Abstract
The septohippocampal pathway, which is mostly comprised of cholinergic and GABAergic projections between the medial septum/diagonal band (MS/DB) and the hippocampus, has an established role in learning, memory and disorders of cognition. In Wernicke-Korsakoff’s syndrome (WKS) and the animal model of the disorder, pyrithiamine-induced thiamine deficiency (PTD), there is both diencephalic damage and basal forebrain cell loss that could contribute to the amnesic state. In the current experiment, we used the PTD animal model to access both cholinergic (choline acetyltransferase [ChAT] immunopositive) and GABAergic (parvalbumin [PV]; calbindin [CaBP]) neuronal loss in the MS/DB in relationship to midline-thalamic pathology. In addition, to gain an understanding about the role of such neuropathology in behavioral dysfunction, animals were tested on a non-rewarded spontaneous alternation task and behavioral performance was correlated to neuropathology. Unbiased stereological assessment of neuronal populations revealed that ChAT-positive neurons were significantly reduced in PTD rats, relative to control pair-fed rats, and thalamic mass and behavioral performance correlated with ChAT neuronal estimates. In contrast, both the PV- and CaBP-positive neurons in the MS/DB were not affected by PTD treatment. These results support an interactive role of both thalamic pathology and cholinergic cell loss in diencephalic amnesia.
Keywords: Thalamus, Septohippocampal pathway, Acetylcholine, GABA, Spontaneous alternation, Rat
Introduction
Basal forebrain damage contributes to memory problems in a number of disorders including Alzheimer’s disease (Whitehouse et al., 1982; Schliebs, 2005) and diencephalic amnesia associated with thiamine deficiency (Wernicke Korsakoff syndrome [WKS] (Schliebs and Arendt, 2006; Savage et al., 2007). The basal forebrain region includes a number of groups of cholinergic neurons (medial septal area, diagonal vertical bands, and nucleus basalis) that project to the hippocampus, amygdala and cortex. The medial septum/diagonal band (MS/DB) complex located in the forebrain is composed of cholinergic and GABAergic neurons that project to the hippocampal formation (Mesulam et al., 1983; Frotscher and Leranth, 1985; Wainer et al., 1985). Lesions of the septal region that disrupt both cell populations—but not either alone--impair spatial memory and also alter theta rhythm in the hippocampus (Pang et al., 2001; Smith and Pang, 2005; Yoder and Pang, 2005). However, many neurological disorders that include neurodegeneration of the cholinergic neurons have sparing of the GABAergic neurons (see Schliebs and Arendt, 2006).
Then again, there is seldom a neurological disorder that has selective damage to a single structure. The role of MS/DB cell loss in relation to other brain pathology has not been extensively assessed. We have previously observed in a rat model of WKS, pyrithiamine-induced thiamine deficiency (PTD), that along with thalamic, cortical and mammillary damage, there is also a loss of cholinergic neurons in the MS/DB (Pitkin and Savage, 2001, 2004; Savage et al., 2007). Subsequently, this pathology leads to selective cholinergic hippocampal dysfunction as assessed by in-vivo microdialysis in PTD-treated rats (Savage et al., 2003; Savage et al., 2007). Furthermore, enhancing hippocampal ACh levels via site-specific administration of physostigmine alleviated the PTD-induced behavioral impairment (Roland et al., 2008). Such results suggest that there is a functional impairment of the septohippocampal pathway in PTD-treated rats. Thus, the behavioral impairment observed in WKS and the PTD model could be a result of combined neuropathology to diencephalic and basal forebrain systems.
Thiamine deficiency, associated with diencephalic amnesia, has been proposed to lead to mitochondrial dysfunction that creates decreased cellular metabolism, glutamate excitotoxicity and oxidative stress in the thalamus, mammillary bodies and other diencephalic structures (Todd and Butterworth, 1999, 2001). The mechanisms of cell death in the MS/DB after thiamine deficiency are unknown. However, thiamine is critical for the production of acetylcholine (ACh). Thiamine acts as a cofactor for the production of the enzymes transketolase, pyruvate dehydrogenase (PDH), and alpha-ketoglutarate dehydrogenase (α-KGDH). PDH is essential in the production of ACh because it converts pyruvate to acetyl-CoA during the process of glycolysis (Todd and Butterworth, 1999). The ratio of acetyl-CoA energy producing and ACh synthesizing capacities may result in differential susceptibility of cholinergic populations to oxidative stress (McKinney, 2005). In addition, cholinergic cells appear to have a higher demand for energy production and are more sensitive to glucose deprivation (Szutowicz et al., 2007). These properties may make some cholinergic populations more sensitive to PTD-related neurodegeneration.
However, we do not know if ChAT immunopositive cells are the only MS/DB cell population sensitive to thiamine deficiency. Within the MS/DB, there is a group of GABAergic neurons that project to the hippocampus, which contain the calcium-binding protein parvalbumin [PV], (Freund, 1989). It has been suggested that the presence of PV in the cell body may protect against calcium-induced neurodegeneration (Heizmann, 1984; Freund et al., 1992). These PV-immunoreactive (PV-ir) neurons, localized mainly in the medial MS/DB regions (Heizmann, 1984), play an essential role in modulating the septal influence on hippocampal activation (Morris et al., 1999). The MS/DB also has a population of GABAergic interneurons that contain the calcium-binding protein calbindin ([CaBP], Freund and Antal, 1988). Within the medial septal region, the majority of CaBP immunoreactivity is found in the MS-lateral septum (LS) border with less reactivity in the more medial MS and DB regions (Jakab and Leranth, 1995). These CaBP-containing cells along the MS/LS border receive projections from the PV-containing MS/DB cells (Kiss et al., 1997) and have a bi-directional connection to the supramammillary nucleus (Borhegyi and Freund, 1998).
There is some evidence that damage to both ACh and GABA neurons of the basal forebrain are needed before some animal models display memory dysfunction (Sotty et al., 2003; Manseau et al., 2005; Smith and Pang, 2005; Yoder and Pang, 2005). The current experiment used unbiased stereology techniques to estimate cell loss of ChAT, PV, and CaBP neuronal populations within the MS/DB after PTD-treatment and related this neuropathology to the loss of midline thalamic tissue. In addition, control and PTD-treated animals were tested on a non-rewarded spontaneous alternation task. Performance on the spontaneous alternation is not a measure of spatial memory per se, rather it has been shown to be related to spatial memory. However, spontaneous alternation is affected by manipulations that alter memory performance on other tasks (lesions of the hippocampus, medial septum, cholinergic drugs, etc.—see Hughes, 2004; Lolonde, 2002). Percent alternation, in both groups of rats was compared to all neuronal estimates as well as thalamic pathology. The goal of this study was to determine the relationship between MS/DB pathology and thalamic damage to further understand how neuropathological interactions produce behavioral impairment. Determining the critical pathological features of diencephalic amnesia is essential for understanding the interdependencies between brain regions as well as the development of pharmocotherapeutics to treat disorders of cognition and memory.
