Abstract
This study was performed in 36 rabbits to investigate the role of midkine (MK) in the resorption of herniated intervertebral discs. The L1-2 disc was excised and immersed in one of three kinds of solution for two hours before relocation into the L4 epidural space. In the MK-treated group, the weight of relocated intervertebral discs decreased more over time than in the control group. Newly formed vessels and inflammatory cells were more frequently observed in the MK-treated group than in the control group two weeks after surgery. The degradation of matrix was more significant in the MK-treated group than in the control group four weeks after surgery. Larger areas were replaced by fibrous tissues in the MK-treated group eight weeks after surgery. Thus, MK can accelerate the resorption of the intervertebral disc relocation to the epidural space. Epidural injection of MK may contribute to the therapy of lumber disc herniation.
Résumé
Le but de ce travail est d’évaluer le rôle du facteur de croissance midkine (MK) dans la résorption progressive des hernies discales. 36 lapins adultes ont été divisés en trois groupes. Les disques intervertébraux L1 et L2 de chaque lapin ont été partiellement incisés après un abord rétropéritonéal. Le matériel discal prélevé a été plongé dans une des trois différentes solutions pendant 2 heures puis repositionnés au niveau de l’espace épidural de L4. Les lapins de chaque groupe ont été sacrifiés pour un examen histologique des disques repositionnés à 1, 2, 4 et 8 semaines après la chirurgie. Dans le groupe traité par le facteur MK, le poids du disque intervertébral réimplanté diminue avec le temps comparé au groupe contrôle. A 2 semaines après la chirurgie, des vaisseaux néo-formés et des cellules inflammatoires ont été observés de façon plus fréquente dans le groupe traité par le facteur MK. A 4 semaines post-opératoires, il existe une dégradation de la matrice significativement plus importante dans le groupe traité par MK que dans le groupe contrôle. Enfin, à 8 semaines après la chirurgie, de vastes plages discales sont remplacées par du tissu fibreux, dans le groupe MK, comparé au groupe contrôle. Le facteur MK accélère donc la résorption du disque vertébral réimplanté au niveau de l’espace épidural. L’injection épidurale du facteur MK pourrait donc contribuer à traiter les hernies discales lombaires.
Introduction
Low back pain and sciatica caused by herniated disc often resolve spontaneously due to natural herniated disc resorption [1–3]. There is an increasing interest in the mechanism of spontaneous resorption of the herniated intervertebral disc seen by computed tomography [4] and magnetic resonance imaging [5]. Various factors may play significant roles in the spontaneous process, such as cytokines, macrophage infiltration and neovascularisation [6, 7]. Recently, angiogenesis received greater attention because evidence suggests that neovascularisation plays an important role in the wound-healing process [8–10]. Thus, neovascularisation in herniated disc appears to be essential for spontaneous resorption of herniated disc.
So far, different growth factors, such as bFGF, VEGF, TGF-α and midkine (MK) have been identified as positive regulators of angiogenesis [11–14]. MK was first identified in embryonal carcinoma cells in the early stages during retinoic acid-induced differentiation [15]. Now, increasing evidence shows that MK plays an important role in angiogenesis, which is an important event for tumour development and the wound-healing process. MK is a heparin-binding growth factor unrelated to fibroblast growth factor and enhances plasminogen activator activity in aortic endothelial cells [16]. The angiogenic activity of MK was confirmed by the rabbit corneal assay. However, the role of MK in angiogenesis of herniated disc resorption has not been reported.
The purpose of this study was to analyse the role of MK in the spontaneous resorption process of herniated disc. We used a hernia model in rabbits, which simulates a sequestrated type of human disc herniation. We investigated the histological change progression and angiogenesis of herniated disc within the epidural space, and the relationship with MK was discussed. The results reported here may be clinically important for non-operative treatment of patients with lumbar disc herniation.
Materials and methods
Animals and materials
Thirty-six healthy, male, New Zealand white rabbits, specific pathogenic free (SPF), each weighing 2.75 ± 0.25 kg, were supplied by Animal Experimental Centre of Medical College, Zhejiang University (Zhejiang, China). Mouse anti-human F VIII factor polyclone antibody and EnVision Kit for immunohistochemistry was obtained from DAKO Corporation. MK protein with biological activity was obtained from R&D Systems (Minneapolis, MN, USA).
