Abstract
We retrospectively reviewed the medical charts of 210 consecutive patients treated for spinal infection in Tottori University Hospital in Japan between 1956 and 2005. Until 1995, spinal infection was under control in this series; however, during the last decade, it has been on the rise. Male predominance had been gradually progressing, and 69% of the patients were male during the last decade. Patients with spinal infection were aging, and the ratio of immunocompromised hosts dramatically increased to 53%. Until 1995, the percentage of patients with tuberculous spondylitis had been declining; however, the incidence has been on the rise during the last ten years. The organism was detected in 64% of patients treated between 1996 and 2005, of which Staphylococcus aureus was detected in 49%. Moreover, methicillin-resistant S. aureus was detected in 61% of patients with S. aureus. There were no immigrants or cases with human immunodeficiency virus in this series.
Résumé
Nous avons examiné de façon rétrospective le dossier de 210 patients consécutifs traités pour une infection vertébrale à l’hôpital universitaire de Tottori au Japon, entre 1956 et 2005. En 1995, les infections rachidiennes dans cette série étaient surveillées, cependant, durant la dernière décade, celles-ci ont augmenté, avec une prédominance du sexe masculin et ce de façon progressive (69% des patients durant ces 10 dernières années). Les patients présentant une infection rachidienne étaient des patients âgés avec diminution des défenses immunitaires. Jusqu’en 1995, le pourcentage des patients présentant une spondylite tuberculeuse a diminué cependant que leur incidence a été en hausse durant ces 10 dernières années. Les micro organismes détectés chez 64% des patients traités entre 1996 et 2005 étaient des Staphylocoques dorés dans 49% des cas. De plus, les staphylocoques methiiR étaient détectés chez 61% des patients présentant une infection à staphylocoques dorés. Il n’y avait, dans cette série aucun immigrant ni aucun cas de patients atteints par le VIH.
Introduction
Spinal infection is a relatively rare condition and accounts for 2–16.7% of all cases of osteomyelitis [6, 11, 14, 19]. In developed countries, improved public hygiene reduced the incidence of spinal infections in the 1970s; however, several papers [2, 3, 7, 19] have warned that spinal infections have been more frequent recently with the increase of immunocompromised hosts, human immunodeficiency virus (HIV) infection and immigrants from developing countries. These conditions make the treatment of spinal infection difficult.
In 2005, there were 8.8 million new patients with tuberculosis (TB) all over the world, and of these, there were 7.4 million in Asia and sub-Saharan Africa [24]. Approximately 1.6 million patients died of TB. In Japan, there were 26,384 new patients with TB (20.6 per 100,000 people) in 2006 [22]. The incidence in Japan, which was 524.2, 172.3, 60.7, 41.9, and 31.0 per 100,000 people in 1960, 1970, 1980, 1990 and 2000, respectively [22], has rapidly decreased over the past 50 years. The number of patients with TB spondylitis in Japan also declined to 233 in 2005 from 734 in 1978 and 276 in 2001 [10, 21].
Even in developed countries, the present conditions and trends in infectious disease differ from country to country. For example, the incidence of methicillin-resistant Staphylococcus aureus (MRSA) depends on the quality of infection control practice [18]. Moreover, the incidence of TB is much higher in Japan than in other developed countries [22]. It was 4.5 times higher than that in Canada, 4.4 times higher than in the USA, 4.0 times higher than in Australia, 2.9 times higher than in Germany and in Italy, 1.6 times higher than in France and 1.5 times higher than in the UK [22, 24].
To our knowledge, there are no previous reports of long-term studies of consecutive patients with spinal infection that have described the trends of this disease. Moreover, there are no previous reports describing recent trends in spinal infections in Japan. This study investigated recent trends in spinal infection, based on the medical charts of the patients in our hospital treated during the past 50 years.
Materials and methods
This study included patients from Tottori University Hospital, a 697-bed hospital providing primary, secondary and tertiary medical care in western Tottori Prefecture, Japan. This hospital covers an area with a population of approximately 300,000, which has changed little since 1945.
