (OR = 3.292). Variables related to surgical technique were the most predictive of polyethylene wear rate.
Résumé
Nous avons analysé les données de 155 révisions d’une prothèse totale de hanche sans ciment de façon à déterminer les facteurs relatifs aux patients et à l’implant concernant l’utilisation du polyéthylène et l’importance de l’ostéolyse (OL). Les 155 modèles de prothèses étaient identiques. Il s’agit d’une analyse informatique. L’augmentation ou la diminution du taux d’usure était associée à la position de la cupule par rapport à la ligne de Kohler, une cupule verticalisée entraînant plus d’usure. De même en ce qui concerne les arthroses d’origine traumatique ou inflammatoire et le poids des patients. On pouvait prévoir d’importantes lésions d’ostéolyse acétabulaire du fait d’une importante augmentation de l’usure du polyéthylène (OR = 5,782 taux d’usure 707, 200 mm3/an) et l’augmentation du poids des patients (OR = 0,905) pour chaque cm. L’augmentation du taux prédictif d’usure avec des lésions osseuses sévères au niveau du fémur l’étaient également (OR = 3,479 d’usure et 200 mm3/an) de même que le positionnement de la cupule en dehors de l’arrière fond acétabulaire (OR = 3,292). Les facteurs d’usure les plus importants sont surtout d’origine technique et secondaires aux problèmes de technique chirurgicale.
Introduction
Debris from polyethylene (PE) wear is believed to be a key factor in the development of osteolysis around total hip arthroplasty (THA) [1]. This debris triggers multiple adverse host reactions that involve signalling pathways and finally result in osteoclast-mediated bone resorption [2]. Wear particles originate predominantly from motion between two opposing, articulating surfaces, but other mechanisms of particle generation are also possible [3]. As the number of primary THAs is increasing and PE remains the main weight-bearing material, interest has increased in understanding its mode of degradation, with the goal of preventing it.
The many factors that influence PE wear in vivo are traditionally divided into those related to the patient, the implant, and the surgery [4]. The known influences on PE wear include level of physical activity, age, gender, primary diagnosis, design-related variables, type of PE, and quality of implant settlements [5–8]. Other factors that may be important, but are not readily analysed, include third body wear, composition of joint fluid, type of lubrication, and individual motion pattern.
In a previous study, we found a high risk for increased wear rate associated with this prosthesis [9]. Moreover, the univariate analyses revealed that wear rate was influenced by primary diagnosis, use of zirconia ceramic heads, medial cup settlement, and date of surgery. However, such analysis was designed neither to give insight into the influence of competing factors nor to quantify the magnitude of each factor’s influence. Therefore, we conducted our study with more patients, using logistic regression to identify the significant predictors of high and low wear rates. The same data were also analysed to identify predictors of severe bone defects at both the acetabular and femoral sites. The identification of such predictors may be useful in making clinical decisions about THA and reducing the need for surgical revisions.
Patients and methods
Patients
Patients undergoing surgical revision of THA between August 2000 and December 2005 were included in the study. The revised cases belonged to a group of patients with ABG I prostheses operated upon at the author’s institution between September 1994 and January 2000 (n = 506). We previously reported on the poor 12-year survival of the ABG I prosthesis [10]. Information was collected on the indication for the original arthroplasty, the time since the arthroplasty, and indication for revision. The ethical committee of the institution approved the study protocol and all revisions were performed under standard conditions with written informed consent of the patients. The majority of revisions (>90%) were performed by a single surgeon.
Prosthesis
The first generation of modular, cementless hip prosthesis (ABG I, Howmedica, Inc., Staines, England) was used [11]. The ABG I prosthesis was designed in the 1980s as a press-fit hemispherical cup and anatomical stem with hydroxyapatite coating. Marketing began in Europe in 1989 and in the Czech Republic in 1993. The first studies on this implant reported excellent early results [12, 13]. Therefore, we opted for this prosthesis in younger patients. All primary procedures were performed by eight experienced surgeons via an anterolateral approach, according to the manufacturer’s instructions. Patients were protected from weight-bearing with crutches for the first four weeks which was followed by a period of partial weight-bearing as tolerated; full loading was permitted 12 weeks after the surgery. All patients had routine perioperative antibiotics and prophylaxis against thromboembolic disease using subcutaneous heparin or low-molecular weight heparin.
All PE liners were ram-extruded from Hostalen GUR 4150 and air-sterilised with 25 kGy gamma irradiation. The PE thickness ranged from 4.9 to 12.9 mm. Both standard (n = 125) and hooded polyethylene liners were implanted (n = 30). In nearly all of the hips a 28-mm femoral head made from cobalt-chromium alloy (n = 143) was inserted, except for 12 cases in which a 28-mm zirconia head was used. Tables 1 and 2 summarise the data that were included in the analyses.