Methods
Subjects
This experiment used 20, 3–4 month old, male Sprague-Dawley rats (PF = 10; PTD = 10). Two sets of animals were used: The first set had a total of 12 animals (PF = 6; PTD = 6) and after behavioral testing their brain sections were used to compare ChAT and PV staining. A second set of animals (PF = 4; PTD = 4) were treated and assessed at a later date using the exact same histological and behavioral procedures. The brains from the second set of animals, along with 4 rats from the first group (PF = 2; PTD = 2) were used to compare ChAT-ir and CaBP-ir staining.
PTD & PF Treatment
At the onset of the treatment phase, both PTD and PF animals had ad libitum access to water and thiamine-deficient chow (Harlan-Teklad Mills, WI). PTD treatment consisted of daily injections of pyrithiamine HBr (0.25 mg/kg, i.p. Sigma Chem. Corp., MO). Pyrithiamine is a thiamine antagonist that inhibits thiamine pyrophosphokinase, the enzyme that catalyzes the production of the coenzyme form of thiamine, thiamine pyrophosphokinase (Butterworth and Heroux, 1989). Within 14–16 days of treatment, PTD subjects developed symptoms of anorexia, ataxia, loss of righting reflexes, and eventually display seizure activity. Animals were monitored bi-hourly for these neurological changes starting on day 13. Within 4 – 4.5 hours of the appearance of seizure activity, all PTD-treated rats were reversed with a large dose of thiamine (100 mg/kg). The described protocol produces reliable diencephalic damage (Langlais and Savage, 1995). The administration of thiamine and return to normal chow completely reversed the acute symptoms within 8 hours and animals typically were fully recovered within 24 hours. The PF control rats were fed daily with thiamine deficient chow equal to the average amount eaten by the PTD-treatment group (to mimic the anorexia effects) and were given daily injections of thiamine HCl (.04 mg/kg i.p.). All rats were given two weeks to recover and return to a normal free-feeding weight. The weights of all animals were monitored daily to assess health status. All rats recovered completely from treatment and were behaviorally tested.
Behavioral Testing
After complete recovery from PTD treatment (approximately 2 weeks) and prior to behavioral testing, every animal was handled for 4 days, 10 minutes each to reduce anxiety to experimentation as well as fasted overnight to increase exploration. Maze testing consisted of animals being placed on the plus-maze to perform non-rewarded spontaneous alternation for 18 minutes. The maze was constructed of wood with clear Plexiglas sidewalls (12 cm high) and a black floor with all arms of equal distance (55 cm) and was elevated 80 cm from the floor. Animals were placed in the center of the maze at the beginning of testing and the experimenter recorded all arm entries. The percent alternation score is equal to the ratio of: (actual alternation/possible alternations) × 100. With the exception of the first arm chosen, every time the animal chose an arm they had the possibility to make an alternation or go straight. Actual alternations were the number of times the animals chose to turn a corner rather than go straight ahead.
Histology
After behavioral testing, animals were transcardially perfused and the brains were removed and placed in a 10% formalin solution for 1 week followed by emersion in a 30% sucrose solution for an additional week. Brains were then frozen and sliced (60 μm) with a sliding microtome (Sm2000r; Lecia Instruments, Germany). Every sixth section starting with the most rostral septal nuclei (1.20 mm from bregma) to the end of the diagonal band (−.26 mm from bregma) was selected for ChAT-immunocytochemistry (ICC) resulting in 4–5 sections per animal with 360 μm in between each section. For PV-ICC or CaBP-ICC, the adjacent section to each section selected for ChAT was selected resulting in 4–5 sections per animal with 360 μm in between each section.
ChAT ICC
In between each step, all sections were washed in phosphate buffer (pH=7.2–7.4) 3 times, for 5 minutes each. First, sections were washed in a H2O2 solution to quench endogenous peroxidases for 20 minutes. Next, sections were incubated for 60 minutes in a blocking solution (3% normal rabbit serum and 0.1% Triton X-100 in PB). Sections were then incubated in the primary goat anti-ChAT (diluted 1:100 in blocking solution; Chemicon International) at 4° C for 24 hours. The primary antibody was followed by biotinylated anti-goat IgG (1:100; Vectastain ABC Kit, Vector Laboratories) for 60 minutes and then Vectastain ABC reagent for 60 minutes. To visualize the ChAT antibody, we used diaminobenzidine (DAB Fast Tablet, Sigma) as a chromogen. Negative control slices were run for both groups and consisted of the same procedure minus the primary antibody.
Parvalbumin (PV) ICC
After ChAT staining, we used adjacent sections from the same animals to stain for PV. Once again, in between each step sections were washed in phosphate buffer 3 times, for 5 minutes each. First the sections were washed in a H2O2 solution to quench endogenous peroxidases for 20 minutes. Next, sections were put into a blocking solution (5% normal goat serum and 0.2% Triton X-100 in PB) for 60 minutes. For PV staining, we used a mouse monoclonal antibody (diluted 1:2500; Swant, Switzerland) at RT for 24 hours and then at 4°C for 24 hours. The primary antibody was followed by biotinylated anti-mouse IgG (1:100; Vectastain ABC Kit, Vector Laboratories) for 60 minutes and by Vectastain ABC reagent for 60 minutes. To visualize the PV antibody, we used DAB with Nickel enhancer (DAB Fast Tablet, Sigma) as a chromogen. Negative control slices were run for both groups and consisted of the same procedure minus the primary antibody.
Calbindin (CaBP) ICC
Due to both ChAT and PV staining in the same set of animals, there were not enough MS/DB sections for CaBP staining in all subjects. Therefore, 4 PF and 4 PTD animals were added to the study to result in a final number of 6 in each group for this stain (2 PF and 2 PTD from the ChAT and PV staining did have enough sections). In between each step sections were washed in phosphate buffer 3 times, for 5 minutes each. Sections were first were washed in a H2O2 solution to quench endogenous peroxidases for 20 minutes. For CaBP staining, we used a mouse monoclonal antibody (diluted 1:400; Sigma Chem. Corp., MO) at RT for 24 hours. The primary antibody was followed by biotinylated anti-mouse IgG (1:100; Vectastain ABC Kit, Vector Laboratories) for 60 minutes and by Vectastain ABC reagent for 60 minutes. To visualize the CaBP antibody, we used DAB (DAB Fast Tablet, Sigma) as a chromogen. Negative control slices were run for both groups and consisted of the same procedure minus the primary antibody.