Animal model
All experiments were carried out in accordance with the Institutional Animal Care and Use Committee, Medical College, Zhejiang University. The 36 rabbits were equally divided into an MK 5-μg group, an MK 20-μg group and a control group. The rabbits were anaesthetised with 30 mg/kg intravenous ketamine. The L1-L2 intervertebral disc (containing the nucleus pulposus and the anulus fibrosus in similar proportion) was exposed and partially incised through an anterior retroperitoneal approach. The harvested disc material was weighed and then immersed in one of the following materials for two hours according to the allocated group:
Physiological saline containing 1% bovine serum albumin (control group; n=12)
Physiological saline containing 5 μg/mL MK and 1% bovine serum albumin (MK 5-μg group; n=12)
Physiological saline containing 20 μg/mL MK and 1% bovine serum albumin (MK 20-μg group; n=12)
After these treatments, the disc material was placed into the posterior epidural space at the L4 vertebra of the identical rabbit through an incision in the ligamentum flavum. All procedures were performed under surgical microscopy. Three rabbits from each group were sacrificed at one, two, four and eight weeks postoperation, and the spinal segment from L4 was harvested in all groups.
Tissue preparation for histology
After being weighed on the same balance, the specimens were fixed in 10% buffered formalin overnight and embedded in paraffin. Then, the samples were cut in the axial plane to 5 mm thickness. Hemotoxylin–eosin (HE) staining was used routinely and the histological change in the harvested discs was examined under a microscope (Shanghai Medical Optical Instruments Factory, Shanghai, China). In other sections obtained at one, two, four, and eight weeks after surgery, immunohistochemical staining was performed to determine basement membrane components of newly formed vessels. The staining procedure was carried out by the EnVision System. Briefly, endogenous peroxidase was inactivated by 3% hydrogen peroxide (H2O2) for ten minutes at room temperature, and the sections were treated with 5% normal rabbit serum for ten minutes at room temperature, followed by incubation with mouse anti-human F VIII factor polyclone antibody for 30 minutes at 37°C. After being washed with PBS (phosphate buffered saline) three times, the slides were incubated with EnVision for 30 minutes at 37°C and then further washed three times with PBS. After that, the sections were reacted with DAB (3, 3’-diaminobenzidine) for two to five minutes to allow examination under a microscope. For negative controls, sections were processed as above but the primary antibody was replaced by TBS (20 mM Tris, 150 mM NaCl). The known F VIII factor positive slice was used as a positive control.
Histological examination and scoring
The number of inflammatory cells, the degree of degradation of the matrix and the area of replacement by fibrous tissue were evaluated by HE staining. The number of newly formed vessels was evaluated by immunohistochemical staining. Each of the parameters measured was scored as described below. The histological evaluation was blindly assigned to two investigators and was averaged for statistical analysis. The axial sections were observed under a photomicroscope at a magnification of 100. Five visual fields of the area of relocated intervertebral disc were selected blindly, and certain parameters thought to be histologically associated with the resorption of disc material in the field of view were measured. Each of the parameters measured was graded to permit average scores. The grading scheme is as follows.
Inflammatory cells (including macrophages, lymphocytes, and fibroblasts) Less than 10 cells grade = 0 10–29 cells grade = 1 30–49 cells grade = 2 50 cells or more grade = 3
Newly formed vessels Less than 10 vessels grade = 0 10–29 vessels grade = 1 30–49 vessels grade = 2 50 vessels or more grade = 3
Degradation of the matrix Almost no change grade = 0Derangement of anulus fibrosus grade = 1Fragmentation of the matrix grade = 2Disappearance of the original matrix grade = 3
Area of replacement by fibrous tissue Less than 10% grade = 0 10–29% grade = 1 30–59% grade = 2 60% or more grade = 3
In addition to these parameters, a percentage decrease of the relocated intervertebral disc was measured for each sample. Each sample was weighed using digital scales (A&D Electronic Balance, Model EW-300B, A&D Engineering Inc., San Jose, USA) with a resolution of 0.1 g to provide the initial weight. Results of each sample were calculated as the average of the five readings. Variation in weight of the relocated intervertebral disc within each sample was expressed as a percentage decrease compared with the weight of the first disc harvested.
Statistical analysis
All data were expressed as mean ± SD. SPSS 10.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. Statistical significance was evaluated by Tukey’s post hoc analysis. P < 0.05 was regarded as statistically significant.
Results
Weight change
The relocated discs were weighed before and after the surgery. The weight of the located discs in each group decreased postoperatively compared with that before surgery (Table 1). The percentage of weight decrease was more significant in weeks 2 and 4 after surgery in the MK 20-μg group when compared with that of the control group (P < 0.05, 0.01). This suggested that in the MK-treated group, the weight of relocated intervertebral disc decreased more over time than in the control group.
Table 1.