Table 1.
Categorical variables included in the study
| Variable | Categories | Number of hips (%) |
|---|---|---|
| Gender | Men | 44 (28) |
| Women | 111 (72) | |
| Preoperative diagnosis | Osteoarthritis | 29 (19) |
| Hip dysplasia | 71 (46) | |
| Osteonecrosis | 30 (19) | |
| Traumatic | 14 (9) | |
| Inflammatory | 6 (4) | |
| SCFE | 5 (3) | |
| Charnley type | A | 53 (34) |
| B | 89 (57) | |
| C | 13 (8) | |
| Liner geometry | Neutral | 125 (81) |
| Hooded | 30 (19) | |
| Head material | CoCr | 143 (92) |
| Zirconia | 12 (8) | |
| Cup abduction angle | Between 30° and 50° | 120 (77) |
| Above 50° | 35 (23) | |
| Cup relation to KL | Laterally | 14 (9) |
| In contact | 27 (17) | |
| Medially | 114 (74) | |
| True acetabular region | Yes | 123 (79) |
| No | 32 (21) | |
| Stability of the implant | Stable | 121 (78) |
| Unstable | 34 (22) | |
| Acetabular BD | Type I, II | 44 (28) |
| Type III | 87 (56) | |
| Type IV, V | 24 (16) | |
| Femoral BD | Type I, II | 127 (82) |
| Type III | 25 (16) | |
| Type IV | 3 (2) |
KL Kohler’s line, BD bone defects, SCFE slipped capital femoral epiphysis
Table 2.
Continuous variables included in a stepwise logistic regression
| Variable | Mean | SD | Range | Median |
|---|---|---|---|---|
| Age at surgery (y) | 46.1 | 6.8 | 25–65 | 46.3 |
| Height (cm) | 165.5 | 8.6 | 149–195 | 164 |
| Weight (kg) | 75 | 14.4 | 42–114 | 73 |
| Body mass index | 27.3 | 4.1 | 16–42.6 | 27.3 |
| Cup size (mm) | 50.3 | 3.81 | 46–60 | 50.0 |
| PE thickness (mm) | 7.03 | 1.93 | 4.9–11.9 | 6.9 |
| Abduction angle of the cup (°) | 45.26 | 7.98 | 28–72 | 45.0 |
| Follow-up (y) | 6.0 | 1.9 | 2.0–10.5 | 6.0 |
| Linear wear (mm) | 2.21 | 1.57 | 0–6.9 | 1.86 |
| Linear wear rate (mm/y) | 0.415 | 0.364 | 0–2.284 | 0.346 |
| Volumetric wear (mm3) | 859 | 781 | 0–6000 | 701 |
| Volumetric wear rate (mm3/y) | 153 | 134 | 0–815 | 115 |
PE polyethylene, SD standard deviation
Wear measurement
After prosthesis extraction, all of the PE liners were immersed in Sekusept aktiv (Ecolab GmbH, Düsseldorf, Germany) for 24 hours and sterilised in formaldehyde for two hours. The wear measurements were performed by one of the authors (VH). Briefly, linear wear, defined as the maximum penetration of the prosthetic head into the polyethylene liner, was determined using a previously reported method that measures the shift of the centre of the prosthetic head from the manufactured to the post-use position. To determine the centre of a ball of known diameter, it is sufficient to measure four surface space coordinates with the touch stylus of the Universal-type measuring microscope (VEB, Carl Zeiss, Jena, Germany). This measurement was made after fixation of the retrieved prosthetic femoral head in the retrieved cup for each of the positions mentioned. Nine space coordinates were determined in practice to improve measurement accuracy. Assuming that the diameter of the cup and the head are known, it is possible to calculate the linear and volumetric wear using a geometrical "two-sphere model" and a special computational algorithm that also takes into account the original position of the prosthetic head in the cup and the cross-sectional profile of the ABG I cup. The accuracy of the described method was previously assessed in a set of 30 retrieved cups, ranging between 1 and 4 μm and 1–9 mm3 for linear and volumetric wear, respectively [14]. The reliability of the method has also been previously reported [15].
