Abstract
Few studies focused on the prevalence of scoliosis and involvement of the spine in patients with fibrous dysplasia (FD) of bone. We examined for FD involvement of the spine and scoliosis in 56 patients affected by FD of bone. Fifty patients were part of a cohort reported in a multicentric study on FD promoted by European Pediatric Orthopedic Society (EPOS) in 1999, and six were new patients. There were 30 females and 26 males (mean age 12.5 years; range 1–42 years). Twenty-three had monostotic FD, 9 polyostotic FD, and 24 McCune-Albright Syndrome (MAS). Scoliosis was observed in 11 cases of polyostotic FD and MAS (33.3%). In seven of the patients with scoliosis (63.3%) spine was involved by FD lesional tissue. FD lesions involved the thoracic or lumbar spine in all patients but one, where cervical spine was also affected. A correlation between scoliosis and either spinal (p < 0.01) or pelvic lesions (p < 0.05) and pelvic obliquity (p < 0.01) was observed. Three of the 11 patients showed familiarity for scoliosis but in 2 of them spine was involved by FD. Scoliosis and spine involvement were never detected in monostotic FD. This study indicates that in FD patients with polyostotic disease (1) the prevalences of FD involvement of the spine and scoliosis are high enough to include spine in the clinico-radiographic survey of these patients, and (2) the involvement of the spine and pelvis by FD lesions and pelvic obliquity are important determinants in the occurrence of scoliosis.
Keywords: Scoliosis, Fibrous dysplasia, Spine, Pelvic obliquity, Bone lesion
Introduction
Fibrous dysplasia (FD) of bone is a genetic, non-inherited disease caused by somatic activating missense mutation of the GNAS gene [21] in which the normal bone-bone marrow organ is replaced by fibro-osseous tissue [2, 11]. FD represents approximately 7% of all benign tumor-like bone lesions and may affect the skeleton either in isolation (monostotic and polyostotic FD) or in variable combination with endocrine and cutaneous abnormalities (McCune-Albright Syndrome, MAS) [1, 2, 11, 12, 21].
Few studies have focused on FD involvement of the spine and on the prevalence of scoliosis in patients with FD. FD has been described in each segment of the spine, with the highest prevalence in the lumbar region [5, 8–10, 13, 15, 18–20, 22, 23]. Malignant sarcomatous transformation of FD of the spine has also been reported [14]. In the pivotal study by Leet et al. [15], in which 62 patients with polyostotic FD were examined by bone scanning, the prevalence of FD involvement of the spine and scoliosis were 63 and 40%, respectively, and development of scoliosis was strongly correlated with the presence of FD lesions in the spine and with pelvic obliquity as well. However in that study, the cohort mainly included patients with a larger disease burden than those who are treated in the community at large. For this reason, we decide to determine retrospectively the prevalence of scoliosis and FD involvement of the spine in a series of 56 patients affected by FD of bone.
Materials and methods
Patients
Fifty-six patients (30 females and 26 males; mean age 12.5 years; range 1–42 years) affected by FD of bone were included in this study. Fifty patients were selected from the cohort reported in a multicentric study on FD promoted by the European Pediatric Orthopedic Society (EPOS) in 1999 [11] based on the availability of medical records and imaging material appropriate for the diagnosis of scoliosis and for the identification of FD lesions in the spine. The remaining six patients were new patients. Back pain, familiarity for scoliosis, “cafè-au-lait spots” and endocrinopathies were registered for the patients in which these information were available.
