Abstract
Rats have long been the animal of choice for research in the field of osteoporosis. In the search for a complementary large animal model the sheep appears useful but hitherto the extent of bone loss from the spine has failed to reach a level that is generally accepted as osteoporotic in humans. Osteoporosis was induced in ten sheep using ovariectomy, low calcium diet and steroid injection for 6 months. Bone samples of iliac crest (IC), lumbar spine (LS), and proximal femur (PF) from the osteoporotic sheep were compared with those from four normal sheep using densitometry, histomorphometry, biochemistry and basic mechanical testing. The differences were examined using an analysis of variance with Tukey–Kramer test. Overall, the bone mineral density at LS and PF decreased more than 25% after treatment. Trabecular bone volume decreased by 29.2, 33.4 and 42.6% in IC, LS and PF, respectively. The failure load of the LS in axial compression was reduced to 2,003 from 6,140 N. The extent of bone loss was sufficient to categorise these sheep as osteoporotic although the pattern of bone loss varied between sites. Reduced mechanical competence in LS confirmed the suitability of this model for evaluation of potential treatments for osteoporosis.
Keywords: Osteoporosis, Animal model, Sheep, Histology, Lumbar spine
Introduction
Various animal models have been utilised to investigate the osteoporotic condition in order to facilitate preclinical testing of pharmaceutical agents and to evaluate treatment options. The ideal animal model should mimic the anatomy, biomechanics, cell biology and pathological changes seen in the human skeleton [40], and at the same time should be reproducible, meet appropriate ethical standards and be economical and efficient. Most basic investigations of osteoporosis have been conducted in the rat [17] but this model has limited utility because it lacks true lamellar bone and hence does not undergo trabecular remodelling in the same way as human bone, especially in younger animals [11]. Long-term bone loss following ovariectomy alone in the rat is not as dramatic as the bone loss in postmenopausal women [37] and cortical bone studies are limited due to the absence of Haversian systems. Importantly the rat is too small to permit major orthopaedic surgical procedures. Consequently, there is a need for a large animal model for use in osteoporosis-related research to facilitate preclinical evaluation of pharmaceutical [12] as well as surgical and mechanical treatments.
In order to meet this demand many large species including dogs, cats, mini pigs, sheep and non-human primates have been used [27]. Of these animals, the sheep may be a useful model for bone related studies for many reasons: (1) the size and mechanical characteristics of the skeleton are comparable to humans which makes their large vertebral bodies more suited to conventional surgical procedures [41, 44]; (2) older sheep display Haversian bone remodelling [41]; (3) they are genetically closer to humans than rodents and mice; (4) ewes ovulate spontaneously and have sex hormone profiles similar to women; (5) the oestrus cycle in Australian Merino sheep is almost continuous [29]; (6) they are docile and easy to handle; (7) they are relatively inexpensive to maintain and are available in large numbers in most countries; (8) they usually present fewer ethical concerns than the use of domestic pets and non-human primates.
The main challenge associated with the use of sheep for osteoporosis studies however is that they do not naturally attain the same degree of bone loss within their adult life (3–8 years) as humans which have almost a 10-fold longer period of development. Previous studies have shown that sheep develop osteopaenia at various sites to different extents following ovariectomy [16, 27, 28, 42], chronic steroid therapy [7, 10] or dietary manipulation [9] or combinations of these treatments [13, 14, 19–21, 29, 32, 33, 42] but invariably either the BMD of the lumbar spine was not investigated as these studies were focussed more on developing models for repair of long bone fractures or if the spine was investigated the reduction of BMD fell substantially short of the generally accepted definition of osteoporosis in humans, which is BMD ≤ 25% of the young normal value [24].
The aim of this study was to develop a model of osteopaenia in mature sheep with the principal characteristics of bone loss in osteoporotic humans. Specifically we wanted to induce at least 25% bone loss in the lumbar spine and proximal femur since these sites have the highest likelihood of fracture in humans [24]. A regimen combining ovariectomy, low calcium diet and weekly steroid injections was used, remaining mindful that it should occur in a relatively short time frame to minimise the impact of the known side effects of chronic steroid treatment. Thus we hypothesised that ewes on this treatment would lose in excess of 25% of bone mass. The densitometric, histomorphometric, biochemical, and biomechanical properties of bone from these skeletal sites were assessed at baseline and after 3 and 6 months to determine the suitability of the sheep as a large animal model of osteoporosis.
Materials and methods
Animal preparation
With Institutional Animal Ethics Committee approval 10 3-year-old Merino ewes (mean weight 52.3 ± 0.1 kg) were bilaterally ovariectomised under general anaesthesia induced by 1 g Pentothal (Jorox Pty. Ltd, Australia) and maintained by 2% Halothane. They were housed in barren pens and fed a manufactured diet of wheat chaff, which is the “above ground” fraction of the cereal plant that remains after removal of the grain following harvest [36] and processing through a 5 mm screen. This diet is composed of 86% dry matter (DM); 2.9% crude protein; 5.8–6.0 Metabolizable Energy (MJkg DM); 0.15–0.18% Calcium; 0.07% Phosphorus; 2.35:1 Ca:P ratio. The ovariectomised sheep received weekly intramuscular injections of 54 mg dexamethasone [6 ml Dexafort (Intervet, Australia)] and 1.5 ml dexamethasone sodium phosphate (Mayne Pharma) for 6 months. After 3 and 6 months five animals were killed by intravenous injection of 7 g sodium pentobarbitone (Lethabarb, Virbac Pty. Ltd. Australia). These sheep comprised the treated group. Four control animals aged 3 years (mean weight 55.1 ± 0.8 kg) underwent sham abdominal surgery and were killed at baseline and after 6 months (two at each time point). The control animals were fed a normal diet and received no steroids. All animals were weighed weekly.