Clinical and radiographic evaluation
All hips included in the study had stable prostheses one year after the index operation based on review of radiology reports. All of the patients were clinically and radiographically examined prior to revision surgery using the same protocol. The Charnley classification was applied to estimate the level of walking capacity, with class A having no disturbance in locomotion, class B with bilateral hip disease and normal findings in other weight-bearing joints, and class C with severe compromise of locomotion due to multiple joint involvement [16]. Anteroposterior pelvic X-rays were performed with the patient in the supine (non-weight-bearing) position. Interpretation of radiographs consisted of the evaluation of the cup position relative to the lateral part of tear drop figure, determination of the cup position relative to the true acetabular region [17], and measurement of the abduction angle of the cup. The position of the cup in relation to the floor of the acetabulum was graded as lateral, in contact, or medial depending on relationship between a most medial part of the cup and Kohler’s line. The abduction angle is the angle formed by a horizontal line along the teardrop, ischial tuberosities or obturator foramina and a line along the open face of the cup. Bone defects were evaluated intraoperatively and expressed in terms of Saleh’s classification at the acetabular site, i.e. no significant bone loss (type I), contained bone loss (type II), moderate uncontained bone loss (type III), severe uncontained bone loss (types IV), and pelvic discontinuity (type V) [18]. At the femoral site, the same classification was used. Finally, the stability of the implant was evaluated intraoperatively.
Statistics
The primary goal of the study was to identify the predictors of high and low rates of PE wear. A stepwise logistic regression analysis was chosen because a high wear rate was defined as >0.2 mm/y or >200 mm3/y; a low wear rate was ≤0.1 mm/y or ≤100 mm3/y. In the logistic regression analysis, the effect of a number of independent variables was determined with wear rate as the dependent variable. To enable this analysis, some variables were recoded and rescaled as either 0 or 1 (Table 1), while others were included as continuous variables (Table 2). As a result, wear data for retrieved ABG I PE liners were simultaneously analysed in relation to variables that could be distinguished by demographic (age, gender, weight, height, primary diagnosis, and the Charnley classification of activity), surgical (abduction angle of the cup, position of the cup relative to the Kohler’s line, and the true acetabular region), and implant variables (size of the cup, thickness of the PE liner, type of PE liner, metallic/ceramic femoral head). A stepwise variable entry continued if the inclusion α value was less than or equal to 0.05. This sequential inclusion/exclusion of independent variables according to stepping criteria eventually leads to the selection of a single “optimal” model [19]. Therefore, variables without significant associations with wear rate were removed from the model. In addition, the data were modelled according to limits for a high wear rate (>100 mm3/y) and a low wear rate (≤80 mm3/y). Also, factors that predicted severe bone defects (dependent variable) were analysed in the same way after wear rate was added to the independent variables listed above. Survival of the index prosthesis was computed using the Kaplan-Meier methods. Survival curves derived for particular wear rate groups were compared by the log rank test. The accepted significance level was 0.05. Statistical analysis was performed with the commercial SPSS 14.0 package (SPSS Inc., Chicago, IL, USA).
Results
The study included 155 patients (44 men, 111 women) who had surgical revisions of THA after a mean of six years from the index operation. The reasons for revision were periprosthetic osteolysis (n = 115, 74%), aseptic loosening of the cup (n = 32, 21%) or stem (n = 2, 1%), and periprosthetic fracture of the femur (n = 6, 4%).
Prosthetic survival analysis
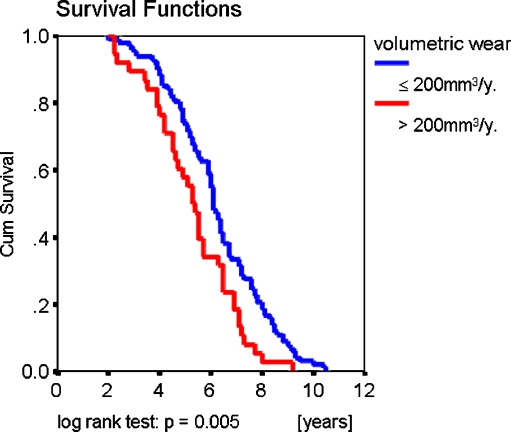
The mean time to revision was 5.96 ± 0.15 years (mean ± standard deviation [SD]). Mean linear and volumetric wear rates were 0.415 mm/y (range 0–2.284, SD 0.364) and 153 mm3/y (range 0–815, SD 134.4), respectively. Wear rate was found to have a significant influence on survival of the prosthesis only when the whole group was divided into those having a wear rate above and below 200 mm3/y (Fig. 1); the other analyses, according to incremental wear rate (i.e. 0.1 mm/y, 0.2 mm/y, 100 mm3/y) failed to reveal significant influence on implant survival.
Fig. 1.