After searching the database of inpatients of our hospital, we retrospectively reviewed the charts of 210 consecutive patients with spinal infection between 1956 and 2005. Patients with surgical site infection or iatrogenic infection, such as associated with discography or epidural block, were excluded. There were 116 men and 94 women, with an average age of 51 years (range: 3–86). The patients were divided into five groups: those admitted to our hospital between 1956 and 1965 (group 1), between 1966 and 1975 (group 2), between 1976 and 1985 (group 3), between 1986 and 1995 (group 4) and between 1996 and 2005 (group 5). We evaluated changes in the numbers of patients, gender, age, involved levels, immunocompromised hosts and organisms among the five groups.
Diagnosis
Patients with severe neck, back or lumbar back pain were suspected of having spinal infection if their erythrocyte sedimentation rate, leukocyte count and/or C-reactive protein (CRP) was increased. If an organism was detected from the specimen obtained by needle biopsy or during surgery, it was easy to diagnose a patient as having spinal infection. Otherwise, a patient was diagnosed as having spinal infection, based on his/her clinical findings, laboratory data and imaging studies. When end plate erosion, destructive change or sclerotic reactive bone formation was observed on plain films, a patient was diagnosed as having pyogenic spondylitis [20]. Computed tomograms (CT) or magnetic resonance images (MRI) frequently show widening of the retropharyngeal space, enlargement of the paravertebral shadow or changes in the psoas shadow in patients with spinal infection [5]. In patients with TB spondylitis, sclerotic reactive bone formation on plain films is seen much later and is less marked than that in those with pyogenic spondylitis [12]. On MRI, the intervertebral disc often shows a normal height and normal signal in patients with TB spondylitis [5].
Regarding imaging tools for diagnosis in our department, only plain films and tomograms were used until around 1980, and in approximately 1980 and 1995, CT and MRI were available, respectively. Before the introduction of MRI, the initial diagnosis of the involved level was difficult. It takes approximately two weeks to show the reaction to spinal infection on plain film or CT [6, 11]; therefore, specimens from the involved level could not be obtained initially, which forced doctors to use antibiotics before obtaining specimens. For these reasons, the collection of the specimen was not performed for all of the patients in groups 1–4. According to their charts, it was performed only for the patients with surgical indication or fistula. After the mid-1990s when MRI became available, we were able to diagnose the involved level immediately. After 2001, a patient suspected as having spinal infection immediately underwent MRI to determine the involved level, and then specimens were collected using CT-guided needle biopsy or needle biopsy under fluoroscopy in addition to blood collection for culture.
Statistical analysis
Differences in age distribution among the groups were statistically analysed using the Kruskal-Wallis test. The differences in the distribution of gender, immunocompromised hosts and mycobacterial infection were tested using the chi-square test. A probability value of less than 0.05 was considered significant. All statistical analysis was performed using SPSS.