BMD measurements
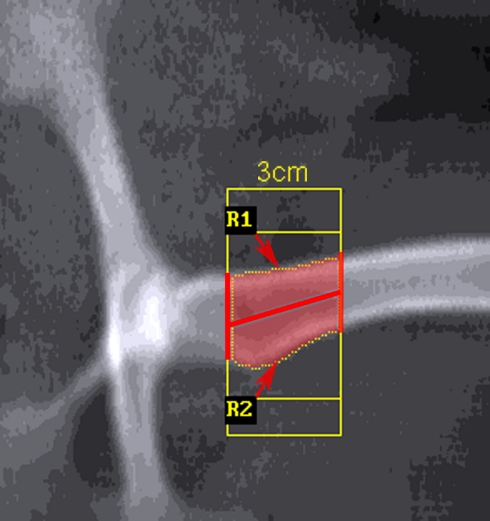
Dual-energy X-ray absorptiometry (DXA) (QDR 2000, Hologic Inc, Waltham, MA, USA) with standard software (Version 7.10b) was used to monitor bone mineral density (BMD) of the lumbar spine (L2–L5) and a defined region of the left proximal femur (vide infra) at the start and 3, 5 and 6 months after the commencement of treatment. The animals were scanned in life under general anaesthesia and again immediately after death using Array Spine mode in the supine position with foam wedges to ensure that true anterior/posterior images were obtained. As femoral BMD could not be determined using standard human regions of interest (ROI) due to the short femoral neck a modified manual method was used. In brief the femur was scanned with the left hind limb abducted from the physiological “flexed” normal position in the sheep, without rotation and perpendicular to the spine, to maximise visibility of the femoral neck. The limb was taped to the scanner bed to prevent movement. The femur was analysed with two fixed subregions extending 3 cm distally from the superior lateral margin of the greater trochanter and bisecting the femoral shaft along the midline (Fig. 1).
Fig. 1.
BMD of femur was analysed using two fixed subregions (R1 and R2) extending 3 cm distally along the femoral shaft from the superior lateral margin of the greater trochanter and bisecting the femoral shaft along the midline. These regions of interest were created on the baseline scan and copied onto each subsequent scan to minimise variation in the region analysed
Biochemical analysis
Venous blood samples were collected weekly to isolate serum for measuring degradation products of Type I collagen c-telopeptide (β-CTX), a marker for bone resorption, by an Elecsys® β-Cross-Laps electrochemiluminescence immunoassay (Roche Diagnostics, Australia).
Histology
Biopsies were taken from the right anterior superior iliac crest (IC) using an 11G Jamshidi trephine at baseline and a second biopsy was taken at necropsy. The entire lumbar spine (LS) from each sheep was harvested after death. Individual vertebral bodies were isolated by removing posterior elements at the pedicle and processed as follows: L1 was wrapped in gauze that was soaked with normal saline and stored at −20°C for subsequent biomechanical testing. A mid-sagittal slice of L2 and IC biopsy were fixed in 10% formalin, dehydrated in graded ethanol solutions and embedded undecalcified in methyl methacrylate (MMA). Mid-sagittal slices of L3 and L4 were decalcified in ethylene diaminotetraacetic acid, processed into paraffin wax, sectioned at 5 μm using a sledge microtome and stained with van Gieson [43]. Sections from L2, L3 and L4 were used to determine cancellous bone structural parameters and cortical thickness. The L6 vertebra was ashed (vide infra) to determine bone mineral content and to validate the DXA data. L5 was reserved frozen as per L1, for prospective studies. A representative sample measuring approximately 10 mm × 10 mm × 2 mm was taken from the intertrochanteric region of the proximal femur (PF) after death to determine cancellous bone structural histomorphometric parameters. These specimens were fixed in 10% formalin and embedded undecalcified in MMA as previously described. A transverse section of the mid femoral shaft was retained in 70% of ethanol for future investigation.
Histomorphometry of cancellous bone
From the undecalcified samples of IC, LS and PF, 5-μm thick histological sections were prepared using a Polycut SP2600 microtome (Leica, Heidelberg, Germany). The sections were stained by the von Kossa (VK) silver impregnation method to determine cancellous bone volume/total volume (BV/TV, %), trabecular thickness (Tb.Th, mm), trabecular separation (Tb.Sp, mm) and trabecular number (Tb.N, number/mm) using a Quantimet 500 computerised semiautomatic image analyser (Leica, Cambridge, UK).
Histomorphometry of cortical bone
Cortical bone thickness was measured from van Gieson-stained sections of L2, L3 and L4 vertebrae. In brief images were captured using the Quantimet 500 Image Analyser and an Olympus BX51 light microscope. Cortical bone was delineated by tracing around, and then removing, the cancellous fraction in the images with a cursor on the screen. Bone volume as a fraction of the total volume was obtained by adjusting the binary threshold to a grey level that detects all of the bone area but not the background [31]. The femoral shaft was cut at the central region using a band saw and the cortical thickness was measured manually at eight regular points around the diameter using digital calipers. The diameters of the medullary cavity and the femoral shaft were measured in a similar manner.
DXA and biomechanical testing
L1 vertebrae were thawed at room temperature in normal saline. BMD was measured in the cranio-caudal plane by dual-energy X-ray absorptiometry (DXA) on a GE-LUNAR Prodigy Vision (Madison, Wisconsin, USA) using small animal software (version 8.10) with results reported using ashed bone calibration. The cranio-caudal plane was selected since the subsequent mechanical testing on the vertebrae was conducted in the same plane. In this way the BMD of the spinous processes was selectively excluded from the analysis.