Wear rate as a function of survival of retrieved ABG I prostheses
Factors that influence high and low wear rates
Among the variables (Tables 1 and 2) that were included in the logistic regression, only four significantly predicted a high or low wear rate. Predictors of accelerated and decelerated linear and volumetric wear are summarised in Table 3. The most surprising finding was a potential role for patient’s height in the wear rate. On the other hand, no role was detected for age, gender, weight of the patient, BMI, Charnley classification of activity, initial PE thickness, type of liner, material of the prosthetic head, and stability of the implant at the time of surgery. Logistic regression also determines the percent of variance in the dependent variable explained by the independent variables to assess the relative importance of the independent variables. The best regression achieved was 15% (Nagelkerke R2 = 0.15), and −2 log likelihood was above 120, indicating a poor fit of the regression model to the wear rate data.
Table 3.
Variables that changed the probability of increased/decreased wear rates
| Wear rate | Variable | Odds ratio | 95% confidence interval | p value | R2 | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| > 0.2 mm/y | Increasing patient’s height by 1 cm | 1.065 | 1.018 | 1.114 | 0.007 | 0.116 |
| One-degree increase in abduction angle | 1.049 | 1.001 | 1.099 | 0.047 | ||
| > 200 mm3/y | Cup placed laterally to KL | 3.896 | 1.130 | 13.426 | 0.031 | 0.114 |
| Increasing patient’s height by 1 cm | 1.045 | 1.000 | 1.092 | 0.050 | ||
| > 100 mm3/y | Increasing patient’s height by 1 cm | 1.052 | 1.008 | 1.099 | 0.021 | 0.137 |
| Cup placed medially to KL | 0.375 | 0.156 | 0.905 | 0.029 | ||
| ≤ 100 mm3/y | Cup placed medially to KL | 2.663 | 1.105 | 6.418 | 0.029 | 0.137 |
| Increasing patient’s height by 1 cm | 0.950 | 0.910 | 0.992 | 0.021 | ||
| ≤ 0.1 mm/y | Trauma, inflammation and SCFE as primary diagnosis | 2.975 | 1.007 | 8.787 | 0.049 | 0.040 |
| ≤ 80 mm3/y | Increasing patient’s height by 1 cm | 0.935 | 0.893 | 0.980 | 0.005 | 0.151 |
R2 Nagelkerke R square, KL Kohler’s line, SCFE slipped capital femoral epiphysis
Factors that predict severe bone defects
Development of bone defects is believed to be a direct consequence of PE wear rate, assuming a positive correlation between higher wear rate and more severe bone defects. Among the factors (Tables 1 and 2), including the linear wear rate/volumetric wear, that were included in the regression analysis, only three significantly predicted the severity of bone defects (Table 4). Of them, the most important factor was PE wear rate. Patients having higher linear/volumetric wear rates had a higher risk for severe acetabular (types IV or V) and femoral bone defects (type III). The other two factors affecting bone defects were the patient’s height and the position of the cup in relation to the true acetabulum. The best regression achieved was 19% (Nagelkerke R2 = 0.191) and −2 log likelihood was 88.8, indicating a better fit of the regression model to the size of osteolysis than to the wear rate data.
Table 4.
Variables that increased/decreased the probability of severe bone defects
| Bone defect | Variable | Odds ratio | 95% confidence interval | p value | R2 | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Acetabulum Saleh type IV, V | Wear rate > 0.2 mm/year | 4.146 | 1.222 | 14.066 | 0.023 | 0.155a |
| Increasing patient’s height by 1 cm | 0.898 | 0.837 | 0.963 | 0.002 | ||
| Wear rate > 200 mm3/year | 5.782 | 1.846 | 18.110 | 0.003 | 0.191b | |
| Increasing patient’s height by 1 cm | 0.905 | 0.841 | 0.973 | 0.007 | ||
| Femur Saleh type III | Wear rate > 0.2 mm/year | 5.862 | 1.584 | 21.699 | 0.008 | 0.141a |
| Higher position of the cup | 3.688 | 1.391 | 9.781 | 0.009 | ||
| Wear rate > 200 mm3/year | 3.479 | 1.279 | 9.465 | 0.015 | 0.173b | |
| Cup above the true acetabulum | 3.292 | 1.153 | 9.397 | 0.026 | ||
R2 Nagelkerke R square
aModel with linear wear rate data inclusion
bModel with volumetric wear rate data inclusion
Discussion
This study confirmed the finding from other studies that certain surgical factors such as position and orientation of the cup are associated with increased wear rates of prosthetic hips [7, 8, 20]. Furthermore, the primary diagnosis and the height of the patient were found to significantly influence wear rate. In addition, a high wear rate was strongly associated with severe osteolysis.