Results
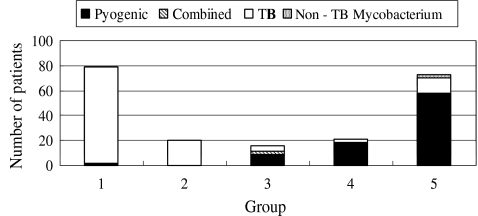
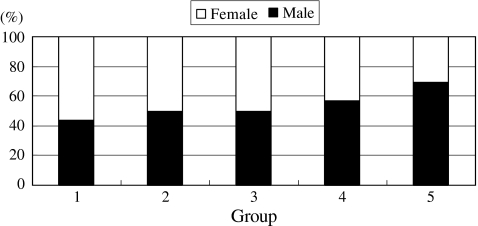
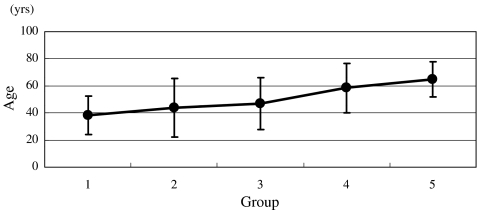
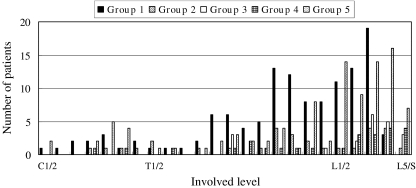
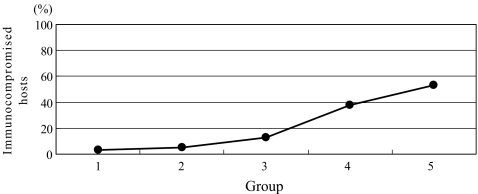
There were 79, 20, 16, 21 and 74 patients in groups 1–5, respectively (Fig. 1). Until 1995, spinal infection had been under control; however, during the last ten years, it has been on the rise. The percentages of male patients were significantly increased (p = 0.043), and 69% (51 patients) of the patients with spinal infection were male between 1996 and 2005 (Fig. 2). The patients with spinal infection became significantly older (p = 0.000) with average ages of 38, 44, 47, 59 and 65 years in groups 1–5, respectively (Fig. 3). The most common levels of spinal infection were between the mid-thoracic and upper lumbar spine in group 1, and between the lower thoracic and lumbosacral spine in group 5 (Fig. 4). The percentages of immunocompromised hosts were 3% (seven patients), 5% (one patient), 13% (two patients), 38% (eight patients) and 53% (39 patients) in groups 1–5, respectively (Fig. 5, Table 1) with a significant difference (p = 0.000).
Fig. 1.
Changes in the numbers of patients with spinal infection. Until 1995, spinal infection was under control; however, during the last 10 years, the incidence has been rising. Furthermore, the percentage of patients with mycobacterial spondylitis had been declining until 1995; however, it has been rising during the last 10 years. TB tuberculosis, combined combined pyogenic and mycobacterial infections
Fig. 2.
Changes in the percentages of male patients. Male predominance has been significantly progressing (p = 0.043), and 69% of patients during the last decade were male
Fig. 3.
Changes in average ages (SD) of the patients with spinal infection. The ages of patients with spinal infection have advanced (p = 0.000)
Fig. 4.
Changes in the involved levels. The most common site of spinal infection was between the mid-thoracic and upper lumbar spine in group 1 and between the lower thoracic and lumbosacral spine in group 5
Fig. 5.
Changes in the percentages of Immunocompromised hosts. Immunocompromised hosts had significantly increased to 53% in 1996–2005 (p = 0.000)
Table 1.
Immunocompromised hosts
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
| Hepatic dysfunction | 2 | 1 | 1 | 3 | 3 |
| Immediate postoperativea | 3 | 1 | 1 | ||
| RA | 1 | 1 | |||
| Respiratory compromise | 1 | ||||
| Cancer | 2 | 7 | |||
| DM | 2 | 15 | |||
| Steroid use | 1 | 8 | |||
| Malignant lymphoma | 3 | ||||
| Renal dysfunction | 2 | ||||
| DIC | 1 |
The numbers indicate the number of the patients. Some patients had two conditions
RA rheumatoid arthritis, DM diabetes mellitus, DIC disseminated intravascular coagulation
aWithin 1 month from the date of operation
The organism was detected in 33% (26 patients), 40% (eight patients), 31% (five patients), 14% (three patients) and 64% (47 patients) in groups 1–5, respectively. In group 5, S. aureus was detected in 49% (23 patients) of the patients in whom an organism was detected (Table 2). Of these, MRSA was detected in 61% (14 patients). MRSA was not detected in groups 1–4. In 36% of the patients in group 5, the organism was not detected mainly because the previous attending doctor or primary care doctor had already administered antibiotics to treat high fever and elevated CRP. The percentages of patients with mycobacterial spondylitis comprised 97% (77 patients), 100% (20 patients), 31% (five patients), 14% (three patients) and 21% (16 patients) of groups 1–5, respectively (Fig. 1) with a significant difference (p = 0.000). After elimination of the cases with a negative bacteriological result, the percentage of patients with mycobacterial spondylitis was 57.7, 50.0, 60.0, 66.7 and 23.4% in groups 1–5, respectively. In groups 1–4, all patients with mycobacterial spondylitis were infected with Mycobacterium tuberculosis, and in group 5, 17.0, 4.3 and 2.1% of the patients with a positive bacteriological result were infected with M. tuberculosis, M. intracellulare and M. avium, respectively.s
Table 2.