Stereology
Unbiased stereology was used to estimate ChAT, PV, and CaBP immunopositive cell counts in the MS/DB. Parameters for sampling were based on previously published studies assessing the number of ChAT positive (Miettinen et al., 2002; Yoder and Pang, 2005; Savage et al., 2007), and PV positive neurons in the basal forebrain (Henderson et al., 2004). A low power image was captured with a digital camera (DVC-1310; DVC Company, Austin Texas) with a 5X lens on a Zeiss microscope (Axioscope 2-plus) with an attached 3-axis motorized stage. A contour was drawn around the region of interest using StereoInvestigator software (MicroBrightField; Williston, VT). Anatomical boundaries used to define the basal forebrain were the corpus callosum on the dorsal aspect and the walls of the lateral ventricles. Sections began with the appearance of the rostral genu of the corpus callosum crossing hemispheres and ended caudally with the last section containing the full anterior commissure [approximately IA 10.6–8.74 mm (Paxinos and Watson, 1986). The neurons were counted with a 40X objective using the optical fractionator approach. The fractionator sampling consists of a section sampling fraction (ssf = 1/6), an area sampling fraction representing a ratio between counting frame size and grid size (asf = 50 μm × 50 μm/100 μm × 100 μm) and a height sampling fraction (hsf = 20 μm/60 μm). The equation for determining cell estimates is: N = ΣQ− × 1/ssf ×1/asf ×1/hsf, where Q is the number of neurons actually counted in the specimens, whereas N represents the total cell estimation.
Intraventricular distances (IVD) measures
In addition, quantitative measures of thalamic tissue loss were obtained. The amount of midline thalamic tissue destroyed can be estimated by measuring the IVD at the midline (Robinson and Mair, 1992; Savage et al., 1999). The IVD (mm) was measured from the floor of the dorsal III ventricle to the roof of the III ventricle at the following approximate interaural (IA) locations: 7.20 mm (at the level where the stria medullaris runs longitudinal to the dorsal surface and the ventral lateral nucleus first appears) and 6.44 mm (at the first appearance of the lateral habenula) according to the atlas of Paxinos and Watson (1986). Quantitative measures were determined by the use of video images taken from a Nikon light microscope (Nikon Eclipse E400; Nikon Instruments; Melville, NY) using a Scion 1394 camera JAVA module (Scion Corp; Fredrick, Maryland), and analyzed using an image analyzer program (IMAGE-J, v.1.34, NIH, Bethesda, MD.) on a Macintosh G4 computer.
Statistical analyzes
Group differences in specific cell phenotype (ChAT, PV, or CaBP) neuronal estimates in the MS/DB and thalamic mass were determined using a one factor (Group: PF vs. PTD) ANOVA for each hostological marker. Behavioral differences in alternation rates and activity (number of arms entered) between the groups were also assessed with a one-factor (Group) ANOVA. Simple regression analyses were conducted to assess correlative relationships between different cell types, cell types and thalamic mass, and between histological measures and behavior.
Results
Histology
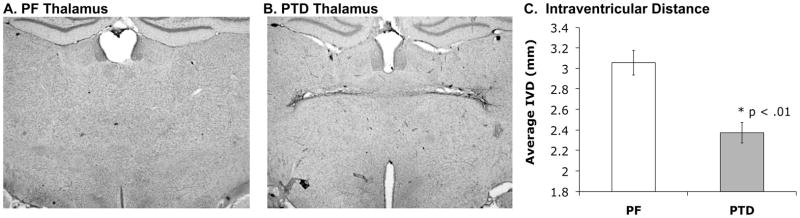
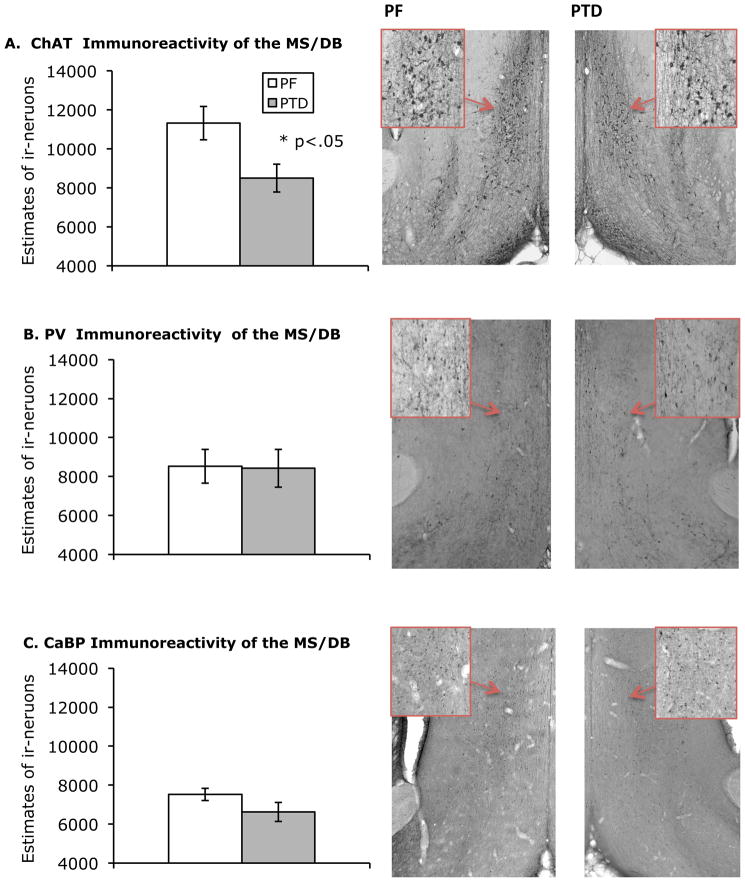
Figure 1-AB shows representative thalamic sections for both PF and PTD animals. There was a significant loss of thalamic mass as measured by IVD (both F’s [1, 10] > 10.7, p’s < .01) in the PTD rats relative to the PF rats (see Figure 1-C). Figure 2 shows the localization of MS/DB ChAT-, PV-, and CaBP-ir staining. The negative controls from ChAT, PV, and CaBP did not display any cell positive staining. In the MS/DB region, PTD rats had significantly less ChAT-positive cells than the PF rats (both F’s [1,10] > 6.40; p < 0.05). In contrast, there was no significant difference in PV-positive cells or CaBP –positive cells between PTD-treated and PF rats (PV:F [1,10]<1; CaBP: F [1,10]= 2.39; p>.15; see Figure 2). The coefficient errors (using Gundersen–Jensen estimator and a smoothness factor of 1; see Slomianka & West, 2005) for each cell population across treatment groups (no differences all p’s>.1) were the following: ChAT=0.085; PV=0.101; CaBP=0.09.
Figure 1.
Representative coronal sections of the thalamus stained with cresyl violet. On the left is the intact thalamus of a PF rat (A) in comparison to a PTD-induced thalamic lesion on the right (B). Intraventicular distance in mm (IVD) was taken from the roof to the floor of the third ventricle on two brain sections per rat. Compared to PF controls, PTD-treated rats displayed a significant loss of thalamic tissue as measured by mean IVD (C). Error bars represent standard error of the mean (SEM).
Figure 2.
Mean number (± SEM) of immunoreactive neurons for (A) choline acetyltransferase (ChAT), (B) parvalbumin immunoreactivity (PV) and the (C) calcium-binding protein calbindin (CaBP) in PF and PTD rats. Photomicrographs of coronal sections of the medial septum/ diagonal band of PF rats (left column) and PTD-treated rats (right column) stained for ChAT, PV, and CaBP taken with a 2X objective. The photomicrograph inserts outlined in red are of neurons taken with a 20X objective. PTD-treated rats displayed a significant reduction in ChAT-immunoreactive MS/DB neurons as compared to PF controls. There were no significant differences between groups in the number of PV- or CaBP immunoreactive MS/DB neurons.