Diagnosis, magnitude, and progression of scoliosis
The patients clinically examined by the authors included 28 already published cases [11] and the six new cases. These patients underwent to a comprehensive anamnestic and clinical evaluation and radiographic analysis including standing postero-anterior and lateral radiographs of the spine and bone scan when either spine curvature or spine FD involvement were clinically suspected (Fig. 1). In the other (22 out of 56) already published cases [11], retrospective material was reviewed and the diagnosis of scoliosis and FD involvement of the spine was based on the available clinical and imaging data. The types of curvature were established according to the traditional anatomically based classification, and the magnitude of the curvature by measurement of the Cobb angle [6]. When possible, radiographs were preferred to bone scan based on the well-known underestimation of the magnitude of the curvature by the latter [15]. Pelvic obliquity was assessed by measuring the angulation between a line drawn along the top of the pelvis and the horizontal plane after appropriate balance of the lower limbs in those cases with leg length discrepancy.
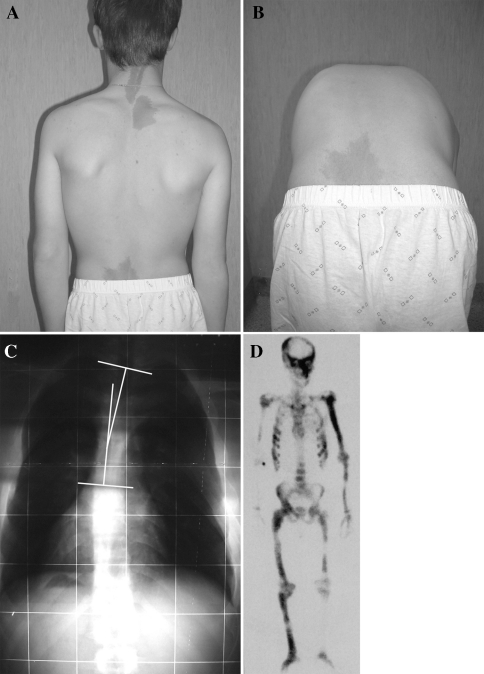
Fig. 1.
Clinical pictures (a, b) showing a patient with MAS in which scoliosis was suspected and confirmed by postero-anterior radiographic examination of the spine (c). Three cafè-au-lait spots are located along the posterior midline (a). The angle of Cobb, measured on the postero-anterior radiograph was T2–T10 15°. By bone scan (d) spine FD involvement can be detected but the diagnosis of scoliosis and, in particular, measure of its magnitude are hard to be established
In two patients with MAS, we had the opportunity to evaluate the radiographic progression of the curves. In both patients spine radiographs performed at the time of diagnosis of FD (4 and 10 years) were available. Radiographs were also obtained at the age of 12 and 23 in the first patient and at the age of 18 and 25 in the second.
Statistical analysis
For all patients with polyostotic FD, we analyzed the association of scoliosis with FD lesions in the long bones of the lower limbs, pelvis, and spine, and with pelvic obliquity. In addition, we analyzed the association of FD involvement of the spine with the involvement of other skeletal segments. The analysis was performed with the Fisher exact test. A probability level of 5% was used to establish significance of differences.
Results
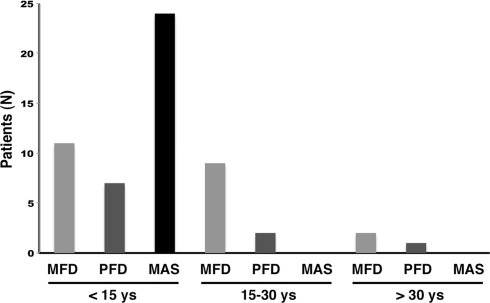
The clinical characteristics of the patients included in this study is summarized in Table 1. Twenty-three patients were affected by monostotic FD (41%), 9 by polyostotic FD (16%) and 24 (43%) by MAS. Of the 56 patients, 53 were younger than 30 years old at the time of diagnosis of FD (Fig. 2). Back pain was not reported by patients with monostotic FD. Among the patients with polyostotic disease, back pain was reported only by those patients with FD involvement of the spine.
Table 1.