After DXA scanning the vertebral endplates were embedded in dental cement (Vertex-Dental B·V., The Netherlands) between two parallel plates and a pure compressive load was applied at 1 mm/min using a Hounsfield HK25T materials testing machine (High Wycombe, UK). A load displacement curve was created, from which ultimate failure load (N) and elastic stiffness (N/mm) were determined. Linear regression analysis was applied to BMD and ultimate failure load data.
Ash weight
Bone mineral content (BMC) was determined ex-vivo in the L6 vertebrae by DXA using the GE-Lunar Prodigy Vision bone densitometer. The vertebrae were weighed and heated over 4 days at temperatures increasing from 160 to 800°C and the proportion of mineral ash in each vertebra was expressed as total weight (g) and ash weight/wet weight (%). Linear regression analysis was applied to BMC and total ash weight data.
Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM). An analysis of variance with Tukey–Kramer post hoc comparison of means (Statistical Analysis Software, SAS Institute, NC, USA) was used to test for differences between the treated and the control groups. Linear regression was carried out using Excel software (Microsoft, Redmond, WA, USA). Minimum statistical significance was set at P < 0.05.
Results
General observations
The average body weight of sheep in the control and treatment (osteoporotic) groups was not significantly different at the beginning of the study and there was no significant difference after 6 months (63.5 ± 1.0 kg, n = 2 and 64.5 ± 4.7 kg, n = 5, respectively). One animal from the 6-month treated group lost 5 kg in weight over 3 weeks towards the end of the study period. It was euthanized 2 weeks earlier than scheduled and was included in the 6 month treated group.
Bone mineral density
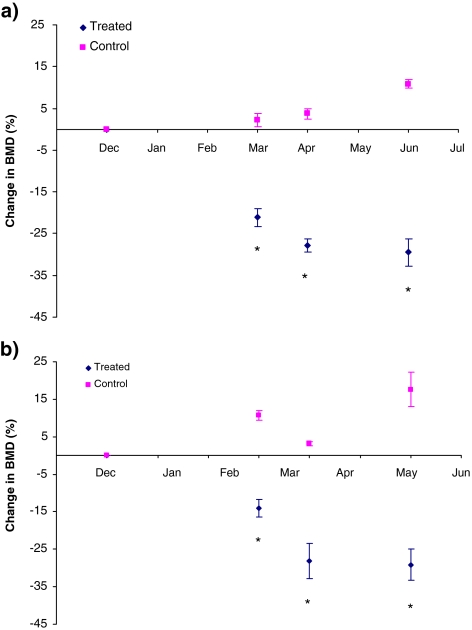
BMD of the (pooled) LS from the treated animals decreased on average by 21.1% after 3 months (P < 0.001) and by 29.5% after 6 months (P < 0.001) from baseline (Fig. 2a). During the last month of treatment BMD of the LS was reduced further by approximately 1% (Fig. 2a). In the 6-month treated sheep BMD of the LS (L1–L5) decreased by 37.5% compared with the 6-month controls (P < 0.001, Fig. 2a).
Fig. 2.
Bone mineral density (BMD) of the lumbar spine (average of L2–L5) (a) and proximal femur (b) in the treated (filled diamond) and the control sheep (filled square) (* P < 0.05). The months are included to emphasise the seasonal fluctuation of BMD
The change in individual vertebrae was not statistically different from the pooled values (data not shown). There was no significant change in LS BMD in the controls after 3 months and only a slight but insignificant increase after 6 months (Fig. 2a). BMD in the PF of the treated animals decreased from baseline by 13.9% after 3 months (P < 0.005) and by 29.1% after 6 months and by 46.3% from the 6-month controls (P < 0.001, Fig. 2b). The decline in PF BMD slowed during the last month of treatment such that there was only a 1.5% reduction in this period (Fig. 2b). The BMD of the PF from the 6-month control sheep increased by 17% compared to the baseline control (NS).
Biochemistry
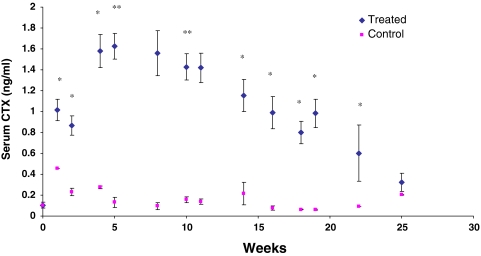
Serum CTX was markedly elevated in the treated animals during the first 5 weeks (P < 0.01) after which it declined towards baseline level near the conclusion of the study (Fig. 3).
Fig. 3.
Type I collagen c-telopeptide (β-CTX) in the serum of the treated (filled diamond) and the control sheep (filled square) (* P < 0.05)
Cancellous bone
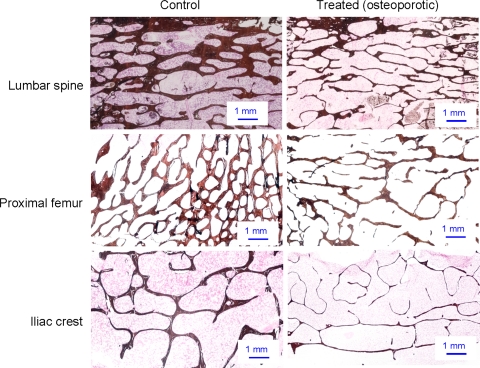
The effect of treatment on the trabecular bone from LS, PF and IC was evident from low power microscopy of stained tissue sections (Fig. 4). Histomorphometric analysis showed that the IC BV/TV decreased by 14.9% after 3 months (NS) and 27.1% after 6 months of continuous treatment (P < 0.01) from baseline (Table 1). Over the same period Tb.Th decreased by 19.0 and 24.7% from baseline (P < 0.01). BV/TV and Tb.Th of IC from the 6-month treated animals were 26.8 and 33.3% lower than the 6-month controls (P< 0.05). There was no significant change in Tb.Sp or Tb.N of IC as a result of treatment from either the baseline or the 6-month controls.