(Note: The loss of ChAT-ir cells, the loss of thalamic mass, and the correlation between these measures-- as well as the behavioral impairment-- in the PTD animals was replicated in the second set of animals (all p’s <.05). Thus, only the data from the first set of animals is shown in figure form.
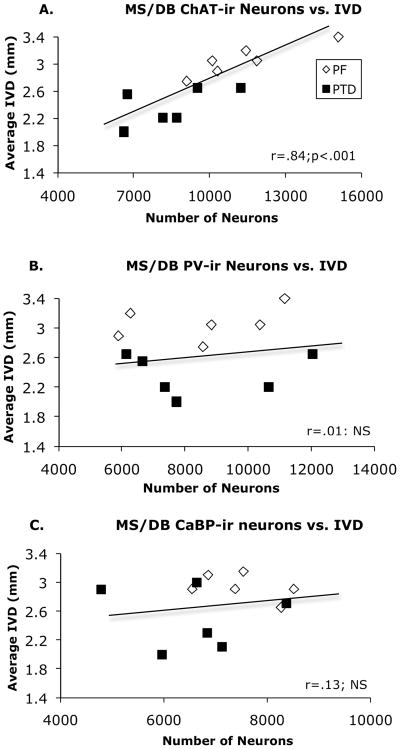
Regression analysis revealed that there was no significant correlation between the number of ChAT-positive and the number of PV-positive neurons in the MS/DB region (r = .12) in PF and PTD rats. However, there was a strong positive correlation between the number of MS/DB ChAT-ir cells and the averaged IVD measurement (r = .84, p < .01; Figure 3-A). In contrast, PV-ir cell number did not correlated with thalamic loss (r = .01; Figure 3-B). There was also no significant correlations between CaBP-ir cell number and the ChAT-ir cell number or IVD score (both r’s < .32, p’s > .2).
Figure 3.
Correlative relationships between ChAT-ir (A), PV-ir (B), and CaBP-ir (C) neuronal estimates and thalamic tissue loss (IVD). The only significant correlation was between the number of ChAT-ir cells and IVD. Trend lines represent the overall regression analysis for the PF and PTD animals.
Spontaneous Alternation
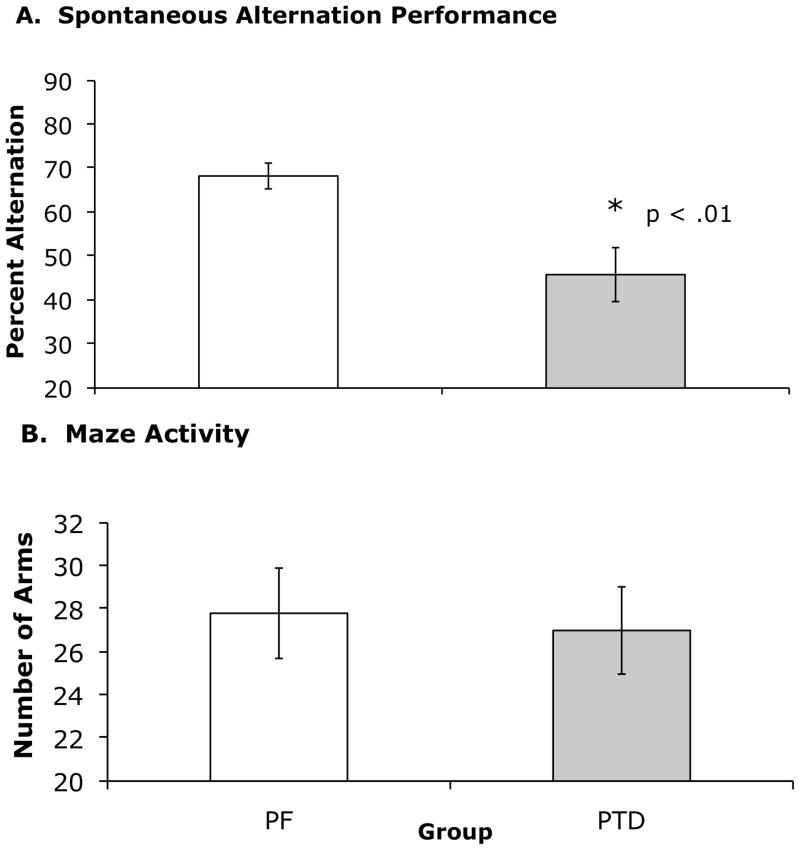
PTD rats in both subsets displayed significantly reduced percent alternation (Figure 4-A) as compared to PF rats (both F’s[1, 10] >10.85; p < 0.01). This decrease in performance cannot be accounted for as a difference in activity because there was no significant difference in number of arms chosen between groups (PF = 27.8 ± 2.12; PTD = 27.0 ± 2.03).
Figure 4.
(A) Spontaneous alternation performance in PF vs. PTD-treated rats. PF rats had a significantly higher mean (±SEM) percent alternation scores than PTD-treated rats. (B) Maze activity, as determined by mean number of arms entered during the 18 minute testing session was not different as a function of Group.
Correlation between behavior and histological measures
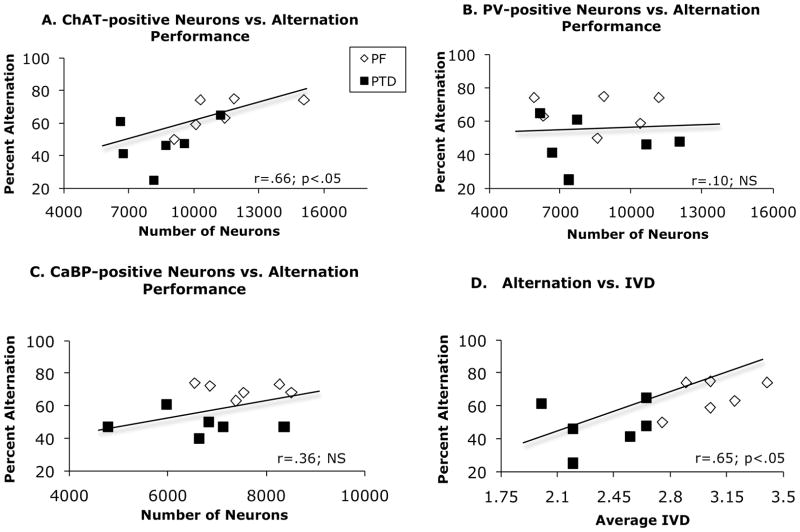
A regression analysis revealed across both PF and PTD rats the number of ChAT-ir neurons in the MS/DB was positively correlated with spontaneous alternation behavior in (r = .66; p < .05; Figure 5-A). Furthermore, the averaged IVD measure was also positively correlated with alternation scores (r = .65; p < .05; Figure 5-D). In contrast, there was no significant correlation between PV-ir neurons (r = .10, p>.2; Figure 5-B) or CaBP–ir neurons (r = .35, p>.2; Figure 5-C) and spontaneous alternation performance across groups. Neither ChAT-, PV- or CaBP-positive neurons or IVD correlated with activity level on the maze across groups (all r’s<.2).