Clinical characteristics of the patients included in this series
| Diagnosis | No. of patients | Gender (M/F) | Mean age at the diagnosis (year) | Age range at the diagnosis (year) |
|---|---|---|---|---|
| Monostotic FD | 23 | 10/13 | 16.9 | 7–42 |
| Polyostotic FD | 9 | 5/4 | 14.3 | 6–34 |
| MAS | 24 | 11/13 | 6.3 | 1–14 |
Fig. 2.
Age distribution of the patients with monostotic FD, polyostotic FD and MAS included in this study
In all patients with monostotic FD, clinical evidence of scoliosis was absent. The FD lesion involved the femur in 14 patients, tibia in 4, humerus in 2, and clavicle, mandible, and rib, in 1 patient each.
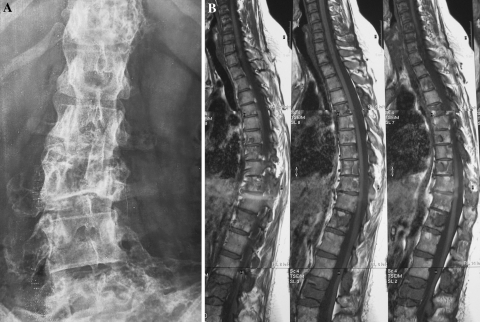
The skeletal segments involved in patients with polyostotic FD are summarized in Table 2. None of these patients showed pelvic obliquity. In one female patient (Table 3; #1), in which the diagnosis of polyostotic FD was made at the age of 18, scoliosis was clinically suspected at the age of 39. A radiograph revealed scoliosis with a lumbar curve L1–L5 of 20° (Fig. 3a). Extensive FD involvement of the cervical- thoraco-lumbar spine was detected by bone scan and MRI (Fig. 3b).
Table 2.
Skeletal segments involved by FD lesion in the patients with polyostotic FD and with MAS included in this study
| Polyostotic FD | MAS | Total | ||||
|---|---|---|---|---|---|---|
| Skeletal segments | Patients | Skeletal segments | Patients | Skeletal segments | Patients | |
| Femur | 8 | 7 | 39 | 24 | 47 | 31 |
| Tibia | 6 | 5 | 26 | 19 | 32 | 24 |
| Fibula | 0 | 0 | 16 | 13 | 16 | 13 |
| Clavicle | 0 | 0 | 2 | 2 | 1 | 1 |
| Scapula | 0 | 0 | 5 | 3 | 5 | 3 |
| Humerus | 3 | 2 | 23 | 14 | 26 | 16 |
| Radius | 1 | 1 | 12 | 10 | 13 | 11 |
| Ulna | 0 | 0 | 9 | 7 | 9 | 7 |
| Skull | 1 | 1 | 15 | 15 | 16 | 16 |
| Ribs | 2 | 2 | 9 | 9 | 11 | 11 |
| Pelvis | 5 | 5 | 18 | 18 | 23 | 23 |
| Spine | 1 | 1 | 6 | 6 | 7 | 7 |
Table 3.
