Abstract
The bone–screw interface has been indicated as the weak link in pedicle screw spine fixation. Bisphosphonate treatment may have the effect of improving bone–screw interface fixation in spine fusion by inhibiting bone resorption. An experimental study was conducted using a porcine model to evaluate the influence of alendronate treatment on bone–pedicle screw interface fixation. Eleven pigs in the treatment group received alendronate 10 mg/day orally for three months postoperatively. The other 11 pigs served as a control group. Posterior lateral fusion with the CD Horizon pedicle screw system was performed with autograft on the lumbar spine on all animals. Biomechanical torsion test and histomorphometric parameters of screw fixation were evaluated three months after the operation. The maximum torque and initial angular stiffness of the treatment group was higher than that of the control group, but there was no statistical significance. The bone–screw contact surface was 23.3 ± 10% for the treatment group and 9.8 ± 5.9% for the control group (P < 0.01). This study indicated that alendronate treatment increased bone purchase of stainless steel screw surfaces.
Introduction
Failure of pedicle screw internal fixation is one of the important factors underlying the nonunion of spinal fusion or failure of an attempted deformity correction [18, 20]. The bone–screw interface has been indicated as the weak link in pedicle screw spinal fixation [3, 5]. The anchor strength of the bone–screw interface is dependent on the total area of bone growth on the screw surface [2, 22]. Osteolysis has been found to be a primary reason for implant loosening. Aseptic loosening is the most common cause of orthopaedic implant failure [10, 28].
Bisphosphonate treatment inhibits osteoclast-induced bone resorption and increases bone quality and density. It has been reported that treatment with bisphosphonates can inhibit bone resorption in the area around the implant [9, 23]. Other reports indicate that bisphosphonate has no influence on bone attachment to the implant surface [6, 17]. Bone resorption and a corresponding decrease in bone quality and density can occur after implant surgery [19, 26]. Extensive bone resorption will reduce the holding strength of pedicle screws. Whether bisphosphonate treatment has the effect of improving bone–screw interface fixation in spine fusion has not been studied. The aim of this study was to evaluate the influence of alendronate treatment on the bone–screw interface fixation of the pedicle screw.
Materials and methods
Twenty-two (11 pairs) female landrace pigs, each three months old and at about 50 kg, were randomly separated into a treatment group and a control group. Posterior lateral fusions with instrument fixation were performed on each pig. The pigs in the treatment group received alendronate (Fosamax Merck Sharp & Dohme B.V., Haarlem, Netherlands) 10 mg/day orally between the two meals, which began on the second postoperative day and lasted for three months.
Surgery was performed at the Institute for Experimental Clinical Research, Aarhus University Hospital. The experiment was approved by the State Inspection Board for Animal Experiments (1998–561–67-CBSC 01101). Autologous bone graft material was harvested from the posterior iliac crest on both sides. Two segmental posterior lateral fusions with the CD Horizon pedicle screw system (Medtronic, Sofamor Danek) were performed at L2/L3 and L5/L6. The type of fusion graft materials used at each level were randomly assigned. At one level, 4 g of autograft was applied on one side and 8 g on the other side. Biphasic calcium phosphate (BCP) was used at the other instrumented level to test the bone growth into the bone graft substitute and is described in another study. The operation was performed in a paired manner between the treatment and control groups in accord with the randomisation. The pigs were kept in individual pens that allowed free walking. All the animals were observed for 12 weeks.
The animals were sacrificed at 12 weeks postoperatively. The spinal segment from L1 to S2 was removed en bloc and stripped of soft tissue. The pedicle screws were harvested together with pedicle and half the vertebral body. The bone structures that covered the screw heads were carefully removed without disturbing the biomechanical property of the screws. In order to avoid influence from bone graft material, only the pedicle screws on the 8-g autograft side in each animal were selected and randomised for biomechanical and histological examination. Torsion testing was performed using an MTS mini Bionix 858 testing machine (MTS Corp., Minneapolis, USA). The head of the pedicle screw was attached by specially designed adapters to the upper load cell [2]. Maximum torque (N-mm) and initial angular stiffness (N-mm/°) were calculated.
