Abstract
Interbody cages are widely used instruments for cervical fusion operations. Long-term follow-up studies are needed to clarify if these devices are dependable. In this prospective study, 79 patients (42 women and 37 men) with a mean age of 51 years operated between January 2000 and December 2005 for treatment of degenerative cervical disc disease and spondylosis associated with radiculopathy or myelopathy were evaluated. Patients underwent two-level contiguous anterior cervical discectomy and fusion operations with standard anterior Smith–Robinson approach. To achieve fusion PEEK cages packed with demineralized bone matrix mixed with autologous blood were used. Clinical outcome was evaluated with Odom’s criteria and results were evaluated as ‘excellent’, ‘good’, ‘fair’ and ‘poor’. Spinal curves, mobility and fusion status were assessed with anterior–posterior and lateral (neutral, flexion and extension) radiographs obtained before surgery and at 3, 12, 24 and 36 months postoperatively. The Ishihara curvature index (ICI) was used for spinal curve evaluation. Lateral dynamic (flexion and extension) radiographs at postoperative 12th month revealed the fusion status classified as 1A, 1B, 2A and 2B. The radiological outcomes were classified as ‘non-fusion’ when 2B healing was observed, and as ‘fusion’ when 1A, 1B or 2A healing was observed at the levels subjected to surgery. According to Odom’s criteria, clinical outcomes were classified as ‘excellent’ or ‘good’ in 69 patients (success rate: 87.3%). Eight patients were graded as ‘fair’ and two as ‘poor’. Preoperative mean ICI was 10.4 ± 3.72 and postoperative mean ICI was 10.1 ± 3.14. The difference was statistically insignificant (P > 0.05); therefore, preoperative lordosis was said to be preserved at final follow-up. Final fusion rate (Types 1A, 1B, and 2A) was 91.7% (145/158 levels). Radiological imaging showed no cage failure or dislodgement and reoperation due to non-fusion was not needed.
Keywords: ACDF, Two-level cervical disc disease, Demineralized bone matrix, Long-term follow-up, PEEK cage
Introduction
Anterior cervical discectomy and interbody fusion (ACDF) has become a standard and a highly successful surgical procedure for degenerative cervical disc disease associated with radiculopathy or myelopathy [10, 20]. The success rates decline in multilevel discectomies as the number of operated levels increase [10].
The literature have supported a 20–27% consistent rate of nonfusion (pseudoarthrosis) for two-level anterior discectomy and autogenous bone fusion [3, 12, 40]. Non-fusion accounts for 80% of spinal surgery failures and graft collapse with autogenous bone is also reported in 20–30% of multilevel fusion operations [18, 30]. There has been an advent of various types of cages to avoid the problems associated with autologous bone grafting. These problems include persistent donor-site pain, infection, hematoma formation, iliac crest fracture and meralgia parasthetica [21]. The use of cages obviates these complications.
Many new interbody fusion cages have been developed, but long-term clinical studies with the use of these material are still scarce [7, 16, 31, 34]. An ideal cage should provide immediate stability, maintain spinal alignement and foraminal height, achieve higher or comparable fusion success rates with autografts [21]. To date, no perfect cage has been produced. Subsidence, migration, and structural failure of the present products [36] have been experienced [6].
Polyetheretherketone (PEEK) is a non-absorbable biopolymer that has been used in a variety of medical devices. It is biocompatible, radiolucent and has modulus of clasticity similar to the bone [21]. PEEK cages are commercially available, but clinically reliable reports of the PEEK cages are rare in the literature [6].
To the authors’ knowledge, a similar study about the long-term clinical and radiological outcome of the two-level contiguous ACDF operations with the use of PEEK cages has not been reported previously [10, 20, 24]. The purpose of this prospective study is to evaluate the effectiveness of ACDF using PEEK cages packed with demineralized bone matrix and autologous blood for two-level degenerative cervical disc disease and to present the long-term clinical and radiological outcomes.