Clinical synopsis of the patients with scoliosis and with or without FD involvement of the spine included in this study
| ID | Age/gender | Diagnosis | Scoliosis | Familiarity for scoliosis | Spine FD (location) | Other FD involved skeletal sites | PO |
|---|---|---|---|---|---|---|---|
| #1 | 18/F | PFD | L1–L5 20° | No | Yes (Cervical, thoracic and lumbar) | Skull, pelvis, ribs | No |
| #2 | 11/F | MAS | T2–T6 40° T7–T12 40° |
Yes | No | L femur, pelvis | No |
| #3 | 3/M | MAS | T4–T10 55° T11–L5 80° |
Yes | Yes (Thoracic and lumbar) | Skull, humeri, R radius, scapulae, pelvis, femura, tibiae, fibulae, | Yes |
| #4 | 9/F | MAS | T7–L1 18° | Yes | Yes (Thoracic and lumbar) | Skull, L scapula, humeri, pelvis, L femur, L tibia | Yes |
| #5 | 4/F | MAS | T4–L3 20° | No | Yes (Thoracic and lumbar) | Pelvis, L femur, L tibia, L fibula | Yes |
| #6 | 7/M | MAS | T7–T11 19° T12–L4 23° |
No | No | Skull, L clavicle, L humerus, ribs, pelvis, femura, tibiae | No |
| #7 | 14/M | MAS | T1–T4 15° | No | Yes (Thoracic and lumbar) | Skull, humeri, R radius, R ulna, ribs, pelvis, R femur, R tibia, R fibula | No |
| #8 | 3/M | MAS | T3–T9 20° T10–L3 25° |
No | No | Skull, humeri, R radius, R ulna, ribs, pelvis, femura, tibiae, fibulae | No |
| #9 | 4/M | MAS | T4–T10 100° T11–L5 100° |
No | Yes (Thoracic and lumbar) | Skull, mandible, L clavicle, scapulae, humeri, radii, ulnae, ribs, pelvis, femura, tibiae, fibulae | Yes |
| #10 | 3/F | MAS | L1–L4 24° | No | No | Skull, humeri, radii, R ulna, ribs, pelvis, femura, tibiae, fibulae | Yes |
| #11 | 14/M | MAS | T2–T10 15° | No | Yes (Thoracic and lumbar) | Skull, L humerus, L radius, L ulna, ribs, pelvis, femura, R tibia, R fibula | No |
PFD polyostotic fibrous dysplasia, MAS McCune-Albright Syndrome, PO pelvic obliquity, R right, L left
Fig. 3.
Patient with polyostotic FD in which the diagnosis was made at the age of 18 (#1 in Table 3). At the age of 39 scoliosis was clinically suspected. a Postero-anterior radiography of the spine confirmed the clinical diagnosis of scoliosis with the curve measuring L1–L5 20°. b MRI showed extensive FD involvement of the thoraco-lumbar spine
The skeletal segments involved in patients with MAS are summarized in Table 2. Spine FD involvement was observed in 6/24 patients. “Cafè-au-lait spots” were present in all the patients in which clinical evaluation was possible (16 patients). The most frequent endocrinopathy was precocious puberty (10 patients). Scoliosis was diagnosed in 10/24 patients with MAS (Table 3: #2–#11). Six of these patients showed FD involvement of the spine and in five of them the involved vertebrae were within the curve. In two patients (Table 3: #3 and #9), thoracic hyperkyphosis was detected on lateral radiographic view. Scoliosis was thoraco-lumbar in four patients (two without spine FD and two with spine FD), thoracic in four (all patients with spine FD), double thoracic in one (without spine FD) and lumbar in one (without spine FD). Five patients showed pelvic obliquity due to scoliosis and lower limb inequality determined by tibia and femur FD affection. In three of the MAS patients with scoliosis familiarity for this condition was observed (Table 3: #2–#4). In two of them (66.6%), however, the spine was affected by FD.
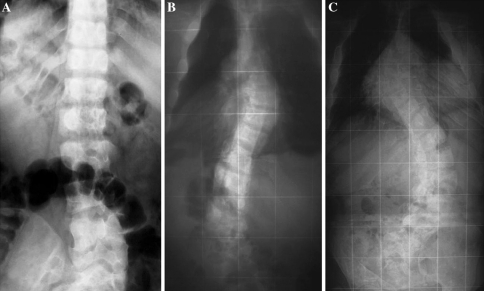
In two patients with MAS diagnosed at the age of 10 and 4, respectively (Table 3: #3 and #9), we had the opportunity to evaluate the progression of the scoliosis. In both cases, in which the most severe curves of this series were observed (T4–T10 55°/T11–L5 80° and T4–T10 100°/T11–L5 100°), FD involvement of the spine was documented by bone scan. Of note, in both cases the scoliosis progressed during skeletal growth and after its cessation as well (Fig. 4).
Fig. 4.