PE wear has been considered a key parameter for periprosthetic osteolysis development, and its multifactorial origin is now generally accepted [5, 21]. In this study we used logistic regression to determine the effect of independent variables on wear rate and on the extension of bone defects. An advantage of our analysis was that the majority of revision operations (>90%) were performed by a single surgeon, and a large number of THAs were included with the in vitro measurement of the wear of retrieved PE liners. In addition, all hips had the same type of implant, which diminished the role for interprosthetic differences as a source of variability. Another theoretical advantage is that the final single model in a stepwise logistic regression model should reliably indicate the most important examined variable.
The strongest coefficient of determination found in the wear-related part of this study was 0.15, which may seem surprisingly low upon initial evaluation. However, this finding is in accordance with other studies, including those that used multiple linear regression models, which are principally different from logistic regression. In a large clinical trial with Duraloc cups, Hopper et al. found that eight factors accounted for 26% of the variance in wear rate [4]. According to Wan et al., the multiple regression model explained only 21.5% of the variation in PE wear rate [8]. Moreover, when wear rate variance was analysed by multiple linear regression in the same patients with bilateral THA, the patient- and implant-related variables accounted for 61% of the variance in wear rate [22]. In clinical terms, these studies show that the majority of the variability in wear rate (e.g. 85% in our study) could not be explained, even under ideal conditions. As a result, several unknown or poorly quantified factors may account for the variability. Such factors may include the quality of the PE, the patient’s activity level, third body wear, conditions of the internal environment or differences in surgical technique.
In this study, the factor that most strongly correlated with wear rate (i.e. had the highest odds ratio) was the position of the cup relative to Kohler’s line. Lateral displacement was a risk factor; medial displacement was a protective factor. This observation is in accordance with the biomechanical concept that the centre of rotation should be as medial as possible to reduce the resultant torque and risk of hardware loosening when the femoral offset is unchanged [23]. Therefore, this study, and others [7, 8, 20, 24], support cup implantation as medially as possible to achieve minimal PE wear.
An increased inclination angle of the cup in the frontal plane (i.e. abduction angle) was significantly associated with a greater wear rate. This finding is in accordance with other studies [8, 25] and may be explained by eccentric sliding of the prosthetic head in conjunction with the impact of the head on a surface much smaller than the head itself. These factors could lead to rim overuse and acceleration of wear rate. However, several studies failed to find a role of abduction angle in the acceleration of wear rate [26–29]. The reasons for these results are not clear, but at least partially may lie in the radiographic origin of wear measurement used in these studies. These radiographic measurements may be less sensitive in detecting wear than in vitro measurements [30].
A most surprising finding in our study was the significant association between patient height and increased wear rate. This finding was also supported by the inverse relation (i.e. decreased probability of low wear with increased height). However, predicting the relationship between patient height and volumetric wear rate is not as simple as one may at first assume, since a number of factors are involved. To our knowledge, the impact of the patient height on wear rate is rarely mentioned in the literature, despite the fact that height directly influences the body weight which, in turn, leads to greater forces applied to the hip [31]. Archard’s equation predicts that the volume of wear per unit of sliding distance will increase in direct proportion to the load [32, 33], (i.e. with the cube of patient height). Moreover, in this study, only 28 mm heads were used. Since the total contact area was, therefore, constant as the weight of the patient increased, the contact stress was greater for taller patients, approximately in proportion to the cube of height. This would be expected to cause a substantially greater volumetric rate of wear in the taller patients. Another factor is the femoral offset, which tends to be greater in taller people [34]. Unfortunately, this parameter cannot be strictly restored in all patients by a single prosthetic design. As a result, inability to restore femoral offset adequately can lead to decreased abductor muscle strength and greater forces across the hip joint [35, 36]. Taken together, if these considerations are correct, strong positive correlation of volumetric wear rate with the height of the patients could be expected [McKellop, H., personal communication]. However, these issues were not investigated in our study and require further research.
Many clinicians and researchers believe the most important factor influencing the wear rate is activity level [6]. We found that Charnley classification of activity is not sensitive enough to explain wear rate variability. Recently, Roder et al. augmented the Charnley classification with a class BB for those with bilateral THA [37]. Even with that modification, we are sceptical of its sensitivity to detect true differences in activity level. Therefore, a new approach to this issue is needed.
Finally, our finding that higher PE wear rates are associated with a risk of severe osteolysis at both the acetabular and femoral sites is in accord with the literature [21, 38]; this finding strongly supports particle disease as the leading mechanism of osteolysis initiation and expansion [1]. On the other hand, the main limitation of our study is that it is based on an implant especially prone to rapid wear and the initiation of OL. The overall 12-year survival for ABG I prostheses, found in our previous study, was 55% [10], which is in agreement with several studies [39–42] but contrasts with others [11, 43, 44]. The reasons for the disparity are still not clear, but this issue is discussed elsewhere [10]. Briefly, it has been disclosed that PE liners made from GUR 415HP resins possess greater biological activity compared to PE made from GUR 1120 [45]. Moreover, when sterilised by gamma irradiation in the air, the PE can undergo significant deterioration of their mechanical and chemical properties after long-term in vivo function [46]. In addition, the multi-hole design of the cup, together with a poor locking mechanism for the PE liners in the ABG I cups, could lead to the repetitive generation of screw-hole fluid pressure at levels greatly exceeding the threshold for OL induction [47]. These characteristics and the fact that only patients with failed implants were included could bias our results by providing an inadequate number of hips with lower wear rates and/or less severe osteolysis.