Fig. 4.
Representative von Kossa and H&E stained sections of trabecular bone from lumbar spine (LS), proximal femur (PF) and iliac crest (IC) from control and treated sheep
Table 1.
Iliac crest, lumbar spine and femoral cancellous bone structural histomorphometry (mean ± SEM) at different time points for the control and the treated sheep
| Baseline | Control | Treated | ||
|---|---|---|---|---|
| 0 month (n = 2) | 6 month (n = 2) | 3 month (n = 5) | 6 month (n = 5) | |
| Iliac crest | ||||
| BV/TV (%) | 18.1 ± 0.7 | 17.9 ± 1.6 | 15.4 ± 0.9 | 13.1 ± 1.2‡,* |
| Tb.Th (mm) | 0.11 ± 0.0 | 0.12 ± 0.0 | 0.09 ± 0.0 | 0.08 ± 0.0‡,* |
| Tb.Sp (mm) | 0.50 ± 0.03 | 0.57 ± 0.1 | 0.49 ± 0.02 | 0.57 ± 0.02 |
| Tb.N (#/mm) | 1.60 ± 0.07 | 1.50 ± 0.2 | 1.72 ± 0.06 | 1.60 ± 0.2 |
| Lumbar spine | ||||
| BV/TV (%) | 28.1 ± 1.7 | 33.9 ± 1.9 | 21.9 ± 0.7 | 19.9 ± 0.7‡,* |
| Tb.Th (mm) | 0.15 ± 0.0 | 0.16 ± 4.6 | 0.11 ± 0.00 | 0.10 ± 0.00‡,* |
| Tb.Sp (mm) | 0.5 ± 0.03 | 0.32 ± 0.03 | 0.41 ± 0.02 | 0.41 ± 0.02 |
| Tb.N (#/mm) | 1.6 ± 0.07 | 2.13 ± 0.2 | 1.97 ± 0.07 | 2.03 ± 0.1 |
| Proximal femur | ||||
| BV/TV (%) | 26.5 ± 4.8 | 31.7 ± 2.1 | 14.7 ± 0.8 | 15.2 ± 1.7†,** |
| Tb.Th (mm) | 0.17 ± 0.05 | 0.15 ± 0.0 | 0.12 ± 0.01 | 0.12 ± 0.00 |
| Tb.Sp (mm) | 0.47 ± 0.02 | 0.33 ± 0.02 | 0.70 ± 0.05 | 0.71 ± 0.08‡,* |
| Tb.N (#/mm) | 1.57 ± 0.2 | 2.08 ± 0.04 | 1.23 ± 0.07 | 1.25 ± 0.1†,** |
Testing the significant differences of the histomorphometry data from the 6-month treated with the baseline († P < 0.05, ‡ P < 0.01) and 6-month control sheep (* P < 0.05, ** P < 0.01)
The pooled LS BV/TV decreased by 22.1 and 29.2% after 3 and 6 months of treatment, respectively (P < 0.01). Tb.Th in the LS decreased by 26.7 and 33.4% over the same time period (P < 0.01). BV/TV and Tb.Th in the LS from the 6-month treated animals were 26.8 and 33.3% lower than the 6 months controls (P < 0.05). Tb.Sp and Tb.N were not significantly altered (Table 1).
BV/TV in the PF decreased by 44.5 and 42.6% from the baseline controls (P < 0.05) and Tb.Th decreased from baseline by 29.4% after 3 and 6 months of treatment (NS). BV/TV in the PF decreased after 3 and 6 months treatment by 48.9 and 51.1% relative to the 6-month controls (P < 0.01). Tb.Sp increased by 48.9% after 3 and 6 months of treatment and Tb.N decreased by 21.7 and 20.4% over the same time period from baseline (P < 0.05) (Table 1). Tb.Sp of trabecular bone from the 6-month treated sheep increased by 115% from the 6-month controls while Tb.N was reduced by 39% (P < 0.01).
Cortical bone
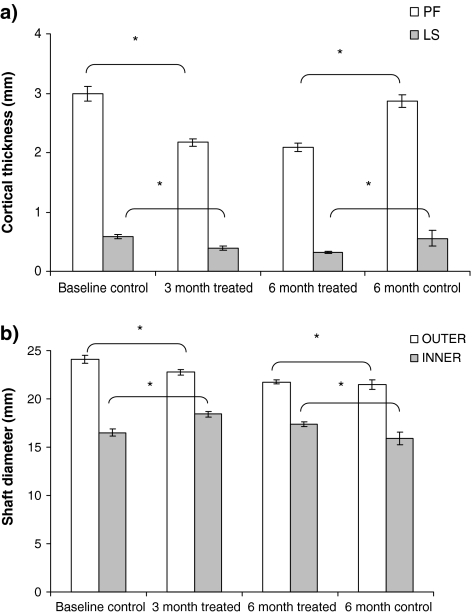
Cortical bone thickness in L2, L3 and L4 vertebrae (pooled) decreased from baseline by 34% after 3 months and by 47% after 6 months of treatment (Fig. 5a, P < 0.05). The difference between the 6-month treated sheep and controls was 44%. After 3 and 6 months, cortical bone of the femoral shaft thinned by 27 and 30%, respectively, from the baseline controls (P < 0.01) (Fig. 5a). The diameter of the medullary cavity of the femoral shaft increased over 13% after 6 months of treatment compared with the 6-month controls (P < 0.05, Fig. 5b). The cortical bone of the femoral shaft in the 6-month treated animals was 27% thinner than the 6-month controls (P < 0.05).