Development and progression of scoliosis in a 25-year-old boy with MAS (#3 in Table 3). At the age of 10 (a), scoliosis is absent. At the age of 18 (b), scoliosis was present with curves measuring T4–T10 36° and T11–L5 50° (b). At the age of 25 (c), scoliosis is significantly worsened with curves measuring T4–T10 55° and T11–L5 80° and both thoracic and lumbar vertebral bodies are markedly deformed
Analysis of the patients with polyostotic disease (PFD and MAS) included in our series revealed that the prevalence of scoliosis was 33.3% (11 patients out of 33), that the spine was involved with FD lesions in 21.2% (7 patients out of 33) and that scoliosis and FD involvement of the spine were associated with one another in 63.6% (7 patients out of 11) (Table 4). Scoliosis was significantly more frequent in patients with FD involvement of spine compared to patients without (100 vs. 15.3%, p < 0.01) and in patients with FD involvement of the pelvis (47.8 vs. 0%, p < 0.05) and pelvic obliquity compared to patients with horizontal pelvis (100 vs. 21.4%, p < 0.01). In addition, FD involvement of the spine was significantly more frequent in patients with FD involvement of skull (37.5 vs. 5.8%, p < 0.05) and pelvis (30.4 vs. 0%, p = 0.05) compared to patients without.
Table 4.
Prevalence of scoliosis and FD involvement of the spine in the patients included in this study
| Scoliosis | Spine FD | Scoliosis + Spine FD | |
|---|---|---|---|
| Monostotic FD | 0/23 | 0/23 | 0/23 |
| Polyostotic FD | 1/9 (11.1%) | 1/9 (11.1%) | 1/1 (100%) |
| MAS | 10/24 (41.6%) | 6/24 (25.0%) | 6/10 (60.0%) |
| Polyostotic FD + MAS | 11/33 (33.3%) | 7/33 (21.2%) | 7/11 (63.6%) |
Of the 11 patients with scoliosis, one (Table 3: #2) was surgically treated by instrumented spinal fusion. This patient was affected by MAS, and showed a double thoracic curve not associated with spine FD involvement. Three years after surgery the clinical and radiographic outcome is still excellent. Scoliosis was not treated in the other 10 patients because deformity was mild (Table 3: #1, #4–#8, #10, #11) or the surgical procedure was considered unsafe for cardiopulmonary problems (Table 3: #3 and #9).
Discussion
Our study confirms recent data that indicated that the involvement of the spine by FD lesions, and scoliosis in patients with FD, are both more common than previously appreciated. Interestingly, based on the data from our series, both spinal involvement and scoliosis occur only in polyostotic FD/MAS. In fact, none of the 23 patients with monostotic FD had either scoliosis or involvement of the spine. This finding is in keeping with data in the literature, indicating that the prevalence of spinal involvement in monostotic FD is extremely low [9]. In our study, the prevalence of scoliosis in patients with polyostotic disease was 33.3% (11/33 patients) and that of FD involvement of the spine was 21.2% (7/33 patients). These values were much lower compared to those reported by Leet et al. [15]. The greater prevalence of both FD involvement of the spine and scoliosis in the study by Leet might be due to inclusion of patients with larger disease burden in this series compared to ours, which more directly reflects the characteristic of unselected patients that are seen and treated in the community at large. However, the analysis of our data confirms the greater frequency of scoliosis in patients with FD involvement of the spine and with pelvic obliquity previously reported. In addition, we found that scoliosis was significantly more frequent in patients with FD involvement of the pelvis compared to patients with uninvolved pelvis. Compared to the study of Leet et al. [15], in which no association was found between FD of the spine and FD lesions in other skeletal segments, we found that FD involvement of the spine is more frequent in patients with FD lesions in the pelvis and, in particular, in the skull. This finding suggests that involvement of the spine may occur as part of a distinct axial pattern of lesion distribution across the skeleton.