We can conclude that PE wear rate was most significantly influenced by the position of the acetabular component. Based on this, we recommend placing the PE cup (centre of rotation) as medially as possible to allow for an abduction angle below 50° without compromising bone coverage of the cup. Further studies are needed to confirm whether patient height influences PE wear rate and to investigate the mechanism of this possible influence.
Acknowledgements
This study was supported by the grant of Ministry of Education, Youth and Sports of the Czech Republic, OC 168 533.
The authors wish to thank Dr. H. McKellop for his advice regarding the biomechanics of the hip and mechanisms of wear and Dr. I. Cechova for assistance. Part of this work was presented at the 8th Meeting of European Federation of National Associations of Orthopaedics and Traumatology (EFORT), May 11–15, 2007, Florence, Italy.
References
- 1.Pearle AD, Crow MK, Rakshit DS, Wohlgemuth J, Nestor BJ. Distinct inflammatory gene pathways induced by particles. Clin Orthop Relat Res. 2007;458:194–201. doi: 10.1097/BLO.0b013e3180320ae8. [DOI] [PubMed] [Google Scholar]
- 2.Drees P, Eckardt A, Gay RE, Gay S, Huber LC. Mechanisms of disease: molecular insights into aseptic loosening of orthopedic implants. Nat Clin Pract Rheumatol. 2007;3:165–171. doi: 10.1038/ncprheum0428. [DOI] [PubMed] [Google Scholar]
- 3.McKellop HA. The lexicon of polyethylene wear in artificial joints. Biomaterials. 2007;28:5049–5057. doi: 10.1016/j.biomaterials.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 4.Hopper RH, Jr, Engh CA, Jr, Fowlkes LB, Engh CA. The pros and cons of polyethylene sterilization with gamma irradiation. Clin Orthop Relat Res. 2004;429:54–62. doi: 10.1097/01.blo.0000150112.34736.82. [DOI] [PubMed] [Google Scholar]
- 5.Schmalzried TP, Dorey FJ, McKellop H. The multifactorial nature of polyethylene wear in vivo. J Bone Joint Surg Am. 1998;80:1234–1242. doi: 10.2106/00004623-199808000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Schmalzried TP, Shepherd EF, Dorey FJ, Jackson WO, Rosa M, et al. The John Charnley Award. Wear is a function of use, not time. Clin Orthop Relat Res. 2000;381:36–46. doi: 10.1097/00003086-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Wroblewski BM, Siney PD, Fleming PA. Wear of the cup in the Charnley LFA in the young patient. J Bone Joint Surg Br. 2004;86:498–503. [PubMed] [Google Scholar]
- 8.Wan Z, Boutary M, Dorr LD. The influence of acetabular component position on wear in total hip arthroplasty. J Arthroplasty. 2008;23:51–56. doi: 10.1016/j.arth.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Gallo J, Havranek V, Cechova I, Zapletalova J. Influence of demographic, surgical and implant variables on wear rate and osteolysis in ABG I hip arthroplasty. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:135–141. doi: 10.5507/bp.2006.021. [DOI] [PubMed] [Google Scholar]
- 10.Gallo J, Langova K, Havranek V, Cechova I. Poor survival of ABG I hip prosthesis in younger patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:163–168. doi: 10.5507/bp.2008.027. [DOI] [PubMed] [Google Scholar]
- 11.Oosterbos CJ, Rahmy AI, Tonino AJ, Witpeerd W. High survival rate of hydroxyapatite-coated hip prostheses: 100 consecutive hips followed for 10 years. Acta Orthop Scand. 2004;75:127–133. doi: 10.1080/00016470412331294365. [DOI] [PubMed] [Google Scholar]
- 12.Garcia Araujo C, Fernandez Gonzalez J, Tonino A. Rheumatoid arthritis and hydroxyapatite-coated hip prostheses: five-year results. International ABG Study Group. J Arthroplasty. 1998;13:660–667. doi: 10.1016/S0883-5403(98)80010-7. [DOI] [PubMed] [Google Scholar]
- 13.Rossi P, Sibelli P, Fumero S, Crua E. Short-term results of hydroxyapatite-coated primary total hip arthroplasty. Clin Orthop Relat Res. 1995;310:98–102. [PubMed] [Google Scholar]
- 14.Gallo J, Havranek V, Hrabovsky M. Measurement of retrieved polyethylene cup by universal microscope. Fine Mech Opt. 2003;48:333–338. [Google Scholar]