The causative organisms
| Causative organism | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|
| Staphylococcus aureus | 9 | 3 | 1 | 0 | 23 |
| Staphylococcus epidermidis | 0 | 0 | 0 | 0 | 2 |
| Escherichia coli | 0 | 0 | 0 | 0 | 3 |
| Pseudomonas aeruginosa | 1 | 0 | 1 | 0 | 1 |
| Enterobacter cloacae | 0 | 0 | 0 | 0 | 1 |
| Streptococcus viridans | 0 | 0 | 0 | 0 | 1 |
| Streptococcus hemolyticus | 1 | 0 | 0 | 0 | 0 |
| Streptococcus spp. | 0 | 1 | 0 | 1 | 1 |
| Enterococcus faecalis | 0 | 0 | 0 | 0 | 1 |
| Enterococcus spp. | 0 | 0 | 0 | 0 | 1 |
| Klebsiella pneumoniae | 0 | 0 | 1 | 0 | 1 |
| Citrobacter amalonaticus | 0 | 0 | 0 | 0 | 1 |
| Bacteroides spp. | 0 | 0 | 0 | 0 | 1 |
| Alcaligenes spp. | 0 | 0 | 1 | 0 | 0 |
| Mycobacterium tuberculosis | 15 | 4 | 3 | 2 | 7 |
| Mycobacterium intracellulare | 0 | 0 | 0 | 0 | 2 |
| Mycobacterium avium | 0 | 0 | 0 | 0 | 1 |
The numbers indicate the number of the patients in whom the organisms were detected
There were no HIV-positive patients or intravenous drug abusers in this series, and all patients were Japanese.
Discussion
Our study has two limitations. Firstly, we retrospectively reviewed only patients admitted to our hospital. Our data reflected the history and incidence in only a small area in Japan. Moreover, there is a possibility that not all of the patients with spinal infection occurring in this area consulted or were referred to our hospital. We could not review the past situation before 1990; however, recently, almost all patients with spinal infection are referred to our hospital. Our data may not reflect the true trends; however, we consider it important to investigate the history and incidence of spinal infection in an area over a prolonged period such as 50 years. Secondly, there is a possibility that some patients were diagnosed incorrectly. If the organism could not been detected, a patient was diagnosed as having spinal infection, based only on his/her clinical findings, laboratory test and imaging studies. In addition, there is a possibility that early doctors differentiated wrongly between pyogenic and mycobacterial spondylitis for some patients with a negative bacteriological result.
The incidence of patients with spinal infection has been reported to have increased [2, 3, 7]. This is probably a result of an aging population, easy access to better diagnostic methods including MRI and an increase in the prevalence of immunocompromised hosts [2, 17]. In this study, spinal infection had been decreasing until 1995; however, during the last ten years, it has been on the rise. Also, the ages of patients with spinal infection have gradually risen, and the percentage of immunocompromised hosts dramatically increased to over 50% during the past ten years.
The percentage of male patients in this study was 69%, which was similar to that in previous studies reporting a range between 58 and 91% [2, 9, 16]. Krogsgaard and colleagues [17] reported that male patients were dominant, 58% of the patients in 1991–1993, whereas the incidence was not significantly different between male and female patients in 1978–1982. Jacofsky and Currier [14] indicated that male predominance was due to the large number of intravenous drug abusers. In our study in which there were no intravenous drug abusers, we could not identify a reason for male predominance.