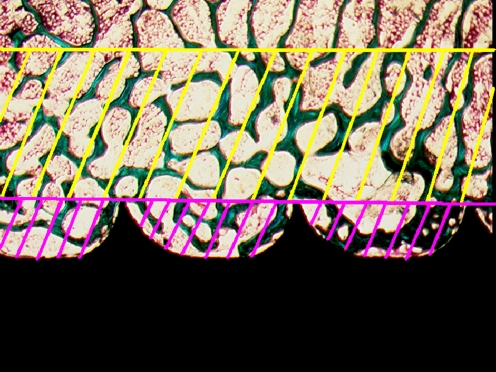
The samples were dehydrated in graded ethanol (70–99%) and embedded in methylmethacrylate. The screw sample was cut longitudinally. Two 150 μm thick sections around the axis of the screw were selected, ground and polished to a thickness of 50 μm using a micro-grinding system (EXAKT-Micro Grinding System, Norderstedt, Germany). The section surface was counterstained with 4% light green for two minutes. Bone growth on a screw surface was defined as bone in direct contact with the screw surface and expressed as a percentage of the total screw surface inside the area examined. Blinded quantitative evaluation of the area of bone growth on the pedicle screw surface was performed using the linear intercept technique and a software program (CAST-Grid, Olympus Denmark A/S, Glostrup, Denmark). Trabecular bone volume was evaluated in the area between the screw threads. A line was drawn between the peaks of threads. Bone volume was determined using the point counting technique expressed as a percentage of total volume of the structure within the area between the threads and enveloped by the line. The bone volume outside this line but in the area within 2 mm was also evaluated and used as the reference for the bone volume evaluation in the area between the threads (Fig. 1). Evaluations of both bone volume and bone growth on the screw surface were performed on the body part of the screw, 12 threads per screw. The test systems for the evaluations were calibrated to have more than 200 intercepts or points counted for each parameter per specimen [8].
Fig. 1.
Bone volume was calculated for both the reference area (area with yellow lines, 2 mm in width) and the area between the screw threads (area with violet lines) (n = 10 in both treatment and control groups)
Comparison of histological data between treatment and control groups was performed using unpaired Students t-test. The biomechanical test data first underwent log-transformation and was then analysed using an unpaired Students t -test. The results are given as mean ± SD. A 5% two-tailed limit of statistical significance was used for all calculations. Statistical analyses were performed using the SPSS 10.0 software package (SPSS Inc., Chicago, IL, USA).
Results
The mean body weight of the animals was 47 ± 1.4 kg before operation and 61 ± 2.4 kg at the time of sacrifice. There was no statistical difference between treatment and control groups. One animal was excluded from the control group after discovery of a local infection. One animal was excluded from the treatment group owing to failure of sample preparation for histological evaluation.
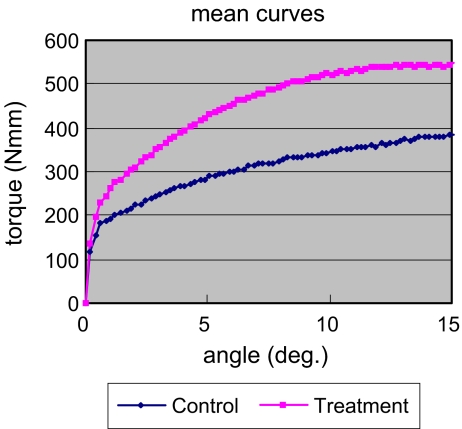
The maximum torque was 594.7 ± 254.2 (N-mm) for the treatment group (n = 11) and 414.6 ± 204.5 (N-mm) for the control group (n = 10) (P = 0.077). The initial angular stiffness was 786.6 ± 360.4 (N-mm/deg) for the treatment group and 696.4 ± 376.9 (N-mm/deg) for the control group (P value not significant [NS]) (Fig. 2).The bone–screw contact surface was 23.3 ± 10% for the treatment group and 9.8 ± 5.9% for the control group (P < 0.01).
Fig. 2.
The mean curves of the angular torque show the treatment group tolerated higher maximum torque (P = 0.077) (n = 10 in the control group; n = 11 in the treatment group)
The mean bone volume between the threads was 52.4 ± 8.3% for the treatment group and 45.4 ± 6.9% for the control group (P = 0.056) (Fig. 3). The mean bone volume of the reference area was 34.3 ± 3.2% for the treatment group and 34.7 ± 3.6% for the control group (NS). A significant difference in bone volume was found between the referenced area and the area between the threads (P < 0.001) in both the treatment and control groups.