Materials and methods
This prospective study was performed with 79 patients (42 women and 37 men) with a mean age of 51 years (range, 37–72 years), who underwent two-level contiguous ACDF operations between January 2000 and December 2005, with the use of PEEK cages (Spine Next, Bordeaux-France and Eurospine, L’Hay-Les-Roses, France) packed with demineralized bone matrix (DBM) (Grafton, Osteotech, Eatontown, NJ, USA) mixed with autologous blood.
Patients with tumors, fractures, or infections were excluded. Only patients operated for degenerative cervical disc disease and spondylosis associated with radiculopathy or myelopathy were included. Conforming to international ethical standards, all patients were given detailed information on the operation, the follow-up protocol and radiological investigations, and signed consent was obtained from every patient. Indications for operation were intractable radiculopathy and/or myelopathy due to compression proven with the preoperative radiographs (anteroposterior and lateral) and magnetic resonance images (MRI) .
Surgical procedure was standard anterior Smith–Robinson approach. The disc, posterior longitudinal ligament, and osteophytes, including the posterior part of the uncinate process were removed microsurgically. The upper and lower endplates were prepared by removing the overlying cartilage preserving the hardest subchondral bone. Vertebral bodies were distracted with a Caspar distractor and an optimal PEEK cage was selected. The inner cavity of the PEEK cage was filled with DBM mixed with 1 ml of autologous venous blood supplied by anesthesiology team during the operation. Eventually PEEK cages were inserted into the disc space. All patients used Philadelphia collar for 6 weeks postoperatively. Neck exercises were initiated 6 weeks after surgery and a normal activity level was progressively resumed.
Odom’s criteria was used to evaluate the clinical outcomes. The results were classified as ‘excellent’ (no complaints related to the cervical lesion with a smooth return to daily activities and work); ‘good’ (intermittent complaints related to the cervical lesion without any serious difficulties in performing daily activities and work); ‘fair’ (patient satisfied with the postoperative improvement of the subjective symptoms but with difficulties in performing daily activities and work); or ‘poor’ (deterioration or no improvement of the symptoms after the surgery) [21, 33].
Spinal curves, mobility and fusion status were assessed with anterior–posterior and lateral (neutral, flexion and extension) radiographs. Spine radiographs were obtained before surgery and at 3, 12, 24 and 36 months postoperatively. An independent radiologist evaluated these radiographs without the knowledge of clinical outcome.
Lateral X-rays were used to evaluate the spinal curve pre and post-operatively. The Ishihara curvature index (ICI) was used for this evaluation [17]. A straight line was drawn from the posterior border of the dens to the posterior border of C7. Another line was drawn from the posterior border of C4 perpendicular to the first line, in which the intersected length was measured in millimeters as the degree of spinal curvature. A positive intersected length indicates the degree of lordosis. If the intersected length is negative, it indicates kyphosis. When the intersected length is zero, the spinal curve is referred to as straight.
Statistical analysis was performed to compare preoperative and postoperative ICI scores with dependent t tests using SPSS V.12.0 (SPSS Inc., Chicago, IL, USA).
Lateral dynamic (flexion and extension) radiographs obtained at postoperative 12th month were used to evaluate fusion at the operated levels. The operative levels were deemed to be fused if there were no movement on dynamic views. Additionally, the radiological images were evaluated using the classification of anterior fusion proposed by Vavruch et al. [38]. In this classification Type 1A is defined as bridging bone anterior and through the disc space; 1B as bridging bone anterior but not through the disc space; 2A as bridging bone not anterior but through the disc space; and 2B as no bridging bone at all. The radiological outcomes were classified as ‘non-fusion’ when 2B healing was observed, and as ‘fusion’ when 1A, 1B or 2A healing was observed at the levels subjected to surgery.
Results
Mean follow-up period was 31.3 months (ranged from 15 to 36 months). 87% of the begining population (79 cases) completed the 36-month follow-up and these formed the study population. C5–6 and C6–7 were the most common operated levels. Details of the treated levels are shown in Table 1.
Table 1.