- 15.Gallo J, Havranek V, Zapletalova J, Mandat D. Measurement of acetabular polyethylene wear of total hip replacement, using a universal measuring microscope. Characteristics of measurements. Acta Chir Orthop Traumatol Cech. 2006;73:28–33. [PubMed] [Google Scholar]
- 16.Charnley J. The long-term results of low-friction arthroplasty of the hip performed as a primary intervention. J Bone Joint Surg Br. 1972;54:61–76. [PubMed] [Google Scholar]
- 17.Pagnano W, Hanssen AD, Lewallen DG, Shaughnessy WJ. The effect of superior placement of the acetabular component on the rate of loosening after total hip arthroplasty. J Bone Joint Surg Am. 1996;78:1004–1014. doi: 10.2106/00004623-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Saleh KJ, Holtzman J, Gafni A, Saleh L, Davis A, et al. Reliability and intraoperative validity of preoperative assessment of standardized plain radiographs in predicting bone loss at revision hip surgery. J Bone Joint Surg Am. 2001;83:1040–1046. doi: 10.2106/00004623-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Zhang W, Bakhai A. Comparison of Bayesian model averaging and stepwise methods for model selection in logistic regression. Stat Med. 2004;23:3451–3467. doi: 10.1002/sim.1930. [DOI] [PubMed] [Google Scholar]
- 20.Karachalios T, Hartofilakidis G, Zacharakis N, Tsekoura M. A 12- to 18-year radiographic follow-up study of Charnley low-friction arthroplasty. The role of the center of rotation. Clin Orthop Relat Res. 1993;296:140–147. [PubMed] [Google Scholar]
- 21.Wilkinson JM, Hamer AJ, Stockley I, Eastell R. Polyethylene wear rate and osteolysis: critical threshold versus continuous dose-response relationship. J Orthop Res. 2005;23:520–525. doi: 10.1016/j.orthres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Orishimo KF, Sychterz CJ, Hopper RH, Jr, Engh CA. Can component and patient factors account for the variance in wear rates among bilateral total hip arthroplasty patients? J Arthroplasty. 2003;18:154–160. doi: 10.1054/arth.2003.50023. [DOI] [PubMed] [Google Scholar]
- 23.Johnston RC, Brand RA, Crowninshield RD. Reconstruction of the hip. A mathematical approach to determine optimum geometric relationships. J Bone Joint Surg Am. 1979;61:639–652. [PubMed] [Google Scholar]
- 24.Hirakawa K, Mitsugi N, Koshino T, Saito T, Hirasawa Y, et al. Effect of acetabular cup position and orientation in cemented total hip arthroplasty. Clin Orthop Relat Res. 2001;388:135–142. doi: 10.1097/00003086-200107000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Patil S, Bergula A, Chen PC, Colwell CW, Jr, D’Lima DD. Polyethylene wear and acetabular component orientation. J Bone Joint Surg Am. 2003;85(Suppl 4):56–63. doi: 10.2106/00004623-200300004-00007. [DOI] [PubMed] [Google Scholar]
- 26.Del Schutte H, Jr, Lipman AJ, Bannar SM, Livermore JT, Ilstrup D, et al. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621–626. doi: 10.1016/S0883-5403(98)80003-X. [DOI] [PubMed] [Google Scholar]
- 27.Nevelos JE, Ingham E, Doyle C, Nevelos AB, Fisher J. The influence of acetabular cup angle on the wear of "BIOLOX Forte" alumina ceramic bearing couples in a hip joint simulator. J Mater Sci Mater Med. 2001;12:141–144. doi: 10.1023/A:1008970027306. [DOI] [PubMed] [Google Scholar]
- 28.Kligman M, Michael H, Roffman M. The effect of abduction differences between cup and contralateral acetabular angle on polyethylene component wear. Orthopedics. 2002;25:65–67. doi: 10.3928/0147-7447-20020101-18. [DOI] [PubMed] [Google Scholar]
- 29.Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. Highly cross-linked polyethylene in cemented THA: randomized study of 61 hips. Clin Orthop Relat Res. 2003;417:126–138. doi: 10.1097/01.blo.0000096802.78689.45. [DOI] [PubMed] [Google Scholar]
- 30.Chuter GS, Cloke DJ, Mahomed A, Partington PF, Green SM. Wear analysis of failed acetabular polyethylene: a comparison of analytical methods. J Bone Joint Surg Br. 2007;89:273–279. doi: 10.1302/0301-620X.89B2.18158. [DOI] [PubMed] [Google Scholar]