Fig. 5.
a Cortical bone thickness in vertebral bodies and proximal femur in the treated and control sheep (P < 0.05). b Diameter of the medullary cavity and the femoral shaft of the treated and control sheep (P < 0.05)
Biomechanical testing
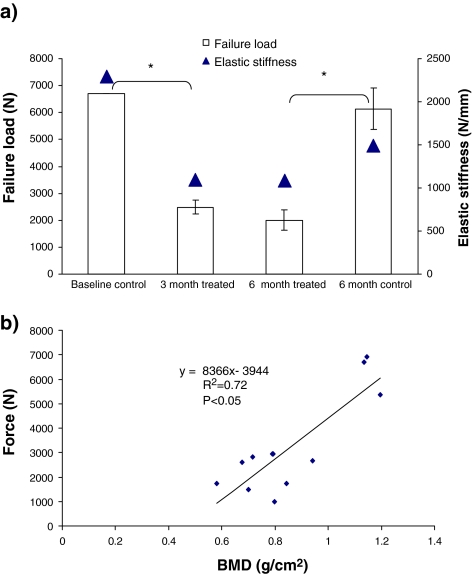
After 3 and 6 months of treatment failure load (N) of the L1 vertebrae was reduced to 2,490 and 2,003 N from 6,590 to 6,140 N in the baseline and 6-month controls, respectively (P < 0.05). Elastic stiffness decreased in both 3 and 6-month treated groups (NS) (Fig. 6a). Failure load and BMD were positively correlated (R2 = 0.72, P < 0.01) (Fig. 6b).
Fig. 6.
a Failure load (N) (* P < 0.05) and elastic stiffness (NS) of lumbar (L1) vertebrae in the treated and control sheep; b Regression curve showing a significant positive correlation between bone mineral density (BMD) and failure load (N) in the L1 vertebrae of the treated sheep (R2 = 0.72)
Ash weight
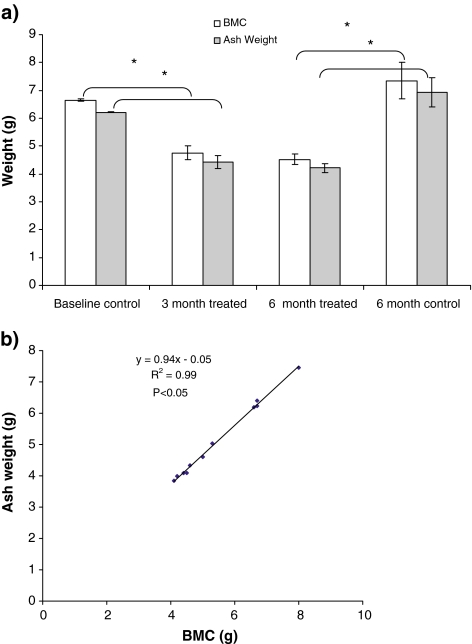
BMC and total ash weight of the L6 vertebrae decreased on average by 38.5 and 39.1%, respectively, after 6 months of treatment compared with the 6-month controls (P < 0.01) (Fig. 7a). Ash weight of L1 correlated with BMC determined ex vivo (R2 = 0.99, P < 0.001, Fig. 7b).
Fig. 7.
a Ash weight (%) and bone mineral content (BMC) of L6 vertebrae in the treated and the control sheep. (* P < 0.05); b Regression curve showing a significant positive correlation between BMC and ash weight of L6 (R2 = 0.99)
Discussion
Perhaps the most significant observation from this study, which was undertaken to characterise a potential large animal model of osteoporosis, was the progressive reduction in BMD at two remote anatomical sites (the lumbar spine and proximal femur) within 3 months of commencing treatment, with a sustained effect after 6-months. In general the combination of long-term corticosteroid, minimal dietary calcium and total ovariectomy that resulted in this bone deficit did not adversely affect the general health of the sheep, and even though all animals gained weight during the course of the study they were not obese. Bone loss at both anatomical sites was in excess of 25% (after 5 months of treatment) which is sufficient to categorise these animals as osteoporotic [24] supporting the stated hypothesis and suggests that the mechanism of bone loss could differ temporally as well as anatomically.
Bone loss has been reported previously at various skeletal sites in sheep following ovariectomy [5, 16, 27, 28, 42], chronic steroid therapy [7, 10] or dietary manipulation [9], but in none of these studies, could these animals be classified as osteoporotic by standard definitions. Other studies have implemented combination treatments to induce osteoporosis in sheep [13, 14, 19–21, 29, 32, 33] and although such treatments have resulted in further bone loss compared to the single treatments, lumbar spine BMD was either not reduced significantly [13, 29, 32, 33] or it did not achieve the minimum of 25% bone loss [20, 21] required to classify them as osteoporotic. The current study was undertaken in an attempt to achieve more substantial bone loss in the lumbar spine of domestic sheep, a large animal that is frequently used for experimental studies. Based on an existing osteoporosis-induction protocol [18–20] that showed the greatest extent of bone loss further modifications were made in the Australian Merino sheep, in particular to the diet but perhaps more importantly to the type of steroid administered, with special emphasis on the dosing regimen.