Weight percentage of the relocated discs compared with that before surgery
| Group | Weeks after surgery | |||
|---|---|---|---|---|
| One | Two | Four | Eight | |
| Control group | 95.2 ± 1.8 | 82.5 ± 1.6 | 70.9 ± 1.3 | 34.2 ± 8.5 |
| MK 5-μg group | 94.1 ± 3.8 | 80.6 ± 4.3 | 64.2 ± 6.8 | 24.8 ± 8.1 |
| MK 20-μg group | 91.2 ± 2.3 | 72.6 ± 2.5* | 51.2 ± 6.4** | 14.0 ± 5.0** |
MK midkine
*P<0.05, **P<0.01 (compared with the control group)
Newly formed vessels
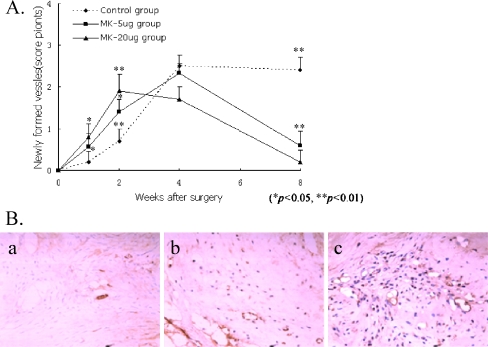
The number of newly formed vessels in the relocated discs increased gradually at first, and then decreased within the four weeks after surgery. In the control group, vessels were formed in the grafted intervertebral disc (Fig. 1A) at two weeks postoperation. The number of newly formed vessels in the MK-treated groups was significantly larger than that in the control group (Fig. 1B, P < 0.01, 0.05). The number in the MK 20-μg group was also larger than that in the MK 5-μg group (Fig. 1B; P < 0.05).
Fig. 1.
A The number of newly formed vessels in the MK-treated groups. The number of newly formed vessels in the MK-treated groups was significantly larger than that in the control group (P < 0.01, 0.05). B Two weeks after surgery (EnVision method ×200). Few newly formed vessels observed in the relocated discs in the control group (a). More newly formed vessels observed in the relocated discs in the MK 5-μg group (b). Many newly formed vessels observed in the relocated discs in the MK 20-μg group (Envision Method ×20) (c)
Inflammatory cells
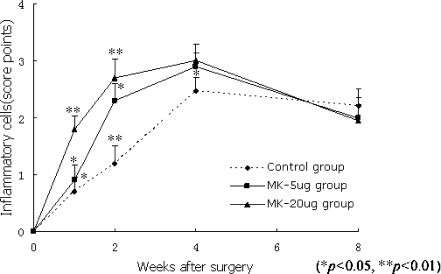
The relocated discs were infiltrated by inflammatory cells including small round cells, macrophages, and fibroblasts after surgery. The number of inflammatory cells in the relocated discs increased gradually at first, and then decreased within the four weeks after surgery. At two weeks postoperation, the number of inflammatory cells in the MK-treated groups was significantly larger than that in the control group (Fig. 1B, parts b and c; P < 0.01, 0.05). The number in the MK 20-μg group was also greater than that in the MK 5-μg group (Fig. 2; P < 0.05).
Fig. 2.
At one and two weeks postoperation, the number of inflammatory cells in the MK-treated groups was significantly larger than that in the control group (P < 0.01, 0.05). The number in the MK 20-μg group was also greater than that in the MK 5-μg group (P < 0.05)
Degradation of the matrix
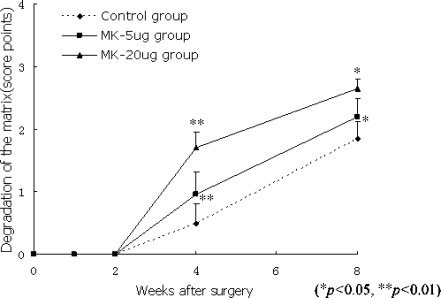
The matrix of the relocated disc showed derangement and loosening of collagen fibres two weeks after surgery. At week 4, fragmentation and disappearance of the matrix were also observed (Fig. 3; P < 0.05). The degradation was more significant in the MK 20-μg group when compared with that of the control group, suggesting a faster degradation of the disc than in the control group.
Fig. 3.