Details of treated levels with fusion rates when both levels are fused
| Levels | Number of cases | Rate to total (%) | Number of fusions | Rate of fusion (%) |
|---|---|---|---|---|
| C3–4/4–5 | 8 | 10.10 | 8 | 100 |
| C4–5/5–6 | 17 | 21.50 | 15 | 88.20 |
| C5–6/6–7 | 52 | 65.80 | 45 | 86.50 |
| C6–7/C7–Th1 | 2 | 2.50 | 2 | 100 |
| Total/mean | 79 | 100 | 70 | 88.60 |
None of the patients suffered neurological deterioration due to operation. There were no complications during the immediate postoperative period, and the radiographs confirmed the appropriate positioning of the cages.
Cages used had all same depth (12 mm) and width (12 mm). Cage heights varied due to the presence of spondylosis and mobility of the vertebral bodies during retraction. Details are given in Table 2.
Table 2.
Cage heights for levels
| Mean cage heights and ranges (mm) | |
|---|---|
| C3–4 | 4.75 (4–5) |
| C4–5 | 5.11 (4–6) |
| C5–6 | 4.91 (4–6) |
| C6–7 | 5.07 (4–6) |
| C7–Th1 | 5.00 (5) |
According to Odom’s criteria, clinical outcomes were classified as ‘excellent’ or ‘good’ in 69 patients (success rate: 87.30%). Eight patients were graded as ‘fair’ and two as ‘poor’ though these ten patients achieved solid fusion at the final follow-up. Analysis of radiographic data of these ten patients showed that the post-operative segmental lordosis and the post-operative disc height were the same after surgery. The clinical results are given in Table 3. ‘Excellent’ and ‘good’ results are called as satisfactory outcomes. Patients were significantly better after the operation clinically (P < 0.05).
Table 3.
Clinical outcomes regarding Odom’s criteria
| Clinical outcomes due to Odom’s criteria | Number and rate of patients |
|---|---|
| Excellent | 27 (34.17%) |
| Good | 42 (53.16%) |
| Fair | 8 (10.12%) |
| Poor | 2 (2.53%) |
| Total | 79 |
Preoperative mean ICI was 10.40 ± 3.72 and postoperative mean ICI was 10.10 ± 3.14. The difference was statistically insignificant (P > 0.05); therefore, preoperative lordosis was said to be preserved at final follow-up.
At the final follow-up, the fusion rate (Types 1A, 1B, and 2A) was 91.70% for levels (145/158 levels) and 88.60% for cases (70/79 cases). The rate of Type 1A fusion was 37.30% (59/158 levels) (Fig. 1), the rate of Type 1B fusion was 40.50% (64/158 levels), the rate of Type 2A fusion was 13.90% (22/158 levels), and the rate of Type 2B non-fusion was 8.20% (13/158 levels) (Fig. 2). There were nine patients with non-fused levels: two non-fused levels in four patients (C5–6 and C6–7 levels) and one non-fused level in five patients (C4–5, C5–6 or C6–7 level). Development of fusion rates in the follow-up period is denoted in Table 4. In long-term follow-up, imaging showed no cage failure or subsiding. Reoperation for non-fusion was not needed. In addition, no cage mobility was observed on dynamic radiographs.
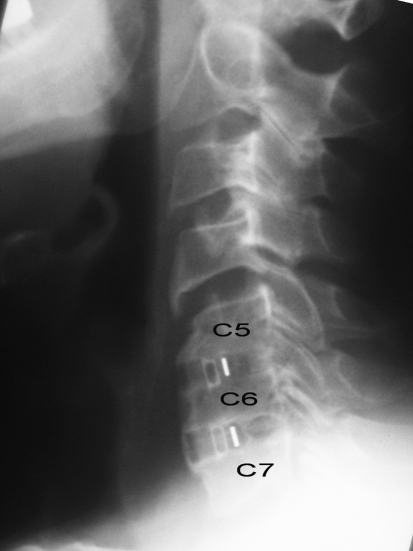
Fig. 1.