The harmful effects of corticosteroids on bone physiology are well documented but the combination of Dexafort® and dexamethasone sodium phosphate used in this study, providing both rapid onset and prolonged activity, effectively reduces the daily dose by 25% as well as reducing the stress associated with the daily injections. This is a significant advance on previous studies not only because it has resulted in more substantial and significant bone loss but it has been achieved with less steroid which must improve animal welfare. Furthermore, it could be speculated that the Australian Merino sheep is a more suitable breed than Swiss mountain sheep since its oestrus cycle is almost continuous as in humans [29] which may be an advantage in an animal model of human disease.
DXA scanning is a relatively non-specific measure of bone composition but the strong correlation between BMD and ash weight in the lumbar spine demonstrated its reliability in this model. It is interesting to note that the reduction of BMD in the proximal femur of the treated animals was greater than in the axial skeleton after 6 months compared with the 6 months control animals. It has been reported that in women aged over 65 years the average reduction in femoral neck BMD is −2.6 compared to −1.7 in the lumbar spine (L1–L4) [15], which is comparable to this sheep model.
The study ran for 6 months as scheduled but the minimal change in BMD of the lumbar spine and the proximal femur during the last month of treatment suggests that 5 months of continuous treatment may be sufficient to develop osteopenia in this model. Furthermore significant changes in BMD between the baseline and 6 month control animals may indicate some seasonal influence on bone status as described previously [9, 16, 29, 42] and for this reason it is essential to include appropriate control groups to make valid comparisons[41].
Other measurement instruments such as serum CTX, which reflects total collagen breakdown and therefore is a surrogate measure of bone turnover, contribute to understanding how bone loss occurs. The sharp rise in CTX during the early weeks of treatment indicates that systemic bone resorption has been elevated initially, consistent with observations in humans with glucocorticoid-induced osteoporosis [4, 22]. After the initial increase, however, the level of CTX started to decrease until after 6 months there was no significant difference between controls and treated animals. It has been suggested that the continued bone loss in glucocorticoid-induced osteoporosis was the result of impaired bone formation rather than bone resorption [7, 34]. Furthermore the pattern of CTX changes seen in this study was also similar to the changes reported in sheep after ovariectomy alone [8]. It has been suggested the loss of oestrogen secretion after ovariectomy will increase the bone turnover as shown by the increase in bone markers [8]. Consequently, combined of the influence of glucocorticoid and lack of oestrogen secretion may explain the return of CTX to a normal level after the initial peak. However, biochemical markers of bone turnover, such as CTX, appear to be useful in assessing early response to therapy, and they are weakly related to subsequent changes in BMD [5].
The histomorphometric data indicate that cancellous bone volume at three anatomically distinct sites (iliac crest, proximal femur and lumbar spine) was significantly reduced in all treated animals. In the iliac crest and the lumbar spine this was due mainly to thinning of existing trabecular elements rather than a decrease in connectivity. This has also been reported after corticosteroid therapy in humans and rats [6, 26]. In contrast there was thinning as well as wholesale loss of trabeculae (and hence connectivity) in the proximal femur, more like the situation observed in postmenopausal osteoporosis [1]. We speculate on the basis of these findings that the mechanism of bone loss at the tissue level could differ not only with duration of treatment but also between different skeletal sites. Based on this hypothesis the impact of treatment would be more marked in the proximal femur than either the lumbar spine or the iliac crest but the underlying mechanism of bone loss in this model needs to be validated. Nonetheless it lends further support to previous observations of a site-specific effect of bone loss in sheep models [20, 42].
The effects of this treatment regimen are not confined to cancellous bone. Cortical bone thickness in the femoral shaft and lumbar spine was reduced significantly after 6 months of continuous treatment. This is substantially more than the 8% reduction reported in diaphyseal cross sections of tibiae of sheep after ovariectomy and 6 months of glucocorticoid treatment [34]. Glucocorticoid treatment in rats causes increased bone resorption in the endosteal region resulting in thinning of the cortical walls [30]. Cortical bone thinning in the distal forearm of postmenopausal asthmatic patients treated with glucocorticoids has been reported [38, 39]. Thus it is likely that the effects observed in the femoral shaft of the treated sheep were the result of endosteal bone resorption, a notion supported by the increased medullary area of the femoral shaft. The extent of cortical bone loss is clearly site specific, similar to that seen with the cancellous bone of the lumbar spine and the proximal femur.
Clinical studies have shown that reduced BMD is associated with increased susceptibility to vertebral fracture [3, 35]. Consequently, the significant reduction in BMD as early as 3 months from the start of induction could account for the substantial changes in biomechanical properties of bone in this model. There was also a strong relationship between bone density and bone strength in the lumbar spine, consistent with previous reports [25]. Furthermore, reduced cortical bone thickness would impair the biomechanical properties of bone in this model since the relationship of cortical BMD and geometry to bone strength has also been documented [2]. Other factors such as trabecular architecture, tissue properties and microdamage are important for maintaining the mechanical integrity of bone [23] and should be investigated further in this model. Nonetheless, the significant impact of osteoporosis on the mechanical loading characteristics of the lumbar spine confirms the suitability of this model for developing and testing new surgical strategies for treating vertebral osteoporosis.
This large animal model does not mimic either post-menopausal or corticosteroid-induced osteopenia in humans. Nor is that the intention. It is a model of severe bone loss that is achieved in a relatively short period and one that could be utilised for preclinical trials of specific pharmacologic or surgical treatments or intervention for osteoporosis of the lumbar spine as well as other clinically relevant sites [13]. Despite the obvious advantages of this model there is still limited information about bone physiology in sheep generally and the underlying mechanisms of bone loss in particular. Thus further studies, including molecular, dynamic histomorphometry and in vitro investigations, are needed to further characterise this animal model.