At week 4, fragmentation and disappearance of the matrix were observed (P < 0.05). The degradation was more significant in the MK 20-μg group when compared with that of the control group (P < 0.05)
Replacement by fibrous tissue
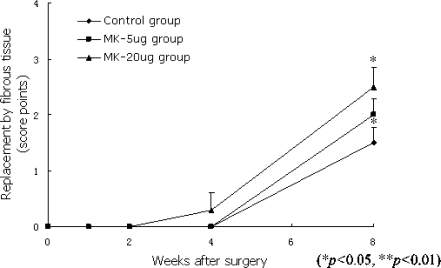
The number of fibroblasts increased surrounding the discs after surgery. The relocated intervertebral disc started to be replaced by the fibrous tissue at four weeks postoperation. Eight weeks after surgery, the replacement of the relocated intervertebral discs by fibrous tissues in the MK 20-μg group was more significant when compared with that in the control group (P < 0.05; Fig. 4).
Fig. 4.
Eight weeks after surgery, the replacement of the relocated intervertebral discs by fibrous tissues in the MK 20-μg group was significant when compared with that in the control group (P < 0.05)
Discussion
Imaging techniques, such as magnetic resonance imaging and computed tomography, have revealed the gradual disappearance or decrease in the size of herniated intervertebral discs, which is associated with improvement of clinical symptoms. The regression is more significant in epidurally displaced (extruded and sequestrated) herniated disc [17]. It has been reported that newly formed vessels and macrophages play a role in herniated disc resorption [18]. Various factors such as MK, bFGF and other cytokines have been identified as positive regulators of angiogenesis [11–14] and may be important in the mechanism of herniated intervertebral disc resorption.
It is generally believed that spontaneous resorption of herniated disc is induced by inflammation because the immune system recognises the disc as a foreign body. However, most of the infiltrating cells surrounding the herniated discs were macrophages instead of lymphocytes. It is suggested that the inflammation is induced not only by an autoimmune response, but also by a wound-healing process [7, 18]. Angiogenesis is a critical component in the wound-healing process as well as a necessary procedure leading to new blood vessel formation [19]. MK, a heparin-binding growth/differentiation factor, appears to play an important role in angiogenesis and wound healing [20, 21]. It was reported that an angiogenic role of MK enhanced tumour growth in MCF-7 breast carcinoma cells due to MK high expression correlated with increased vascular density and endothelial proliferation [14]. Meanwhile, a previous study by Dai et al. showed that angiogenesis was suppressed by antisense oligonucleotides targeting MK both in HepG2-induced chick chorioallantoic membrane and in situ human hepatocellular carcinoma [22]. Exposure to blood circulation is essential for spontaneous resorption of herniated discs, and we speculate that MK enhances neovascularisation and increases the inflammatory cell supply route. The migration of leukocytes into inflammatory foci is governed by cell-specific chemoattractants called chemokines. Recently, Takada et al. found that MK is a chemotactic factor for neutrophils [14]. Thus, MK release might also assist infiltration of inflammatory cells.
Under physiological conditions, the vasculature is quiescent in the normal adult. Only 0.01% of endothelial cells in a normal adult vessel are in the cell division cycle at any given time [23]. However, in response to an appropriate angiogenic stimulus, the endothelial cells can become activated to grow new vessels. In this paper, we first analysed the role of MK on angiogenesis in herniated disc resorption. Our results suggest that MK could promote the angiogenesis and, in turn, accelerate the resorption of the discs.
However, as is known, sequestrated discs comprise a small percent of all types of disc herniation. The term ‘disc herniation’ is a broad and general term that includes three specific types of disc lesions: protrusion, extrusion and sequestration. Our further studies will explore the possible role of MK in these kinds of disc herniation and investigate the correct dose of MK in different kinds of disc herniation.
For the first time, we have directly addressed the potential therapeutic role of MK on herniated vertebral discs, and suggested that epidural injection of MK has the possibility of being developed as an effective method for treatment without surgical intervention.
Acknowledgements
This work was supported by the Zhejiang Province Medicine and Sanitation Research Foundation (2004B185) and the National Natural Science Foundation of China (30772534).
Contributor Information
Guoshun Zhou, Email: guoshun_zhou@yahoo.cn.
Licheng Dai, Phone: +86-572-2033020, FAX: +86-572-2033020, Email: dlc@hzhospital.com.