Figure 5.
Correlative relationships between spontaneous alternation performance and neuronal estimates of ChAT-ir (A); PV-ir (B), and CaBP-ir (C), as well as thalamic tissue loss (IVD; D). Across groups, significant positive correlations were found between spontaneous alternation performance and ChAT-ir cell count and IVD. There were no significant correlations between GABA-ir cell counts and behavior. Trend lines represent the overall regression analysis for the combination of PF and PTD animals.
Discussion
Two important points were revealed from examining the relationship between loss of MS/DB projection neurons, thalamic tissue damage and behavioral impairment in the animal model of WKS. First, neither PV- nor CaBP-ir GABA neurons in the MS/DB were significantly affected by thiamine deficiency. Second, although previous studies have documented cholinergic MS/DB neuronal loss (Pitkin and Savage, 2001, 2004), those studies did not assess how the basal forebrain cell loss was related to other neuropathology or behavioral dysfunction. Our data revealed a close relationship between the number of cholinergic neurons in the MS/DB, thalamic shrinkage and behavioral impairment in the PTD model. Below, we specifically address these points.
In the MS/DB, pyrithiamine treatment resulted in selective cell loss of ChAT-ir neurons that project to the hippocampus. Thiamine deficiency did not cause neurodegeneration of PV- or CaBP-positive MS/DB neurons. These findings provide evidence that cholinergic neurons are especially sensitive to the neurodegeneration caused by thiamine deficiency. Parvalbumin is found in those MS/DB GABAergic neurons that project to the hippocampus (Freund, 1989; Kermer et al., 1995), whereas CaBP is found in GABAergic interneurons as well as supramammillary complex projection neurons (Kiss et al., 1997; Borhegyi and Freund, 1998). Such calcium-binding proteins have been shown to have calcium-buffering properties, in other words they help bind and remove excess calcium in the cell (Baimbridge et al., 1982; Heizmann, 1984). Therefore, calcium-binding proteins may be protecting these GABAergic neurons from excitotoxic damage (Freund et al., 1992). However, there glutamatergic neurons in the basal forebrain (Freund and Antal, 1988; Manseau et al., 2005) and we do not know if these neurons are sensitive to thiamine deficiency.
Given that there was no reduction in PV- or CaBP-positive neuronal numbers in the PTD model, it makes sense that estimates of these cell populations did not correlate with ChAT-ir cell loss, thalamic tissue loss or performance on the spontaneous alternation task. These findings are consistent with the data demonstrating that lesioning of the GABAergic septohippocampal neurons or selectively lesioning the PV-ir MS/DB neurons does not affect spatial memory performance (Pang et al., 2001; Smith and Pang, 2005).
In contrast, loss of ChAT-ir neurons after thiamine deficiency does correlate with behavioral impairment and thalamic tissue loss. The degree of ChAT cell loss (about 25–30%) in this study is similar to what we have obtained in previous studies (Pitkin and Savage, 2001, 2004; Savage et al., 2007). Furthermore, the degree of behavioral impairment observed on the spontaneous alternation task after PTD treatment (Savage et al., 2003; Roland et al., 2008; Vetreno et al., 2008) and the degree of thalamic tissue loss was also similar to what was recorded in previous studies (Robinson and Mair, 1992; Langlais and Savage, 1995). Our correlative analysis revealed that thiamine-deficiency induced neuropathology of MS/DB cholinergic neurons and thalamic tissue loss are both strongly related to each other and loss in either region predict behavioral impairment. It should be noted that thalamic tissue loss was assessed by measuring contraction of tissue, in brain samples that underwent a chemical processing that induces shrinking. Thus, care was taken to be surethat tissue from both groups was processed together. However, the IVD measure had been used by several labs and has been shown to be a sensitive marker of thalamic pathology in the PTD-treated rat (Robinson & Mair, 1992; Langlais & Savage, 1995; Pitkin & Savage, 2001; 2004).
There has been some conflicting clinical data regarding the role of basal forebrain cholinergic cell loss and the production of amnesia in alcohol-induced WKS. Arendt et al. (1995) quantified overall basal forebrain cell loss and found that, depending of the severity of the amnesia, WKS patients displayed a 25–45% decrease in cells with the most severe memory loss being shown at 30% or greater cell loss. The cell loss observed in the WKS patients was almost entirely attributably (over 90%) to cholinergic cells of the MS/DB; however, these patients also displayed cell loss in the vertical limb of the diagonal band (VDB) and the nucleus basalis of Meynert (NBM) (Arendt et al., 1995). Using WKS as one of multiple disorders of basal forebrain pathology, a correlation was found between the loss of cholinergic basal forebrain neurons and cognitive disturbance on the Mini mental exam (Schliebs and Arendt, 2006). Although, Cullen et al. (1997) found a comparable amount of cholinergic cell loss in the NBM, they did not find a difference in NBM cell loss in alcoholics with Wernicke encephalitis (WE- non amnesic) and alcoholics with WKS (amnesic). They found there was significant NBM cholinergic cell loss, relative to aged matched controls, regardless of whether the patient was classified as amnestic or not. Given that Arendt et al. (1995) included the MS, VDB and NBM groups, whereas Cullen et al. (1997) only included the NBM, the MS/DB cholinergic cell loss could correlate with a specific type of cognitive impairment while cholinergic cell loss in the NBM is responsible for a different type of cognitive dysfunction. Cullen et al. (1997) does mention that alcoholic WE patients without Korsakoff’s syndrome display attentional difficulties.
In animals studies there is evidence that suggest ACh plays a specific role in attenuating associative interference rather than a general role in supporting acquisition of associative memories—but this effect occurs outside the basal forebrain (DeRosa, Hassellmo & Baxter, 2001). Intense activation of cholinergic forebrain projection neurons could be important for encoding new stimuli in discrimination tasks (DeRosa & Hassellmo, 2000). Although spontaneous alternation performance is not a discrimination task per se, alternation across arms requires a rat to discriminate between arms on the basis of which arms were most recently visited. Such alternation behavior has been described to be memory-dependent action to the environment driven by a natural response to novelty (Hughes, 2004). Thus, a loss of MS/DB neurons as seen in the PTD animals could contribute to such a behavioral deficit as suggested by the correlative analysis.