Several studies have shown the familial nature of idiopathic scoliosis supporting the concept that a genetic component plays a role in its development [3, 7, 14]. Based on data from our series, familiarity was observed in 3 of the 11 patients with scoliosis with a prevalence (27.2%) comparable to that of general population [17]. Because the prevalence of scoliosis in polyostotic FD is much greater than that in otherwise normal children and adolescents, in which it has been estimated to be lower than 3% [4, 16], FD might play an additive role in the development of scoliosis in those FD patients in which familiarity for scoliosis does exist, but the numbers of this study do not allow a final statement.
The natural history of the spinal pathology in FD is unknown because no prospective study has been published. In two MAS patients of our series, we observed a rapid progression of the curves occurring during skeletal growth and after its cessation. The extensive FD involvement of the spine in both patients suggest that FD-dependent vertebral shape changes and spine deformity may contribute to the progression of the curves after the end of skeletal growth as well when FD lesions are notoriously thought to stop their growth [2]. Obviously, the analysis of larger series of patients are mandatory to confirm this view.
One of the main problem in patients with spine FD and scoliosis regards the opportunity and feasibility of surgical treatment. Because in our series, only one of the 11 patients with scoliosis was surgically treated, a final statement cannot be definitely established.
Our data confirm that scoliosis is an important clinical feature in the spectrum of FD with polyostotic bone involvement. For this reason, patients with polyostotic FD need to undergo appropriate clinico-radiographic investigation for FD involvement of the spine and scoliosis. Bone scan and radiographic examination of the spine in both postero-anterior and lateral projections must be included in the evaluation of each FD patient with polyostotic involvement. Once the diagnosis of scoliosis and/or spine FD involvement is made, each individual FD patient should be closely monitored to evaluate progression of scoliosis and to establish the feasibility of a surgical treatment.
Acknowledgments
The authors wish to thank Edward W. Bray (Shriners Hospitals, Greenville, South Carolina, USA), Ulrich G. Exner (Balgrist Orthopedic University Health Centre, Zurich, Switzerland), Pamela Gehron Robey (Craniofacial and Skeletal Diseases Branch, National Institute of Dental and Craniofacial Research, National Institute of Health, Bethesda, Maryland, USA), Franz Grill and Oswald Pinggera (Orthopadisches Spital Speising GmbH, Vienna, Austria), Roberto Lala (Division of Pediatric Endocrinology, Regina Margherita Hospital, Torino, Italia), Marco Massobrio (Department of Orthopedic Surgery, La Sapienza University, Rome, Italy), Slawomir Snela (Szpital Wojewodski, Rzeszow, Poland), Christos Zambakidis (P&A Kiriakou Children’s Hospital, Athens, Greece), and Paolo Bianco (Department of Experimental Medicine, La Sapienza University, Rome, Italy) for their adhesion and participation to the multicenter study on fibrous dysplasia promoted by the European Pediatric Orthopaedic Society in 1999.