Solid fusion in both C5–6 and C6–7 levels
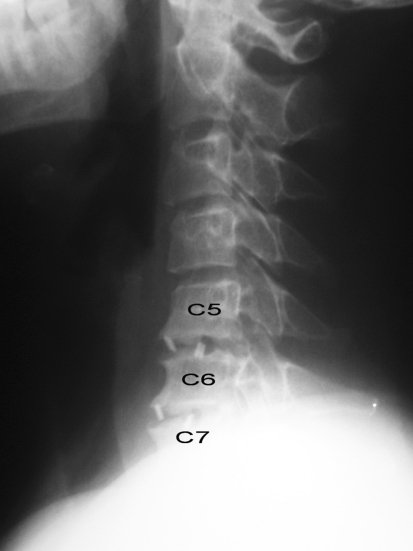
Fig. 2.
Clear bone development in both levels but no bridging at all. Patient still suffered neck pain at postoperative third year
Table 4.
Development of fusion rates (for individual levels)
| Months | ||||
|---|---|---|---|---|
| 3 | 12 | 24 | 36 | |
| Fusion rates (%) | 53.79 | 69.62 | 91.70 | 91.70 |
Discussion
ACDF has become a well-established operative procedure used to treat a variety of cervical spinal disorders. The success of this procedure relies on thorough decompression and development of a solid osseous fusion [26, 38]. But a few issues, of which the optimal graft material and the necessity for additional stabilization compose the majority are still controversial [20].
Traditionally, cervical stabilization biomechanics have been investigated in three topics: single level, two levels, multi (more than two) levels. Due to the different structural behaviour of the above mentioned, particularly in regard to adjacent segment mobility and the need of additional instrumentation (plates), in this study only contiguous two level ACDF cases were included [8].
Although tricortical autograft harvested from iliac crest as interbody fusion material can provide satisfactory clinical results and fusion rates, donor-site complications are around 20% [1, 21]. Brown and Savolainen reported 97–98% fusion rate in patients who underwent fusion procedures with autografts [10, 28]. In their series Sampath et al. denoted 92–100% fusion rate and 70–90% neurological and symptomatic improvement for single-level discectomy with autogenous bone fusion [26, 27].
The morbidity of the iliac bone harvest can often tarnish these results [25, 32, 35]. The literature also reports a consistent rate of 10–12% non-fusion for single-level anterior discectomy and autogenous bone fusion, 20–27% for two-level, and approximately 30–56% for three-level fusions [12, 38]. It is clear that the success rates decline as the number of operated levels increase. A similar comparison study found a 25% or greater graft collapse in 40% of patients treated with autografts, but no graft collapse was found in patients who received an anterior plate [15].
To eliminate autograft-related problems, interbody cages were developed for fusion operations. Use of cages resulted in a high fusion rate but there were no statistically significant differences in operation time, length of hospitalization, pain reduction or clinical results. Ludek et al. compared a historical group of patients receiving tricortical iliac autografts to those with cages packed with smaller autografts [22]. The cage group had less donor site pain and a better fusion rate with better preservation of postoperative lordosis and increase in disc space. Savolainen et al. also reported that these radiological differences were not related to the clinical results [2]. In our study, no cage failure or migration even in patients with non-fused levels, was encountered. Cages were found to preserve the spinal lordosis and the foraminal height [5, 6, 11].
Cervical interbody cages have been developed to provide immediate stability and high fusion rates with and without supplemental fixation [20]. In the light of the literature, in multilevel ACDF procedures, augmentation with plate fixation, may seem to be preferable due to higher fusion and lower reoperation rates, and better pain relief [4, 29]. In spite of these benefits, anterior fixation plates have the potential for morbidity. Plate complication rate varies from 2.2 to 24.0% and includes screw pullout, screw breakage, injury of the laryngeal nerve, injury of oesophagus, prolonged dysphagia, injury of spinal cord or root, injury of vertebral artery, and wound infection [9, 10]. Additionally, the operative time is usually longer.
Titanium, carbon fiber, and PEEK are most commonly used material for cage production [2, 13, 31]. The use of a titanium cage may lead to vertebral body collapse if the end plate is over degraded during discectomy. In addition, radiological metallic artifacts may complicate imaging. Furthermore, radiotransparent carbon fiber cages have been used widely, but synovitis and the lymphatic spread of fiber debris may be found after intra-articular procedures [6, 23].