Our laboratory has previously quantified ChAT-positive neurons in the NBM and found no difference in estimates between PTD and PF rats (Savage et al., 2007). The different groups of basal forebrain cholinergic neurons demonstrate a differential vulnerability to neurotoxic events (Boegman et al., 1992; Wenk and Willard, 1998). One difference between MS/DB and one subpopulation of NBM cholinergic neurons in the rodent is presence of the p75-NGF receptor. The maintenance and survival of MS/DB cholinergic cells in the rodent is dependent on the neurotrophin, nerve growth factor (NGF), which is synthesized in the hippocampus by GABAergic interneurons (Hartikka and Hefti, 1988; Lauterborn et al., 1993; Acsady et al., 2000). The cholinergic fibers that project from the MS/DB to the hippocampus retrogradely transport NGF to the medial septum where it binds to the p75 receptor (Acsady et al., 2000). Unlike the MS/DB cholinergic neurons, in the rodent the NBM cholinergic neurons that project to the amygdala do not express the p75-NGF receptor (Berger-Sweeney et al., 1994; Heckers et al., 1994), which may make them less sensitive to the neuronal death caused by PTD-treatment (Savage et al., 2007).
Thus, p75-NGF receptors on cholinergic neurons may make them sensitive to thiamine-deficiency induced neurotoxicity. One of the main functions of the p75-NGF receptor is regulating both cell death and survival (Kaplan and Miller, 2000; see Twiss et al., 2006 for a review). Degenerative neurological diseases, such as Alzheimer’s and Parkinson’s disease, preferentially display degeneration in those cell populations that contain the p75-NGF receptor (Woolf et al., 1989; Wang et al., 2008). Excitotoxin-induced lesion and ischemia have both shown to increase the density of the p75-NGF receptor in basal forebrain cholinergic neurons and this overexpression is associated with cell death (Dunnett et al., 1991; Rabadizadeh et al., 1993; Weiss et al., 1994; Oh et al., 2000). Currently, the present data suggest that cell loss in the MS/DB region is not due to widespread cell death in the basal forebrain, but is specific to the ChAT-positive cells of the MS/DB.
The neuroanatomical damage seen after PTD treatment is most likely due to glutamate excitotoxicity and/or ischemia (Todd and Butterworth, 1999, 2001). It has been well documented that glutamate excitotoxicity is responsible for thalamic lesions in the PTD model of WKS (Langlais and Zhang, 1993; Zhang et al., 1995) and cholinergic neurons are particularly sensitive to glutamate excitotoxicity (McKinney, 2005). The extent of thalamic damage caused by excessive rises of glutamate during thiamine deficiency is commonly determined by measuring thalamic mass (IVD) (Robinson and Mair, 1992; Savage et al., 1999). We found a significant correlation between thalamic tissue loss and behavioral impairment. Furthermore, there was a strong correlation between IVD score and the number of MS/DB ChAT-ir cells. This data suggest that degree of thalamic damage is coupled to the loss of cholinergic neurons in the MS/DB. Additionally, both of these measures correlate with the measure of behavioral dysfunction on the spontaneous alternation task. Previous studies have demonstrated that this type of behavioral dysfunction in PTD rats is correlated to blunted hippocampal ACh release (Savage et al., 2003) and behavioral performance can be restored in PTD rats by increasing hippocampal ACh levels (Roland et al., 2008). Such results suggest that cholinergic septohippocampal dysfunction significantly contributes to the amnesic syndrome seen after thiamine deficiency.
What we do not know is whether there is a common causal neurotoxic mechanism across the MS/DB and thalamus or if the neuropathology occurs independently. Both thalamic tissue loss and cholinergic cell loss in the MS/DB are key features of diencephalic amnesia associated with thiamine deficiency, it is important to note that damage to both structures occurs in parallel. The present result of such a strong correlation between thalamic mass and cholinergic neurons in the MS/DB is suggestive of a common pathogenic link. However, additional studies need to address why the cholinergic population of neurons in the MS/DB are selectively sensitive to thiamine deficiency induced neurotoxicity.
Lesion of either the medial thalamus (Mair and Lacourse, 1992; Savage et al., 1997; Burk and Mair, 1998) or the MS/DB cholinergic nuclei (Walsh et al., 1996; Chang and Gold, 2004) alone can produce significant behavioral impairment, but the severity of behavioral impairments seen in the PTD model tends to be greater and broader—suggesting a synergistic interaction between neuropathology in that model. The present study contributes to our understanding of diencephalic amnesia produced by thiamine deficiency through demonstrating that damage to the cholinergic septohippocampal pathway as well as thalamic tissue loss predict behavioral dysfunction. In contrast, both the GABAergic interneurons (CaBP-ir) and projection neurons (PV-ir) are spared. Thus, therapeutic approaches should address both of these neuropathological outcomes that occur in diencephalic amnesia associated with alcoholism and thiamine deficiency.
Acknowledgments
This work was funded by NINDS Grant RO1 NS054272 to LMS. The authors would like to thank Ryan Vetreno and Jess Blackwolf for their help with behavioral testing and histology. Jessica Roland is now at the Department of Veterans Affairs in East Orange, NJ and the University of Medicine & Dentistry of New Jersey.
References
- Acsady L, Pascual M, Rocamora N, Soriano E, Freund TF. Nerve growth factor but not neurotrophin-3 is synthesized by hippocampal GABAergic neurons that project to the medial septum. Neuroscience. 2000;98:23–31. doi: 10.1016/s0306-4522(00)00091-9. [DOI] [PubMed] [Google Scholar]
- Arendt T, Bruckner MA, Bigl V, Marcova L. Dendritic Reorganisation in the Basal Forebrain Under Degenerative Conditions and Its Defects in Alzheimer’s Disease. II. Ageing, Korsakoffs Disease, Parkinson’s Disease, and Alzheimer’s Disease. J Comp Neurol. 1995;351:189–222. doi: 10.1002/cne.903510203. [DOI] [PubMed] [Google Scholar]
- Baimbridge KG, Miller JJ, Parkes CO. Calcium-binding protein distribution in the rat brain. Brain Res. 1982;239:519–525. doi: 10.1016/0006-8993(82)90526-1. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Heckers S, Mesulam MM, Wiley RG, Lappi DA, Sharma M. Differential effects on spatial navigation of immunotoxin-induced cholinergic lesions of the medial septal area and nucleus basalis magnocellularis. J Neurosci. 1994;14:4507–4519. doi: 10.1523/JNEUROSCI.14-07-04507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boegman RJ, Jhamandas K, Cockhill J. Differential effect of excitotoxins in the basal forebrain on choline acetyltransferase activity in the cortex and amygdala. Ann N Y Acad Sci. 1992;648:254–255. doi: 10.1111/j.1749-6632.1992.tb24548.x. [DOI] [PubMed] [Google Scholar]