Spine infection most commonly occurs in the lumbar spine [13, 20]. In a literature review [20], the lumbar spine was involved in 48% of 294 cases, thoracic in 35% and cervical in 6.5%. Hadjipavlou et al. [13] reported their series covering 1986–1996 and stated that the locations of spondylodiscitis were the lumbar spine in 56.1%, thoracic in 35.6% and cervical in 10.2%. In this study, the most commonly involved levels of spinal infection in 1996–2005 were similar to those in previous reports. However, the percentage of cases with lumbar spine involvement was higher than that in other reports and increased to 81% in 1996–2005 from 58% in 1956–1965.
In European countries, the incidence of TB increased recently because of the HIV epidemic and increased immigration from countries with a high TB prevalence [4]. The number of HIV-positive people in Japan was reported to be 8,344 at the end of 2006 [1]. Its prevalence (0.0065%) was much lower than that in Western and Central Europe (0.3%) and in North America (0.8%) [23]. In Japan, there are fewer immigrants than there are in other developed countries; however, the percentage of immigrants among TB patients is increasing [22].
The incidence of MRSA infections has been reported to be increasing [15]. MRSA accounted for 10–30% of all S. aureus isolates from patients with spinal infection [13]. In this study, MRSA was detected in 61% of patients with S. aureus. MRSA osteomyelitis was a well-recognised late sequel of inadequately treated bacteraemia [8]. The reasons for the higher proportion compared to that in other reports remain unclear; however, it might be ascribed to inadequate treatment of a previous infectious state. Moreover, there was a greater variety of organisms detected in 1996–2005 than in other decades (Table 2). To select appropriate antibiotics, organism detection is very important; therefore, specimens should be collected from the infection focus using CT-guided needle biopsy or needle biopsy under fluoroscopy in addition to blood collection for culture prior to administration of antibiotics.
In conclusion, until the mid-1990s, spinal infection was under control in this series; however, during the last decade, the incidence has been rising. We also demonstrated that the mean age of patients with spinal infection is rising and that immunocompromised hosts have dramatically increased.
Acknowledgement
This work was presented in part at the 36th annual meeting of the Japanese Society for Spine Surgery and Related Research, Kanazawa, Japan, 26–27 April 2007, and EuroSpine 2007, Brussels, Belgium, 3–6 October 2007. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1.AIDS Prevention Information Network. AIDS epidemic update in Japan: 2006 (in Japanese). Available at: http://api-net.jfap.or.jp/htmls/frameset-03.html Accessed 11 Dec 2008
- 2.Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990–95. Scand J Infect Dis. 2001;33:527–532. doi: 10.1080/00365540110026566. [DOI] [PubMed] [Google Scholar]
- 3.Collert S. Osteomyelitis of the spine. Acta Orthop Scand. 1977;48:283–290. doi: 10.3109/17453677708988770. [DOI] [PubMed] [Google Scholar]
- 4.Colmenero JD, Jiménez-Mejías ME, Reguera JM, et al. Tuberculous vertebral osteomyelitis in the new millennium: still a diagnostic and therapeutic challenge. Eur J Clin Microbiol Infect Dis. 2004;23:477–483. doi: 10.1007/s10096-004-1148-y. [DOI] [PubMed] [Google Scholar]
- 5.Currier BL, Kim CW, Eismont FJ. Infections of the spine. In: Herkowitz HN, Garfin SR, Eismont FJ, Bell GR, Balderston RA, editors. Rothman-Simeone the spine. 5. Philadelphia: Saunders-Elsevier; 2006. pp. 1265–1316. [Google Scholar]