In the present study, PEEK cages were applied in all operations. PEEK is a semicrystalline polyaromatic linear polymer that provides a good combination of strength, stiffness, toughness, and environmental resistance with biocompatible, non-absorbable, and corrosion-resistant abilities [10, 19, 39, 40]. Furthermore, the cage structure (two titanium spikes on the upper and bottom frame, in addition to the retention teeth on the surface of the upper and bottom frame) offers a fixation mechanism, which is similar to the functions of a plate and screws [10]. Volume-related stiffness of the PEEK cage was shown to be higher than that of iliac bone in all directions. [14]. In addition, the PEEK cage is radiolucent and does not produce artefacts, it is easy to evaluate fusion status on radiographs and CT scans.
Cage-assisted ACDF has proven to be a safe and effective procedure for the treatment of degenerative cervical disc disease. In our series, the fusion rate was 91.7% which is comparable with the literature. There were nine patients with non-fusion. Despite the radiological failure, no clinical signs were observed in these patients during the follow-up period.
Usually, cages used in ACDF are packed with bone grafts, demineralized bone matrix, bone morphogenetic protein or acrylate to reach early and solid fusion. If the autologous bone graft is obtained not from the operation site, donor-site complications are still unavoidable. However, in most of these studies, cages packed with cancellous bone were used. Recent reports about the use of bone morphogenetic protein emphasize the high complication rates [37]. Studies about acrylate packing are scarce. To minimize the extent of surgery, and to avoid donor-site complications, we packed the cages with DBM mixed with autologous blood.
The purpose of this study was to evaluate the long-term outcomes of ACDF with PEEK cages. Most studies have presented short-term results and have demonstrated an improvement in pain, neurological deficit and functional disability [24]. In the literature, long-term follow-up studies are mostly retrospective with no assessment of functional disability and therefore of limited potential for firm conclusions on the effectiveness of the treatment [24]. In our study, clinical outcomes were rated as excellent or good in 69 out of 79 patients (success rate: 87.3%). Analysis of radiographic data of these unsatisfied ten patients showed that the post-operative segmental lordosis and the post-operative disc height were kept after surgery. All these cases suffered radiculopathy without neck pain giving rise to the thought that dissatisfaction might have been obviated with the implantation of higher cages to achieve a better foraminal space.
To the authors’ knowledge, long-term clinical and radiological results of two-level ACDF with the use of PEEK cages and DBM have not been reported in the literature.
Conclusion
The results obtained in this study are encouraging and provide an impetus to the use of interbody cage in the treatment of two-level degenerative cervical disc disease.
In the light of our findings, we conclude that interbody fusion with PEEK cages packed with DBM is a safe and a good option for the treatment of patients with contiguous two-level cervical disc disease. It creates cervical lordosis, provides space for cord and root decompression, facilitates radiological follow-up, obviates the complications of graft harvest and leads to satisfactory outcomes without the need of any additional device. The controversies about the effect of packing material to the fusion status need to be eloborated with further studies.
References
- 1.Arringon ED, Smith VJ, Chambers HG, et al. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 2.Bartels RH, Donk R, Azn RD. Height of cervical foramina after anterior discectomy and implantation of a carbon fiber cage. J Neurosurg. 2001;95:40–42. doi: 10.3171/spi.2001.95.1.0040. [DOI] [PubMed] [Google Scholar]