References
- 1.Albright F, Butler AM, Hampton AO, Smith P. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females. Report of five cases. N Engl J Med. 1937;216:727–746. [Google Scholar]
- 2.Bianco P, Robey PG, Wientroub S. Fibrous dysplasia. In: Glorieux FH, Pettifor J, Juppner H, editors. Pediatric bone—biology and disease. New York: Academic Press; 2003. pp. 509–539. [Google Scholar]
- 3.Bonaiti C, Feingold J, Briard ML, Lapeyre F, Rigault P, et al. Genetics of idiopathic scoliosis. Helv Paediatr Acta. 1976;31:229–240. [PubMed] [Google Scholar]
- 4.Bunnell WP. The natural history of idiopathic scoliosis before skeletal maturity. Spine. 1986;11:773–776. doi: 10.1097/00007632-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Chow LT, Griffith J, Chow WH, Kumta SM. Monostotic fibrous dysplasia of the spine: report of a case involving the lumbar transverse process and review of the literature. Arch Orthop Trauma Surg. 2000;120:460–464. doi: 10.1007/PL00013774. [DOI] [PubMed] [Google Scholar]
- 6.Cobb JR (1948) Outline for the study of scoliosis. In: instructional course lectures, The American Academy of Orthopaedic Surgeons. Ann Arbor, Edwards JV vol 5, pp 261–275
- 7.Czeizel A, Bellyei A, Barta O, Magda T, Molnar L. Genetics of adolescent idiopathic scoliosis. J Med Genet. 1978;15:424–427. doi: 10.1136/jmg.15.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehara S, Kattapuram SV, Rosenberg AE. Fibrous dysplasia of the spine. Spine. 1992;17:977–979. doi: 10.1097/00007632-199208000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Guille JT, Bowen JR. Scoliosis and fibrous dysplasia of the spine. Spine. 1995;20:248–251. doi: 10.1097/00007632-199501150-00024. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman KL, Bergman AG, Kohler S. Polyostotic fibrous dysplasia with severe pathologic compression fracture of L2. Skeletal Radiol. 1995;24:160–162. doi: 10.1007/BF00198085. [DOI] [PubMed] [Google Scholar]
- 11.Ippolito E, Bray EW, Corsi A, Maio F, Exner UG, et al. Natural history and treatment of fibrous dysplasia of bone: a multicenter clinicopathologic study promoted by the European Pediatric Orthopedic Society. J Pediatr Orthop B. 2003;12:155–177. doi: 10.1097/00009957-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Jaffe HL. Fibrous dysplasia. Tumors and tumorous conditions of the bones and joints. Philadelphia: Lea & Febiger; 1958. pp. 117–142. [Google Scholar]
- 13.Janus GJ, Engelbert RH, Pruijs JE. Instrumentation for correction and fixation of scoliosis in fibrous dysplasia of the thoracolumbar spine. Eur Spine J. 1998;7:260–262. doi: 10.1007/s005860050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesling KL, Reinker KA. Scoliosis in twins. A meta-analysis of the literature and report of six cases. Spine. 1997;22:2009–2014. doi: 10.1097/00007632-199709010-00014. [DOI] [PubMed] [Google Scholar]
- 15.Leet AI, Magur E, Lee JS, Wientroub S, Robey PG, et al. Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis. J Bone Joint Surg Am. 2004;86:531–537. [PubMed] [Google Scholar]
- 16.Lonstein JE, Carlson JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am. 1984;66:1061–1071. [PubMed] [Google Scholar]
- 17.Martin Martin J, Rodriguez Blanco C, Eguren Hernandez EM, Diaz Polegre R, de Leon Garcia F, et al. Family prevalence of idiopathic scoliosis. An Esp Pediatr. 1997;46:148–150. [PubMed] [Google Scholar]
- 18.Nabarro MN, Giblin PE. Monostotic fibrous dysplasia of the thoracic spine. Spine. 1994;19:463–465. doi: 10.1097/00007632-199402001-00016. [DOI] [PubMed] [Google Scholar]
- 19.Resnik CS, Lininger JR. Monostotic fibrous dysplasia of the cervical spine: case report. Radiology. 1984;151:49–50. doi: 10.1148/radiology.151.1.6701333. [DOI] [PubMed] [Google Scholar]
- 20.Troop JK, Herring JA. Monostotic fibrous dysplasia of the lumbar spine: case report and review of the literature. J Pediatr Orthop. 1988;8:599–601. doi: 10.1097/01241398-198809000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 22.Wright JFC, Stoker DJ. Fibrous dysplasia of the spine. Clin Radiol. 1988;39:523–527. doi: 10.1016/S0009-9260(88)80222-8. [DOI] [PubMed] [Google Scholar]
- 23.Yalniz E, Er T, Ozyilmaz F. Fibrous dysplasia of the spine with sarcomatous transformation: a case report and review of the literature. Eur Spine J. 1995;4:372–374. doi: 10.1007/BF00300303. [DOI] [PubMed] [Google Scholar]