- 6.Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br. 1979;61:47–55. doi: 10.1302/0301-620X.61B1.370121. [DOI] [PubMed] [Google Scholar]
- 7.Espersen F, Frimodt-Møller N, Thamdrup Rosdahl V, et al. Changing pattern of bone and joint infections due to Staphylococcus aureus: study of cases of bacteremia in Denmark, 1959–1988. Rev Infect Dis. 1991;13:347–358. doi: 10.1093/clinids/13.3.347. [DOI] [PubMed] [Google Scholar]
- 8.Fowler VG, Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27:478–486. doi: 10.1086/514686. [DOI] [PubMed] [Google Scholar]
- 9.Friedman JA, Maher CO, Quast LM, et al. Spontaneous disc space infections in adults. Surg Neurol. 2002;57:81–86. doi: 10.1016/S0090-3019(01)00681-4. [DOI] [PubMed] [Google Scholar]
- 10.Fujita M, Saito M, Niiro N, et al. Bone and joint tuberculosis (in Japanese) Seikeigeka. 2004;55:1068–1073. [Google Scholar]
- 11.Garcia A, Grantham SA. Hematogenous pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1960;42-A:429–436. [PubMed] [Google Scholar]
- 12.Guri JP. Pyogenic osteomyelitis of the spine. Differential diagnosis through clinical and roentgenographic observations. J Bone Joint Surg. 1946;28:29–39. [PubMed] [Google Scholar]
- 13.Hadjipavlou AG, Mader JT, Necessary JT, et al. Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25:1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Jacofsky D, Currier BL. Infections of the spine. In: Fardon DF, Garfin SR, Abitbol JJ, Boden SD, Herkowitz HN, Mayer TG, editors. Orthopaedic knowledge update spine 2. Rosemont: American Academy of Orthopaedic Surgeons; 2002. pp. 431–442. [Google Scholar]
- 15.Johnson AP, Pearson A, Duckworth G. Surveillance and epidemiology of MRSA bacteraemia in the UK. J Antimicrob Chemother. 2005;56:455–462. doi: 10.1093/jac/dki266. [DOI] [PubMed] [Google Scholar]
- 16.Kemp HBS, Jackson JW, Jeremiah JD, et al. Pyogenic infections occurring primarily in intervertebral discs. J Bone Joint Surg [Br] 1973;55:698–714. [PubMed] [Google Scholar]
- 17.Krogsgaard MR, Wagn P, Bengtsson J. Epidemiology of acute vertebral osteomyelitis in Denmark: 137 cases in Denmark 1978–1982, compared to cases reported to the National Patient Register 1991–1993. Acta Orthop Scand. 1998;69:513–517. doi: 10.3109/17453679808997789. [DOI] [PubMed] [Google Scholar]
- 18.Myatt R, Langley S. Changes in infection control practice to reduce MRSA infection. Br J Nurs. 2003;12:675–681. doi: 10.12968/bjon.2003.12.11.11317. [DOI] [PubMed] [Google Scholar]
- 19.Ross PM, Fleming JL. Vertebral body osteomyelitis: spectrum and natural history. A retrospective analysis of 37 cases. Clin Orthop. 1976;118:190–198. [PubMed] [Google Scholar]
- 20.Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis. 1979;1:754–776. doi: 10.1093/clinids/1.5.754. [DOI] [PubMed] [Google Scholar]
- 21.Statistics of TB 2006 (in Japanese) Tokyo: Japan Anti-Tuberculosis Association; 2006. [Google Scholar]
- 22.The Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association. Statistics of TB 2007. Available at: http://www.jata.or.jp/rit/re/epro1_top.htm Accessed 11 Dec 2008
- 23.Uniting the World against AIDS/World Health Organization. AIDS epidemic update: December 2006. Available at: http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2006/Default.asp Accessed 11 Dec 2008
- 24.World Health Organization. Global tuberculosis control—surveillance, planning, financing. WHO Report 2007. Available at: http://www.who.int/tb/publications/global_report/2007/en/index.html Accessed 11 Dec 2008