- 3.Bohlman HH, Emery SE, Goodfellow DB, et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy: long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg. 1993;75:1298–1307. doi: 10.2106/00004623-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Castro FP, Holt RT, Majd M, et al. A cost analysis of two anterior cervical fusion procedures. J Spinal Disord. 2000;13:511–514. doi: 10.1097/00002517-200012000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Cho DY, Lee WY, Sheu PC. Treatment of multilevel cervical fusion with cages. Surg Neurol. 2004;62:378–385. doi: 10.1016/j.surneu.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Cho DY, Liau WR, Lee WY, Liu JT, Chiu CL, Sheu PC. Priliminary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc disease. Neurosurgery. 2002;51:1343–1350. doi: 10.1097/00006123-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Cook SD, Dalton JE, Tan EH, Tejeiro WV, Young MJ, Whitecloud TS., 3rd In vivo evaluation of anterior cervical fusions with hydroxyapatite graft material. Spine. 1994;19:1856–1866. doi: 10.1097/00007632-199408150-00011. [DOI] [PubMed] [Google Scholar]
- 8.Dang AB, Hu SS, Tay BK. Biomechanics of the anterior longitudinal ligament during 8 g whiplash simulation following single- and contiguous two-level fusion: a finite element study. Spine. 2008;33(6):607–611. doi: 10.1097/BRS.0b013e318166e01d. [DOI] [PubMed] [Google Scholar]
- 9.Das K, Couldwell WT, Sava G, et al. Use of cylindrical titanium mesh and locking plates in anterior cervical fusion. J Neurosurg. 2001;94(1 suppl):174–178. doi: 10.3171/spi.2001.94.1.0174. [DOI] [PubMed] [Google Scholar]
- 10.Demircan MN, Kutlay AM, Colak A, Kaya S, Tekin T, Kibici K, Ungoren K. Multilevel cervical fusion without plates, screws or autogenous iliac crest bone graft. J Clin Neurosci. 2007;14:723–728. doi: 10.1016/j.jocn.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Eck KR, Lenke LG, Bridwell KH, et al. Radiographic assessment of anterior titanium mesh cages. J Spinal Disord. 2000;13:501–510. doi: 10.1097/00002517-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Emery SS, Fisher RS, Bohlman HH. Three-level anterior cervical discectomy and fusion. Spine. 1997;22:2622–2625. doi: 10.1097/00007632-199711150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Giovanni P, Raffaele F, Giuseppina I. Preliminary experience with anterior cervical microdiscectomy and interbody titanium cage fusion in patients with cervical disc disease. Surg Neurol. 2000;53:417–426. doi: 10.1016/S0090-3019(00)00207-X. [DOI] [PubMed] [Google Scholar]
- 14.Gu YT, Jia LS, Chen TY. Biomechanical study of a hat type cervical intervertebral fusion cage. Int Orthop. 2007;31(1):101–105. doi: 10.1007/s00264-006-0141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hacker RJ. Threaded cages for degenerative cervical disease. Clin Orthop Relat Res. 2002;394:39–46. doi: 10.1097/00003086-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Hacker RJ, Cauthen JC, Gilbert TJ, Griffith SL. A prospective randomized multicenter clinical evaluation of an anterior cervical fusion cage. Spine. 2000;25:2646–2655. doi: 10.1097/00007632-200010150-00017. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara A. Roentgenographic studies on the normal pattern of cervical curvature. Nippon Seikeigeka Gakkai Zasshi. 1968;42:1033–1044. [PubMed] [Google Scholar]
- 18.Katsuura A, Hukuda S, Imanaka T, et al. Anterior cervical plate used in degenerative disease can maintain lordosis. J Spinal Disord. 1996;9:470–476. doi: 10.1097/00002517-199612000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Katzer A, Marquardt H, Westendorf J, Wening JV, Foerster G. Polyetheretherketone-cytotoxicity and mutagenicity in vitro. Biomaterials. 2002;23(8):1749–1759. doi: 10.1016/S0142-9612(01)00300-3. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni AG, Hee HT, Wong HK. Solis cage (PEEK) for anterior cervical fusion: preliminary radiological results with emphasis on fusion and subsidence. Spine J. 2007;7:205–209. doi: 10.1016/j.spinee.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Liao JC, Niu CC, Chen WJ, Chen LH (2008) Polyetheretherketone (PEEK) cage filled with cancellous allograft in anterior cervical discectomy and fusion. Int Orthop 32(5):643–648 [DOI] [PMC free article] [PubMed]