Acknowledgments
The authors thank Dr Ian Parkinson for assistance in statistical analysis; Mr Adnan Mulaibrahimovic for the ash weight analyses; the staff of the Veterinary Services Division, IMVS for animal husbandry; Ms Olga Theodorakakos for assistance with bone histomorphometry and Dr Nick Burgan for advice and assistance with mechanical testing.
Footnotes
The contribution of the second author “H. Beard” in this paper was as equal to the contribution of the first author “M. R. Zarrinkalam”.
References
- 1.Aaron JE, Makins NB, Sagreiya K (1987) The microanatomy of trabecular bone loss in normal aging men and women. Clin Orthop Relat Res 260–271 [PubMed]
- 2.Augat P, Reeb H, Claes LE. Prediction of fracture load at different skeletal sites by geometric properties of the cortical shell. J Bone Miner Res. 1996;11:1356–1363. doi: 10.1002/jbmr.5650110921. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Cummings SR, Genant HK, et al. Axial and appendicular bone density predict fractures in older women. J Bone Miner Res. 1992;7:633–638. doi: 10.1002/jbmr.5650070607. [DOI] [PubMed] [Google Scholar]
- 4.Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002;966:73–81. doi: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 5.Chailurkit LO, Ongphiphadhanakul B, Piaseu N, et al. Biochemical markers of bone turnover and response of bone mineral density to intervention in early postmenopausal women: an experience in a clinical laboratory. Clin Chem. 2001;47:1083–1088. [PubMed] [Google Scholar]
- 6.Chappard D, Legrand E, Basle MF, et al. Altered trabecular architecture induced by corticosteroids: a bone histomorphometric study. J Bone Miner Res. 1996;11:676–685. doi: 10.1002/jbmr.5650110516. [DOI] [PubMed] [Google Scholar]
- 7.Chavassieux P, Buffet A, Vergnaud P, et al. Short-term effects of corticosteroids on trabecular bone remodeling in old ewes. Bone. 1997;20:451–455. doi: 10.1016/S8756-3282(97)00016-1. [DOI] [PubMed] [Google Scholar]
- 8.Chavassieux P, Garnero P, Duboeuf F, et al. Effects of a new selective estrogen receptor modulator (MDL 103, 323) on cancellous and cortical bone in ovariectomized ewes: a biochemical, histomorphometric, and densitometric study. J Bone Miner Res. 2001;16:89–96. doi: 10.1359/jbmr.2001.16.1.89. [DOI] [PubMed] [Google Scholar]
- 9.Chavassieux P, Pastoureau P, Boivin G, et al. Dose effects on ewe bone remodeling of short-term sodium fluoride administration—a histomorphometric and biochemical study. Bone. 1991;12:421–427. doi: 10.1016/8756-3282(91)90031-D. [DOI] [PubMed] [Google Scholar]
- 10.Chavassieux P, Pastoureau P, Chapuy MC, et al. Glucocorticoid-induced inhibition of osteoblastic bone formation in ewes: a biochemical and histomorphometric study. Osteoporos Int. 1993;3:97–102. doi: 10.1007/BF01623380. [DOI] [PubMed] [Google Scholar]
- 11.Erben RG. Trabecular and endocortical bone surfaces in the rat: modeling or remodeling? Anat Rec. 1996;246:39–46. doi: 10.1002/(SICI)1097-0185(199609)246:1<39::AID-AR5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.FDA Division of Metabolism and Endocrine Drug Products (1994) Food and Drug Administration Guidelines for preclinical and clinical evaluation of agents used in the prevention or treatment of postmenopausal osteoporosis, Washington, DC
- 13.Goldhahn J, Jenet A, Schneider E, et al. Slow rebound of cancellous bone after mainly steroid-induced osteoporosis in ovariectomized sheep. J Orthop Trauma. 2005;19:23–28. doi: 10.1097/00005131-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Goldhahn J, Neuhoff D, Schaeren S, et al. Osseointegration of hollow cylinder based spinal implants in normal and osteoporotic vertebrae: a sheep study. Arch Orthop Trauma Surg. 2006;126:554–561. doi: 10.1007/s00402-006-0185-7. [DOI] [PubMed] [Google Scholar]
- 15.Greenspan SL, Maitland-Ramsey L, Myers E. Classification of osteoporosis in the elderly is dependent on site-specific analysis. Calcif Tissue Int. 1996;58:409–414. doi: 10.1007/BF02509439. [DOI] [PubMed] [Google Scholar]
- 16.Hornby SB, Ford SL, Mase CA, et al. Skeletal changes in the ovariectomised ewe and subsequent response to treatment with 17 beta oestradiol. Bone. 1995;17:389S–394S. doi: 10.1016/8756-3282(95)00316-6. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Shen Y, Wronski TJ. Time course of femoral neck osteopenia in ovariectomized rats. Bone. 1997;20:55–61. doi: 10.1016/S8756-3282(96)00317-1. [DOI] [PubMed] [Google Scholar]
- 18.Lill CA, Fluegel AK, Schneider E. Effect of ovariectomy, malnutrition and glucocorticoid application on bone properties in sheep: a pilot study. Osteoporos Int. 2002;13:480–486. doi: 10.1007/s001980200058. [DOI] [PubMed] [Google Scholar]
- 19.Lill CA, Fluegel AK, Schneider E. Sheep model for fracture treatment in osteoporotic bone: a pilot study about different induction regimens. J Orthop Trauma. 2000;14:559–565. doi: 10.1097/00005131-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Lill CA, Gerlach UV, Eckhardt C, et al. Bone changes due to glucocorticoid application in an ovariectomized animal model for fracture treatment in osteoporosis. Osteoporos Int. 2002;13:407–414. doi: 10.1007/s001980200047. [DOI] [PubMed] [Google Scholar]