- 31.Pierrynowski MR, Galea V. Enhancing the ability of gait analyses to differentiate between groups: scaling gait data to body size. Gait Posture. 2001;13:193–201. doi: 10.1016/S0966-6362(01)00097-2. [DOI] [PubMed] [Google Scholar]
- 32.Vassiliou K, Unsworth A. Is the wear factor in total joint replacements dependent on the nominal contact stress in ultra-high molecular weight polyethylene contacts? Proc Inst Mech Eng Part H-J Eng Med. 2004;218:101–107. doi: 10.1243/095441104322983997. [DOI] [PubMed] [Google Scholar]
- 33.Jin ZM, Stone M, Ingham E, Fisher J. Biotribology. Curr Orthop. 2006;20:32–40. doi: 10.1016/j.cuor.2005.09.005. [DOI] [Google Scholar]
- 34.Charles MN, Bourne RB, Davey JR, Greenwald AS, Morrey BF, et al. Soft-tissue balancing of the hip: the role of femoral offset restoration. Instr Course Lect. 2005;54:131–141. [PubMed] [Google Scholar]
- 35.Bicanic G, Delimar D, Delimar M, Pecina M (2008) Influence of the acetabular cup position on hip load during arthroplasty in hip dysplasia. Int Orthop (in press). doi:10.1007/s00264-008-0683-z [DOI] [PMC free article] [PubMed]
- 36.Sakalkale DP, Sharkey PF, Eng K, Hozack WJ, Rothman RH. Effect of femoral component offset on polyethylene wear in total hip arthroplasty. Clin Orthop Relat Res. 2001;388:125–134. doi: 10.1097/00003086-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Roder C, Staub LP, Eichler P, Widmer M, Dietrich D, et al. Avoiding misclassification bias with the traditional Charnley classification: rationale for a fourth Charnley class BB. J Orthop Res. 2006;24:1803–1808. doi: 10.1002/jor.20224. [DOI] [PubMed] [Google Scholar]
- 38.Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17:649–661. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- 39.Kim WY, Muddu BN. Eleven-year results of the ABG I hip replacement. Int Orthop. 2006;30:182–184. doi: 10.1007/s00264-006-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blacha J. High osteolysis and revision rate with the hydroxyapatite-coated ABG hip prostheses: 65 hips in 56 young patients followed for 5–9 years. Acta Orthop Scand. 2004;75:276–282. doi: 10.1080/00016470410001204. [DOI] [PubMed] [Google Scholar]
- 41.Delank KS, Drees P, Menzel N, Hansen T, Duschner H, et al. Increased polyethylene wear after cementless ABG I total hip arthroplasty. Arch Orthop Trauma Surg. 2006;126:509–516. doi: 10.1007/s00402-006-0168-8. [DOI] [PubMed] [Google Scholar]
- 42.Badhe S, Livesley P. Early polyethylene wear and osteolysis with ABG acetabular cups (7- to 12-year follow-up) Int Orthop. 2006;30:31–34. doi: 10.1007/s00264-005-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castoldi F, Rossi R, Russa M, Sibelli P, Rossi P, et al. Ten-year survivorship of the Anatomique Benoist Girard I total hip arthroplasty. J Arthroplasty. 2007;22:363–368. doi: 10.1016/j.arth.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Canales Cortes V, Panisello Sebastia JJ, Herrera Rodriguez A, Peguero Bona A, Martinez Martin A, et al. Ten-year follow-up of an anatomical hydroxyapatite-coated total hip prosthesis. Int Orthop. 2006;30:84–90. doi: 10.1007/s00264-005-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ingram J, Matthews JB, Tipper J, Stone M, Fisher J, et al. Comparison of the biological activity of grade GUR 1120 and GUR 415HP UHMWPE wear debris. Bio-Med Mater Eng. 2002;12:177–188. [PubMed] [Google Scholar]
- 46.Kurtz SM, Rimnac CM, Hozack WJ, Turner J, Marcolongo M, et al. In vivo degradation of polyethylene liners after gamma sterilization in air. J Bone Joint Surg Am. 2005;87:815–823. doi: 10.2106/JBJS.D.02111. [DOI] [PubMed] [Google Scholar]
- 47.Kurtz SM, Harrigan TP, Herr M, Manley MT. An in vitro model for fluid pressurization of screw holes in metal-backed total joint components. J Arthroplasty. 2005;20:932–938. doi: 10.1016/j.arth.2004.11.003. [DOI] [PubMed] [Google Scholar]