- Borhegyi Z, Freund TF. Dual projection from the medial septum to the supramammillary nucleus in the rat. Brain Res Bull. 1998;46:453–459. doi: 10.1016/s0361-9230(98)00038-0. [DOI] [PubMed] [Google Scholar]
- Burk JA, Mair RG. Thalamic Amnesia Reconsidered: Excitotoxic Lesions of the Intralaminar Nuclei, but Not the Mediodorsal Nucleus, Disrupt Place Delayed Matching-to-Sample Performance in Rats (Rattus norvegicus) Behav Neurosci. 1998;112:54–67. [PubMed] [Google Scholar]
- Butterworth RF, Heroux M. Effect of Pyrithiamine Treatment and Subsequent Thiamine Rehabilitation on Regional Cerebral Amino Acids and Thiamine-Dependent Enzymes. J Neurochem. 1989;52:1079–1084. doi: 10.1111/j.1471-4159.1989.tb01850.x. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Impaired and Spared Cholinergic Functions in the Hippocampus After Lesions of the Medial Septum/Vertical Limb of the Diagonal Band With 192 IgG-Saporin. Hippocampus. 2004;14:170–179. doi: 10.1002/hipo.10160. [DOI] [PubMed] [Google Scholar]
- Cullen KM, Halliday GM, Caine D, Kril JJ. The nucleus basalis (Ch4) in the alcoholic Wernicke-Korsakoff syndrome: reduced cell number in both amnesic and non-amnesic patients. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:315–320. doi: 10.1136/jnnp.63.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa E, Hasselmo ME, Baxter MG. Contribution of the cholinergic basal forebrain to proactive interference from stored odor memories during associative learning in rats. Behav Neurosci. 2001;115(2):314–27. [PubMed] [Google Scholar]
- DeRosa E, Hasselmo ME. Muscarinic cholinergic neuromodulation reduces proactive interference between stored odor memories during associative learning in rats. Behav Neurosci. 2000;114:32–41. [PubMed] [Google Scholar]
- Dunnett SB, Everitt BJ, Robbins TW. The basal forebrain-cortical system: interpreting the functional consequences of excitotoxic lesions. Trends in Neuroscience. 1991;14:494–501. doi: 10.1016/0166-2236(91)90061-x. [DOI] [PubMed] [Google Scholar]
- Freund TF. GABAergic septohippocampal neurons contain parvalbumin. Brain Res. 1989;478:375–381. doi: 10.1016/0006-8993(89)91520-5. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Letters to Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Freund TF, Ylinen A, Miettinen RA, Pitkanen A, Lahtinen H, Baimbridge KG, Riekkinen PJ. Pattern of Neuronal Death in the Rat Hippocampus After Status Epilepticus, Relationship to Calcium Binding Protein Content and Ischemic Vulnerability. Brain Res Bull. 1992;28:27–38. doi: 10.1016/0361-9230(92)90227-o. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic Innervation of the Rat Hippocampus as Revealed by Choline Acetyltransferase Immunocytochemistry: A Combined Light and Electron Microscopic Study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Hartikka J, Hefti F. Development of septal cholinergic neurons in culture: plating density and glial cells modulate effects of NGF on survival, fiber growth, and expression of transmitter-specific enzymes. J Neurosci. 1988;8:2967–2985. doi: 10.1523/JNEUROSCI.08-08-02967.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Ohtake T, Wiley RG, Lappi DA, Geula C, Mesulam MM. Complete and selective cholinergic denervation of rat neocortex and hippocampus but not amygdala by an immunotoxin against the p75 NGF receptor. J Neurosci. 1994;14:1271–1289. doi: 10.1523/JNEUROSCI.14-03-01271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann CW. Parvalbumin, an intracellular calcium-binding protein; distribution, properties and possible roles in mammalian cells. Experientia. 1984;40:910–921. doi: 10.1007/BF01946439. [DOI] [PubMed] [Google Scholar]
- Henderson Z, Fiddler G, Saha S, Boros A, Halasy K. A parvalbumin-containing, axosomatic synaptic network in the rat medial septum: relevance to rhythmogenesis. Eur J Neurosci. 2004;19:2753–2768. doi: 10.1111/j.0953-816X.2004.03399.x. [DOI] [PubMed] [Google Scholar]
- Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:494–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Leranth C. Septum. In: Paxinos G, editor. The Rat Nervous System. San Diego: Academic Press; 1995. pp. 405–442. [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transdunction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kermer P, Naumann T, Bender R, Frotscher M. Fate of GABAergic Septohippocampal Neurons After Fimbria-Fornix Transection as Revealed by In Situ Hybridization for Glutamate Decarboxylase mRNA and Parvalbumin Immunocytochemistry. J Comp Neurol. 1995;362:385–399. doi: 10.1002/cne.903620307. [DOI] [PubMed] [Google Scholar]
- Kiss J, Borhegyi Z, Csaki A, Szeiffert G, Leranth C. Parvalbumin-containing cells of the angular portion of the vertical limb terminate on calbindin-immunoreactive neurons located at the border between the lateral and medial septum of the rat. Exp Brain Res. 1997;113:48–56. doi: 10.1007/BF02454141. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Savage LM. Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behav Brain Res. 1995;68:75–89. doi: 10.1016/0166-4328(94)00162-9. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Zhang SX. Extracellular glutamate is increased in thalamus during thiamine deficiency-induced lesions and is blocked by MK-801. J Neurochem. 1993;61:2175–2182. doi: 10.1111/j.1471-4159.1993.tb07457.x. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Tran TND, Isackson PJ, Gall CM. Nerve growth factor mRNA is expressed by GABAergic neurons in rat hippocampus. Neuroreport. 1993;5:273–276. doi: 10.1097/00001756-199312000-00023. [DOI] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Mair RG, Lacourse DM. Radio-frequency lesions of the thalamus produce delayed-nonmatching-to-sample impairments comparable to pyrithiamine-induced encephalopathy in rats. Behav Neurosci. 1992;106:634–645. doi: 10.1037//0735-7044.106.4.634. [DOI] [PubMed] [Google Scholar]
- Manseau F, Danik M, Williams S. A functional glutamatergic neurone network in the medial septum and diagonal band area. J Physiol. 2005;566:865–884. doi: 10.1113/jphysiol.2005.089664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney M. Brain cholinergic vulnerability: Relevance to behavior and disease. Biochem Pharmacol. 2005;70:1115–1124. doi: 10.1016/j.bcp.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Miettinen RA, Kalesnykas G, Koivisto EH. Estimation of the Total Number of Cholinergic Neurons Containing Estrogen Receptor. The Journal of Histochemistry & Cytochemistry. 2002;50:891–902. doi: 10.1177/002215540205000703. [DOI] [PubMed] [Google Scholar]