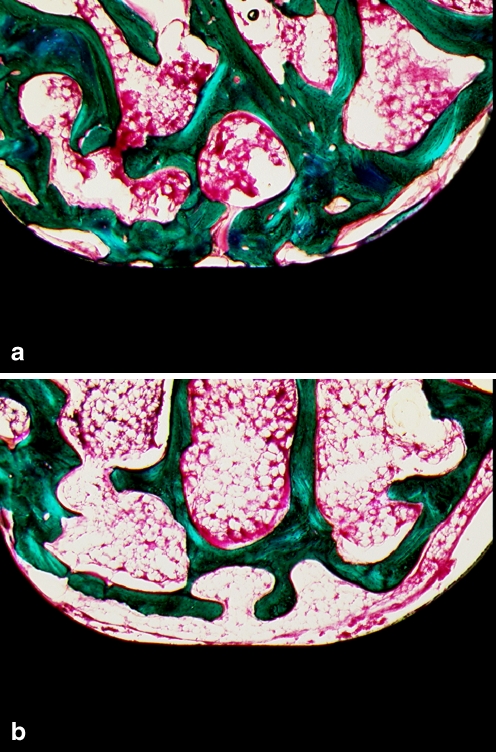
Fig. 3.
Alendronate treatment increased bone growth on the pedicle screw surfaces in the treatment group (a) compared with the control group (b) (original magnification × 100)
Discussion
Bone purchase on pedicle screws is believed to be an important parameter for evaluating the holding strength of implants [11, 12, 30, 32]. In order to improve the holding characteristics of pedicle screws, different tactics have been tried, including alteration in thread design and screw shape as well as surface modification of the screws [4, 13]. However, bone–pedicle screw interface fixation is still not optimal.
Bisphosphonate treatment increases bone quality and density, and it has been widely used clinically for treatment of osteoporosis [25]. Bisphosphonates inhibit bone resorption by being incorporated selectively into osteoclasts and interfering with their resorption activity [15, 21]. The local application of bisphosphonates has been reported to prevent bone resorption following surgery [31]. Bisphosphonate treatment can also prevent peri-implant loosening and local bone loss [23, 27, 29]. Treatment with bisphosphonate shows a tendency to enhance screw-type implant fixation in oophorectomised rats [24]. The use of pharmaceuticals, such as alendronate, to inhibit abnormal bone resorption can be an alternate approach for the optimisation of pedicle screw fixation.
This study was conducted to determine the effect of alendronate on bone attachment to the pedicle screw surfaces. A human pedicle screw device was used in a porcine model under a loaded condition in order to simulate the human surgical situation. Alendronate treatment was administered at a dose of 10 mg daily orally, which is the standard dosage for treatment of osteoporosis in humans. The animal chosen for our research is in relative size and body weight comparable to the human.
Alendronate treatment in this study resulted in increased biomechanical anchor strength at the bone and pedicle screw interface with borderline significance. The control group showed a lower maximum torque and lower initial angular stiffness. Based on the results of histological evaluations, the treatment group had a higher percentage of bone growth on the pedicle screw surface. The bone volume within the area between the screw threads was also high in the alendronate-treatment group (borderline significance). Alendronate treatment for three months enhanced the bone–screw interface fixation in this study.
Screw insertions alter mechanical load and change the mechanical environment for the bone tissue surrounding the screw, which ultimately leads to changes in bone remodelling. The bone structures in contact with the screw surfaces become compacted by screw insertion with micro-fracture and bleeding. The superficial layer of the bone surrounding the screws becomes more active in bone remodelling owing to mechanical compaction and avascularity. Necrotic bone appears to be removed by resorption during bone remodelling. Normal bone remodelling is bone resorption linked to new bone formation by the so-called coupling phenomenon. The most effective approach to prevent implant loosening would be to have a positive balance between bone formation and resorption in the active bone-remodelling time period. Alendronate treatment could protect the bone structure from extra resorption while maintaining the bone volume around the screw surface.
The bone volume in the reference area was not changed by alendronate treatment in this study. The normal trabecular bone remodelling time period is about 150 days in humans. This time period in our porcine model is not known precisely. Alendronate treatment in this study may not be long enough to show treatment effects on normal bone remodelling in the referenced area. The data showed that alendronate had an effect only on the bone structure that was in close contact with a screw. The bone–pedicle screw interface and the area between the screw threads could be the most active bone remodelling area following operation. The higher bone turnover rate might upgrade the alendronate concentration and treatment effects [14].