- 21.MacLeay JM, Olson JD, Enns RM, et al. Dietary-induced metabolic acidosis decreases bone mineral density in mature ovariectomized ewes. Calcif Tissue Int. 2004;75:431–437. doi: 10.1007/s00223-004-0217-7. [DOI] [PubMed] [Google Scholar]
- 22.Manolagas SC, Weinstein RS. New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res. 1999;14:1061–1066. doi: 10.1359/jbmr.1999.14.7.1061. [DOI] [PubMed] [Google Scholar]
- 23.McDonnell P, McHugh PE, O’Mahoney D. Vertebral osteoporosis and trabecular bone quality. Ann Biomed Eng. 2007;35:170–189. doi: 10.1007/s10439-006-9239-9. [DOI] [PubMed] [Google Scholar]
- 24.Melton LJ, III, Chrischilles EA, Cooper C, et al. Perspective. How many women have osteoporosis? J Bone Miner Res. 1992;7:1005–1010. doi: 10.1002/jbmr.5650070902. [DOI] [PubMed] [Google Scholar]
- 25.Mitton D, Rumelhart C, Hans D, et al. The effects of density and test conditions on measured compression and shear strength of cancellous bone from the lumbar vertebrae of ewes. Med Eng Phys. 1997;19:464–474. doi: 10.1016/S1350-4533(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 26.Nakamuta H, Nitta T, Hoshino T, et al. Glucocorticoid-induced osteopenia in rats: histomorphometrical and microarchitectural characterization and calcitonin effect. Biol Pharm Bull. 1996;19:217–219. doi: 10.1248/bpb.19.217. [DOI] [PubMed] [Google Scholar]
- 27.Newman E, Turner AS, Wark JD. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone. 1995;16:277S–284S. doi: 10.1016/8756-3282(95)00026-a. [DOI] [PubMed] [Google Scholar]
- 28.Newton BI, Cooper RC, Gilbert JA, et al. The ovariectomized sheep as a model for human bone loss. J Comp Pathol. 2004;130:323–326. doi: 10.1016/j.jcpa.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 29.O’Connell SL. The sheep as an experimental model for osteoporosis. Department of Medicine. Melbourne: The University of Melbourne; 1999. [Google Scholar]
- 30.Ortoft G, Oxlund H. Qualitative alterations of cortical bone in female rats after long-term administration of growth hormone and glucocorticoid. Bone. 1996;18:581–590. doi: 10.1016/8756-3282(96)00077-4. [DOI] [PubMed] [Google Scholar]
- 31.Parkinson IH, Fazzalari NL. Cancellous bone structure analysis using image analysis. Australas Phys Eng Sci Med. 1994;17:64–70. [PubMed] [Google Scholar]
- 32.Phillips FM, Turner AS, Seim HB, III, et al. In vivo BMP-7 (OP-1) enhancement of osteoporotic vertebral bodies in an ovine model. Spine J. 2006;6:500–506. doi: 10.1016/j.spinee.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Schorlemmer S, Gohl C, Iwabu S, et al. Glucocorticoid treatment of ovariectomized sheep affects mineral density, structure, and mechanical properties of cancellous bone. J Bone Miner Res. 2003;18:2010–2015. doi: 10.1359/jbmr.2003.18.11.2010. [DOI] [PubMed] [Google Scholar]
- 34.Schorlemmer S, Ignatius A, Claes L, et al. Inhibition of cortical and cancellous bone formation in glucocorticoid-treated OVX sheep. Bone. 2005;37:491–496. doi: 10.1016/j.bone.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Spector TD, McCloskey EV, Doyle DV, et al. Prevalence of vertebral fracture in women and the relationship with bone density and symptoms: the Chingford Study. J Bone Miner Res. 1993;8:817–822. doi: 10.1002/jbmr.5650080707. [DOI] [PubMed] [Google Scholar]
- 36.Sundstol F, Owen E (1984) Straw and other fibrous by-products as feed. Elsevier, Amsterdam
- 37.Thompson DD, Simmons HA, Pirie CM, et al. FDA Guidelines and animal models for osteoporosis. Bone. 1995;17:125S–133S. doi: 10.1016/8756-3282(95)97353-H. [DOI] [PubMed] [Google Scholar]
- 38.Tsugeno H, Fujita T, Goto B, et al. Vertebral fracture and cortical bone changes in corticosteroid-induced osteoporosis. Osteoporos Int. 2002;13:650–656. doi: 10.1007/s001980200088. [DOI] [PubMed] [Google Scholar]
- 39.Tsugeno H, Goto B, Fujita T, et al. Oral glucocorticoid-induced fall in cortical bone volume and density in postmenopausal asthmatic patients. Osteoporos Int. 2001;12:266–270. doi: 10.1007/s001980170115. [DOI] [PubMed] [Google Scholar]
- 40.Turner AS. Animal models of osteoporosis—necessity and limitations. Eur Cell Mater. 2001;1:66–81. doi: 10.22203/ecm.v001a08. [DOI] [PubMed] [Google Scholar]
- 41.Turner AS. The sheep as a model for osteoporosis in humans. Vet J. 2002;163:232–239. doi: 10.1053/tvjl.2001.0642. [DOI] [PubMed] [Google Scholar]
- 42.Turner AS, Alvis M, Myers W, et al. Changes in bone mineral density and bone-specific alkaline phosphatase in ovariectomized ewes. Bone. 1995;17:395S–402S. doi: 10.1016/8756-3282(95)00148-7. [DOI] [PubMed] [Google Scholar]
- 43.van Gieson I (1889) Laboratory notes of technical methods for the nervous system. Med J 50:57
- 44.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22:2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]