- Morris NP, Harris SJ, Henderson Z. Parvalbumin-immunoreactive, fast-spiking neurons in the medial septum/diagonal band complex of the rat: intracellular recordings in vitro. Neuroscience. 1999;92:589–600. doi: 10.1016/s0306-4522(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Oh JD, Chartisathian K, Chase TN, Butcher LL. Overexpression of neurotrophin receptor p75 contributes to the excitotoxin-induced cholinergic neuronal death in rat basal forebrain. Brain Res. 2000;853:174–185. doi: 10.1016/s0006-8993(99)02054-5. [DOI] [PubMed] [Google Scholar]
- Pang KCH, Nocera R, Secor AJ, Yoder RM. GABAergic Septohippocampal Neurons Are Not Necessary for Spatial Memory. Hippocampus. 2001;11:814–827. doi: 10.1002/hipo.1097. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1986. [Google Scholar]
- Pitkin SR, Savage LM. Aging potentiates the acute and chronic neurological symptoms of pyrithiamine-induced thiamine deficiency in the rodent. Behav Brain Res. 2001;119:167–177. doi: 10.1016/s0166-4328(00)00350-8. [DOI] [PubMed] [Google Scholar]
- Pitkin SR, Savage LM. Age-related vulnerability to diencephalic amnesia produced by thiamine deficiency: the role of time of insult. Behav Brain Res. 2004;148:93–105. doi: 10.1016/s0166-4328(03)00208-0. [DOI] [PubMed] [Google Scholar]
- Rabadizadeh S, Oh J, Ord T, Zhong L, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by low-affinity nerve growth factor receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- Robinson JK, Mair RG. MK-801 prevents brain lesions and delayed-nonmatching-to-sample deficits produced by pyrithiamine-induced encephalopathy in rats. Behav Neurosci. 1992;106:623–633. [PubMed] [Google Scholar]
- Roland JJ, Mark K, Vetreno RP, Savage LM. Increasing Hippocampal Acetylcholine Levels Enhances Behavioral Performance in an Animal Model of Diencephalic Amnesia. Brain Res. 2008;1234:116–127. doi: 10.1016/j.brainres.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM, Chang Q, Gold PE. Diencephalic Damage Decreases Hippocampal Acetylcholine Release During Spontaneous Alternation Testing. Learn Memory. 2003;10:242–246. doi: 10.1101/lm.60003. [DOI] [PubMed] [Google Scholar]
- Savage LM, Pitkin SR, Knitowski KM. Rats exposed to acute pyrithiamine-induced thiamine deficiency are more sensitive to the amnestic effects of scopolamine and MK-801: examination of working memory, response selection, and reinforcement contingencies. Behav Brain Res. 1999;104:13–26. doi: 10.1016/s0166-4328(99)00049-2. [DOI] [PubMed] [Google Scholar]
- Savage LM, Roland JJ, Klintsova AY. Selective septohippocampal – but not forebrain amygdalar – cholinergic dysfunction in diencephalic amnesia. Brain Res. 2007;1139:210–219. doi: 10.1016/j.brainres.2006.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM, Sweet AJ, Castillo R, Langlais PJ. The effects of lesions to thalamic lateral internal medullary lamina and posterior nuclei on learning, memory and habituation in the rat. Behav Brain Res. 1997;82:133–147. doi: 10.1016/s0166-4328(97)80983-7. [DOI] [PubMed] [Google Scholar]
- Schliebs R. Basal Forebrain Cholinergic Dysfunction in Alzheimer’s Disease –Interrelationship with b-amyloid, Inflammation and Neurotrophin Signaling. Neurochem Res. 2005;30:895–908. doi: 10.1007/s11064-005-6962-9. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm. 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- Slomianka L, West MJ. Estimators of thr precision of stereological estimates: an example based on the CA1 pyramidal cell layer of rats. Neurosci. 2005;136:757–767. doi: 10.1016/j.neuroscience.2005.06.086. [DOI] [PubMed] [Google Scholar]
- Smith HR, Pang KCH. Orexin-saporin lesions of the medial septum impair spatial memory. Neuroscience. 2005;132:261–271. doi: 10.1016/j.neuroscience.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol. 2003;551:927–943. doi: 10.1113/jphysiol.2003.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutowicz A, Bielarczyk H, Gul S, Ronowska A, Pawelczyk T, Jankowska-Kulawy A. Phenotype-dependent susceptibility of cholinergic neuroblastoma cells to neurotoxic inputs. Metab Brain Dis. 2007;21:149–161. doi: 10.1007/s11011-006-9007-4. [DOI] [PubMed] [Google Scholar]
- Todd KG, Butterworth RF. Mechanisms of Selective Neuronal Cell Death due to Thiamine Deficiency. Annals New York Academy of Sciences. 1999:404–411. doi: 10.1111/j.1749-6632.1999.tb07866.x. [DOI] [PubMed] [Google Scholar]
- Todd KG, Butterworth RF. In Vivo Microdialysis in an Animal Model of Neurological Disease: Thiamine Deficiency (Wernicke) Encephalopathy. Methods. 2001;23:55–61. doi: 10.1006/meth.2000.1105. [DOI] [PubMed] [Google Scholar]
- Twiss JL, Chang JH, Schanen NC. Pathophysiological Mechanisms for Actions of the Neurotrophins. Brain Pathol. 2006;16:320–332. doi: 10.1111/j.1750-3639.2006.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Anzalone SJ, Savage LM. Impaired, Spared, and Enhanced ACh Efflux Across the Hippocampus and Striatum in Diencephalic Amnesia is Dependent on Task Demands. Neurobiol Learn Mem. 2008 doi: 10.1016/j.nlm.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer BH, Levey AI, Rye DB, Mesulam MM, Mufson EJ. Cholinergic and noncholinergic septohippocampal pathways. Neurosci Lett. 1985;54:45–52. doi: 10.1016/s0304-3940(85)80116-6. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Herzog CD, Ghandi C, Stackman RW, Wiley RG. Injection of IgG 192-saporin into the medial septum produces cholinergic hypofunction and dose-dependent working memory. Brain Res. 1996;726:69–79. [PubMed] [Google Scholar]
- Wang Y-Q, Bian G-L, Bai Y, Cao R, Chen L-W. Identification and kainic acid-induced up-regulation of low-affinity p75 neurotrophin receptor (p75NTR) in the nigral dopamine neurons of adult rats. Neurochem Int. 2008;53:56–62. doi: 10.1016/j.neuint.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Weiss JH, Yin HZ, Choi DW. Basal forebrain cholinergic neurons are selectively vulnerable to AMPA/kainate receptor-mediated neurotoxicity. Neuroscience. 1994;60:659–664. doi: 10.1016/0306-4522(94)90494-4. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Willard LB. The neural mechanisms underlying cholinergic cell death within the basal forebrain. International Journal of Developmental Neuroscience. 1998;16:729–735. doi: 10.1016/s0736-5748(98)00081-1. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, DeLong MR. Alzheimer’s Disease and Senile Dementia: Loss of Neurons in the Basal Forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Jacobs RW, Butcher LL. The pontomesencephalotegmental cholinergic system does not degenerate in Alzheimer’s disease. Neurosci Lett. 1989;96:277–282. doi: 10.1016/0304-3940(89)90391-1. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Pang KCH. Involvement of GABAergic and Cholinergic Medial Septal Neurons in Hippocampal Theta Rhythm. Hippocampus. 2005;15:381–392. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Weilersbacher GS, Henderson SW, Corso T, Olney JW, Langlais PJ. Excitotoxic cytopathology, progression, and reversibility of thiamine deficiency-induced diencephalic lesions. J Neuropathol Exp Neurol. 1995;54:255–267. doi: 10.1097/00005072-199503000-00012. [DOI] [PubMed] [Google Scholar]