Lugero et al. reported that screw-type implants yielded the greatest formation of bone in both control and osteoporotic animals [16]. A study of screws in thoracolumbar human cadaveric vertebrae revealed that tapping a pilot hole significantly reduces the pullout strength of pedicle screws [7]. With a tapped pilot hole, about 27% of the structure may be additionally removed and in turn cause an 8% loss of pullout strength in porous material [1]. An untapped screw was used in our study. Screw insertion without pre-tapping is, in principle, a press-fit implant. Trabecular bone became compacted by screw insertion. In both the treatment and control groups, bone volume was significantly higher in the area between the screw threads compared with the reference area. An untapped pedicle screw insertion can compact the cancellous bone surrounding the screw and thereby increase the cancellous bone volume in the area between screw threads. Alendronate treatment could protect the bone structure from resorption, and therefore more trabecular bone could remain around the screw surface. Bisphosphonate treatment may be more effective when used in conjunction with untapped screws. High bone volume is supposed to give better support for bone–implant fixation The untapped screw used in this study is therefore most clinically relevant. Limitations of this study include biomechanical load transfer being different in the quadruped animal.
The results indicated that alendronate treatment of 10 mg/daily orally increased bone purchase of stainless steel screw surfaces in this study.
Acknowledgements
The authors thank Anette Milton and Lisa Feng for technical assistance. This study was partly supported by Gigtforeningen (The Association of Rheumatology) (J.nr.233-845-07.06.01). Medtronic Sofamor Danek provided instrumentation for the first 20 operations.
References
- 1.Chapman JR, Harrington RM, Lee KM. Factors affecting the pullout strength of cancellous bone screws. J Biomech Eng. 1996;118:391–398. doi: 10.1115/1.2796022. [DOI] [PubMed] [Google Scholar]
- 2.Christensen FB, Dalstra M, Sejling F. Titanium-alloy enhances bone-pedicle screw fixation: mechanical and histomorphometrical results of titanium-alloy versus stainless steel. Eur Spine J. 2000;9:97–103. doi: 10.1007/s005860050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davne SH, Myers DL. Complications of lumbar spinal fusion with transpedicular instrumentation. Spine. 1992;17:S184–S189. doi: 10.1097/00007632-199206001-00021. [DOI] [PubMed] [Google Scholar]
- 4.DeCoster TA, Heetderks DB, Downey DJ. Optimizing bone screw pullout force. J Orthop Trauma. 1990;4:169–174. doi: 10.1097/00005131-199004020-00012. [DOI] [PubMed] [Google Scholar]
- 5.Dickman CA, Fessler RG, MacMillan M. Transpedicular screw-rod fixation of the lumbar spine: operative technique and outcome in 104 cases. J Neurosurg. 1992;77:860–870. doi: 10.3171/jns.1992.77.6.0860. [DOI] [PubMed] [Google Scholar]
- 6.Frenkel SR, Jaffe WL, Valle CD, et al. The effect of alendronate (Fosamax) and implant surface on bone integration and remodeling in a canine model. J Biomed Mater Res. 2001;58:645–650. doi: 10.1002/jbm.1064. [DOI] [PubMed] [Google Scholar]
- 7.George DC, Krag MH, Johnson CC. Hole preparation techniques for transpedicle screws. Effect on pull-out strength from human cadaveric vertebrae. Spine. 1991;16:181–184. [PubMed] [Google Scholar]
- 8.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 9.Hilding M, Ryd L, Toksvig-Larsen S. Clodronate prevents prosthetic migration: a randomized radiostereometric study of 50 total knee patients. Acta Orthop Scand. 2000;71:553–557. doi: 10.1080/000164700317362163. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs JJ, Sumner DR, Galante JO. Mechanisms of bone loss associated with total hip replacement. Orthop Clin North Am. 1993;24:583–590. [PubMed] [Google Scholar]
- 11.Krag MH. Spinal instrumentation: biomechanics of transpedicle spinal fixation. In: Weinstein JN, Wiesel SW, editors. In the lumbar spine. Philadelphia: W.B. Saunders; 1990. pp. 916–940. [Google Scholar]