References
- 1.Saal JA. Natural history and nonoperative treatment of lumbar disc herniation. Spine. 1996;21(24 Suppl):2S–9S. doi: 10.1097/00007632-199612151-00002. [DOI] [PubMed] [Google Scholar]
- 2.Haro H, Domoto T, Maekawa S, et al. Resorption of thoracic disc herniation. Report of 2 cases. J Neurosurg Spine. 2008;8(3):300–304. doi: 10.3171/SPI/2008/8/3/300. [DOI] [PubMed] [Google Scholar]
- 3.Basaran A, Topatan S. Spinal balloon nucleoplasty: a hypothetical minimally invasive treatment for herniated nucleus pulposus. Med Hypotheses. 2008;70(6):1201–1206. doi: 10.1016/j.mehy.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Delauche-Cavallier MC, Budet C, Laredo JD, et al. Lumbar disc herniation. Computed tomography scan changes after conservative treatment of nerve root compression. Spine. 1992;17(8):927–933. doi: 10.1097/00007632-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita K, Hiroshima K, Kurata A. Gadolinium-DTPA—enhanced magnetic resonance imaging of a sequestered lumbar intervertebral disc and its correlation with pathologic findings. Spine. 1994;19(4):479–482. doi: 10.1097/00007632-199402001-00021. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Yamada M, Ikuta F, Fukuda T, et al. Histologic evidence of absorption of sequestration-type herniated disc. Spine. 1996;21(2):230–234. doi: 10.1097/00007632-199601150-00014. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi S, Yamashita T, Yokogushi K, et al. Immunophenotypic analysis of the inflammatory infiltrates in herniated intervertebral discs. Spine. 2001;26(11):1209–1214. doi: 10.1097/00007632-200106010-00008. [DOI] [PubMed] [Google Scholar]
- 8.Davidson JM, Klagsbrun M, Hill KE, et al. Accelerated wound repair, cell proliferation, and collagen accumulation are produced by a cartilage-derived growth factor. J Cell Biol. 1985;100(4):1219–1227. doi: 10.1083/jcb.100.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebda PA, Klingbeil CK, Abraham JA, et al. Basic fibroblast growth factor stimulation of epidermal wound healing in pigs. J Invest Dermatol. 1990;95(6):626–631. doi: 10.1111/1523-1747.ep12513528. [DOI] [PubMed] [Google Scholar]
- 10.Szabo S, Vattay P, Scarbrough E, et al. Role of vascular factors, including angiogenesis, in the mechanisms of action of sucralfate. Am J Med. 1991;91(2A):158S–160S. doi: 10.1016/0002-9343(91)90469-E. [DOI] [PubMed] [Google Scholar]
- 11.Goldfarb M. The fibroblast growth factor family. Cell Growth Differ. 1990;1(9):439–445. [PubMed] [Google Scholar]
- 12.Ferrara N, Houck K, Jakeman L, et al. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13(1):18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36(2):127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 14.Choudhuri R, Zhang HT, Donnini S, et al. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 1997;57(9):1814–1819. [PubMed] [Google Scholar]
- 15.Kadomatsu K, Tomomura M, Muramatsu T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem Biophys Res Commun. 1988;151(3):1312–1318. doi: 10.1016/S0006-291X(88)80505-9. [DOI] [PubMed] [Google Scholar]
- 16.Mishima K, Asai A, Kadomatsu K, et al. Increased expression of midkine during the progression of human astrocytomas. Neurosci Lett. 1997;233(1):29–32. doi: 10.1016/S0304-3940(97)00619-8. [DOI] [PubMed] [Google Scholar]
- 17.Komori H, Shinomiya K, Nakai O, et al. The natural history of herniated nucleus pulposus with radiculopathy. Spine. 1996;21(2):225–229. doi: 10.1097/00007632-199601150-00013. [DOI] [PubMed] [Google Scholar]
- 18.Minamide A, Hashizume H, Yoshida M, et al. Effects of basic fibroblast growth factor on spontaneous resorption of herniated intervertebral discs. An experimental study in the rabbit. Spine. 1999;24(10):940–945. doi: 10.1097/00007632-199905150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Hunt TK, Knighton DR, Thakral KK, et al. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984;96(1):48–54. [PubMed] [Google Scholar]
- 20.Yoshida Y, Goto M, Tsutsui J, et al. Midkine is present in the early stage of cerebral infarct. Brain Res Dev Brain Res. 1995;85(1):25–30. doi: 10.1016/0165-3806(94)00183-Z. [DOI] [PubMed] [Google Scholar]
- 21.Unoki K, Okubo A, Arimura H, et al. Beneficial effect of a retinoic acid responsive gene product, midkine, on constant light-induced retinal damage in albino mice. Nippon Ganka Gakkai Zasshi. 1995;99(6):636–641. [PubMed] [Google Scholar]
- 22.Dai LC, Wang X, Yao X, et al. Antisense oligonucleotides targeting midkine inhibit tumor growth in an in situ human hepatocellular carcinoma model. Acta Pharmacol Sin. 2007;28(3):453–458. doi: 10.1111/j.1745-7254.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 23.Hobson B, Denekamp J, et al. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984;49(4):405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]