- 22.Ludek V, Rude H, Davood J, Waclaw L, Adel S. A prospective randomized comparison between the Cloward procedure and a carbon fiber cage in the cervical spine. Spine. 2002;27:1694–1701. doi: 10.1097/00007632-200208150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Matge G. Anterior interbody fusion with the BAK-cage in cervical spondylosis. Acta Neurochir (Wien) 1998;140:1–8. doi: 10.1007/s007010050049. [DOI] [PubMed] [Google Scholar]
- 24.Peolsson A, Vavruch L, Hedlund R. Long-term randomised comparison between a carbon fibre cage and Cloward procedure in the cervical spine. Eur Spine J. 2007;16(2):173–178. doi: 10.1007/s00586-006-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawlinson JN. Morbidity after anterior cervical decompression and fusion: the influence of the donor site on recovery, and the results of a trial of Surgibone compared to autologous bone. Acta Neurochir (Wien) 1994;131:106–118. doi: 10.1007/BF01401460. [DOI] [PubMed] [Google Scholar]
- 26.Sampath P, Bendebba M, Davis JD, et al. Outcome of patients treated for cervical myelopathy: a prospective, multicenter study with independent clinical review. Spine. 2000;25:670–676. doi: 10.1097/00007632-200003150-00004. [DOI] [PubMed] [Google Scholar]
- 27.Sampath P, Bendebba M, Davis JD, et al. Outcome in patients with cervical radiculopathy: prospective, multicenter study with independent clinical review. Spine. 1999;24:591–597. doi: 10.1097/00007632-199903150-00021. [DOI] [PubMed] [Google Scholar]
- 28.Savolainen S, Rinne J, Hernesniemi J. A prospective randomized study of anterior single-level disc operations with long-term follow-up: surgical fusion is unnecessary. Neurosurgery. 1998;43:51–55. doi: 10.1097/00006123-199807000-00032. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro S, Connolly P, Donnaldson J, et al. Cadaveric fibula, locking plate, and allogenic bone matrix for anterior cervical fusion after cervical discectomy for radiculopathy or myelopathy. J Neurosurg Spine. 2004;1:160–167. doi: 10.3171/spi.2004.1.2.0160. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro S, Connolly P, Donnaldson J, et al. Cadaveric fibula, locking plate, and allogenic bone matrix for anterior cervical fusion after cervical discectomy for radiculopathy or myelopathy. J Neurosurg. 2001;95(Supp 1):43–50. doi: 10.3171/spi.2001.95.1.0043. [DOI] [PubMed] [Google Scholar]
- 31.Shono Y, McAfee PC, Cunningham BW, Brantigan JW. A biomechanical analysis of decompression and reconstruction methods in the cervical spine. Emphasis on a carbon-fiber-composite cage. J Bone Joint Surg Am. 1993;75(11):1674–1684. doi: 10.2106/00004623-199311000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Silber JS, Anderson DG, Daffner SD. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 33.Song KJ, Lee KB. A preliminary study of the use of cage and plating for single-segment fusion in degenerative cervical spine disease. J Clin Neurosci. 2006;13:181–187. doi: 10.1016/j.jocn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Steffen T, Tsantrizos A, Fruth I, Aebi M. Cages: designs and concepts. Eur Spine J. 2000;9(suppl 1):89–94. doi: 10.1007/PL00010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71(4):677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 36.Tullberg T. Failure of a carbon fiber implant: a case report. Spine. 1993;23:1804–1806. doi: 10.1097/00007632-199808150-00016. [DOI] [PubMed] [Google Scholar]
- 37.Vaidya R, Carp J, Sethi A, Bartol S, Craig J, Les CM. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16(8):1257–1265. doi: 10.1007/s00586-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vavruch L, Hedlund R, Javid D, Leszniewski W, Shalabi A. A prospective randomized comparison between the Cloward procedure and a carbon fiber cage in the cervical spine. Spine. 2002;27(16):1694–1701. doi: 10.1097/00007632-200208150-00003. [DOI] [PubMed] [Google Scholar]
- 39.Wenz LM, Merritt K, Brown SA, Moet A, Steffee AD. In vitro biocompatibility of polyetheretherketone and polysulfone composites. J Biomed Mater Res. 1990;24:207–215. doi: 10.1002/jbm.820240207. [DOI] [PubMed] [Google Scholar]
- 40.Zdeblick TA, Ducker TB. The use of freeze-dried allograft bone for anterior cervical fusions. Spine. 1991;16:726–729. doi: 10.1097/00007632-199107000-00006. [DOI] [PubMed] [Google Scholar]