- 12.Krag MH, Beynnon BD, Pope MH. Depth of insertion of transpedicular vertebral screws into human vertebrae: effect upon screw-vertebra interface strength. J Spinal Disord. 1988;1:287–294. doi: 10.1097/00002517-198800140-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kwok AW, Finkelstein JA, Woodside T. Insertional torque and pull-out strengths of conical and cylindrical pedicle screws in cadaveric bone. Spine. 1996;21:2429–2434. doi: 10.1097/00007632-199611010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Mori S, Kaji Y. Concentration of bisphosphonate (incadronate) in callus area and its effects on fracture healing in rats. J Bone Miner Res. 2000;15:2042–2051. doi: 10.1359/jbmr.2000.15.10.2042. [DOI] [PubMed] [Google Scholar]
- 15.Luckman SP, Hughes DE, Coxon FP. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 16.Lugero GG, Falco Caparbo V, Guzzo ML. Histomorphometric evaluation of titanium implants in osteoporotic rabbits. Implant Dent. 2000;9:303–309. doi: 10.1097/00008505-200009040-00005. [DOI] [PubMed] [Google Scholar]
- 17.Mochida Y, Bauer TW, Akisue T. Alendronate does not inhibit early bone apposition to hydroxyapatite-coated total joint implants: a preliminary study. J Bone Joint Surg. 2002;84A:226–235. doi: 10.2106/00004623-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Ohlin A, Karlsson M, Duppe H. Complications after transpedicular stabilization of the spine. A survivorship analysis of 163 cases. Spine. 1994;19:2774–2779. doi: 10.1097/00007632-199412150-00007. [DOI] [PubMed] [Google Scholar]
- 19.Petersen MM, Lauritzen JB, Pedersen JG. Decreased bone density of the distal femur after uncemented knee arthroplasty. A 1-year follow-up of 29 knees. Acta Orthop Scand. 1996;67:339–344. doi: 10.3109/17453679609002327. [DOI] [PubMed] [Google Scholar]
- 20.Pihlajamaki H, Myllynen P, Bostman O. Complications of transpedicular lumbosacral fixation for non-traumatic disorders. J Bone Joint Surg. 1997;Br 79:183–189. doi: 10.1302/0301-620X.79B2.7224. [DOI] [PubMed] [Google Scholar]
- 21.Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/S8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 22.Sanden B, Olerud C, Johansson C. Improved bone-screw interface with hydroxyapatite coating: an in vivo study of loaded pedicle screws in sheep. Spine. 2001;26:2673–2678. doi: 10.1097/00007632-200112150-00008. [DOI] [PubMed] [Google Scholar]
- 23.Soininvaara TA, Jurvelin JS, Miettinen HJ. Effect of alendronate on periprosthetic bone loss after total knee arthroplasty: a one-year, randomized, controlled trial of 19 patients. Calcif Tissue Int. 2002;71:472–477. doi: 10.1007/s00223-002-1022-9. [DOI] [PubMed] [Google Scholar]
- 24.Tokugawa Y, Shirota T, Ohno K. Effects of bisphosphonate on bone reaction after placement of titanium implants in tibiae of ovariectomized rats. Int J Oral Maxillofac Implants. 2003;18:66–74. [PubMed] [Google Scholar]
- 25.Tonino RP, Meunier PJ, Emkey R, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab. 2000;85:3109–3115. doi: 10.1210/jc.85.9.3109. [DOI] [PubMed] [Google Scholar]
- 26.Venesmaa PK, Kroger HP, Jurvelin JS. Periprosthetic bone loss after cemented total hip arthroplasty: a prospective 5-year dual energy radiographic absorptiometry study of 15 patients. Acta Orthop Scand. 2003;74:31–36. doi: 10.1080/00016470310013617. [DOI] [PubMed] [Google Scholar]
- 27.Venesmaa PK, Kroger HP, Miettinen HJ. Alendronate reduces periprosthetic bone loss after uncemented primary total hip arthroplasty: a prospective randomized study. J Bone Miner Res. 2001;16:2126–2131. doi: 10.1359/jbmr.2001.16.11.2126. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson JM, Hamer AJ, Rogers A. Bone mineral density and biochemical markers of bone turnover in aseptic loosening after total hip arthroplasty. J Orthop Res. 2003;21:691–696. doi: 10.1016/S0736-0266(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson JM, Stockley I, Peel NF, et al. Effect of pamidronate in preventing local bone loss after total hip arthroplasty: a randomized, double-blind, controlled trial. J Bone Miner Res. 2001;16:556–564. doi: 10.1359/jbmr.2001.16.3.556. [DOI] [PubMed] [Google Scholar]
- 30.Wittenberg RH, Shea M, Swartz DE. Importance of bone mineral density in instrumented spine fusions. Spine. 1991;16:647–652. doi: 10.1097/00007632-199106000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Yaffe A, Iztkovich M, Earon Y. Local delivery of an amino bisphosphonate prevents the resorptive phase of alveolar bone following mucoperiosteal flap surgery in rats. J Periodontol. 1997;68:884–889. doi: 10.1902/jop.1997.68.9.884. [DOI] [PubMed] [Google Scholar]
- 32.Zindrick MR, Wiltse LL, Widell EH et al (1986) A biomechanical study of intrapeduncular screw fixation in the lumbosacral spine. Clin Orthop 99–112 [PubMed]