Abstract
The objective of this study is to compare the clinical and radiographic results of ultraporous β-tricalcium phosphate (β-TCP) versus autogenous iliac crest bone graft (ICBG), through prospective randomized pilot study (EBM-Level 1), as graft extenders in scoliosis surgery. In the posterior correction of scoliosis, local bone resected as part of the procedure is used as the base bone graft material. Supplemental grafting from the iliac crest is considered the gold-standard in posterior spinal fusion. However, autograft is not available in unlimited quantities, and bone harvesting is a source of significant morbidity. Ultraporous β-TCP might be a substitute for ICBG in these patients and thus eliminate donor site morbidity. A total of 40 patients with adolescent idiopathic scoliosis (AIS) were randomized into two treatment groups and underwent corrective posterior instrumentation. In 20 patients, ICBG harvesting was performed whereas the other half received β-TCP (VITOSS®) to augment the local bone graft. If thoracoplasty was performed, the resected rib bone was added in both groups. Patients were observed clinically and radiographically for a minimum of 20 months postoperatively, with a mean follow-up of 4 years. Overall pain and pain specific to the back and donor site were assessed using a visual analog scale (VAS). As a result, both groups were comparable with respect to the age at the time of surgery, gender ratio, preoperative deformity, and hence length of instrumentation. There was no significant difference in blood loss and operative time. In nine patients of the β-TCP group and eight patients of the ICBG group, thoracoplasty was performed resulting in a rib graft of on average 7.9 g in both groups. Average curve correction was 61.7% in the β-TCP group and 61.2% in the ICBG group at hospital discharge (P = 0.313) and 57.2 and 54.3%, respectively, at follow-up (P = 0.109). Loss of curve correction amounted on average 2.6° in the β-TCP group and 4.2° in the comparison group (P = 0.033). In the ICBG group, four patients still reported donor site pain of on average 2/10 on the VAS at last follow-up. One patient in the β-TCP group was diagnosed with a pseudarthrosis at the caudal end of the instrumentation. Revision surgery demonstrated solid bone formation directly above the pseudarthrosis with no histological evidence of β-TCP in the biopsy taken. In conclusion, the use of β-TCP instead of ICBG as extenders of local bone graft yielded equivalent results in the posterior correction of AIS. The promising early results of this pilot study support that β-TCP appears to be an effective bone substitute in scoliosis surgery avoiding harvesting of pelvic bone and the associated morbidity.
Keywords: Adolescent idiopathic scoliosis, Spinal fusion, Graft extender, Beta-tricalcium phosphate, Donor site morbidity
Introduction
In posterior spinal fusion (PSF), the local bone that is resected as part of the procedure serves as the base bone graft. However, there is disagreement with respect to the need for, and type of, supplemental graft material to accomplish fusion, particularly regarding long segment spinal fusion in the posterior correction of adolescent idiopathic scoliosis (AIS) [1, 6, 8, 9, 11, 12, 14, 15, 17, 20, 22, 25, 26, 29–31, 33, 34, 37, 38, 42, 45]. Sengupta et al. reported significantly better fusion rates in multilevel posterolateral fusion with autogenous iliac crest bone graft (ICBG) than with local bone alone [42]. Autograft is still the accepted gold-standard in bone grafting [19, 40]. Nevertheless, harvesting autogenous bone from the ilium has shown to be associated with substantial intra- and postoperative morbidity as well as increased length of surgery and blood loss [2, 4, 18, 21, 39, 41, 43, 44, 46]. All this might lead to additional costs, recovery time, and disability. Moreover, appropriate autograft is not available in unlimited quantities.
Therefore, a number of authors advocate allograft augmentation to achieve successful spinal fusion, reporting comparable results to the use of autograft [3, 6, 12, 14, 17, 20]. Concerns, however, exist regarding the efficacy of frozen or freeze dried allograft, the potential of immunogenicity, and the hazard of microbial contamination, though the rate of disease transmission has proven to be low [1, 13, 24, 26, 47]. In addition, the use of allograft is associated with considerable expense, and patients’ reservations towards allograft implantation may play an important role as well [23]. Consequently, the development of reliable and cost effective bone substitutes is a fundamental need of spine surgery.
A promising solution might be the use of osteoconductive synthetic bone substitutes, which consist of hydroxyapatite, tricalcium phosphate, calcium sulfate, or a mixture of these minerals. Combined with local bone graft and/or bone marrow aspirate (BMA) from vertebral bodies or the iliac crest the resulting composite features osteoinductive and osteogenic potential as it contains relevant growth factors as well as osteoprogenitor cells [5, 7, 16, 19, 28, 32, 35, 36]. The application of these materials as a graft extender in PSF has been reported to be favorable, however, most study results are preliminary [8, 9, 11, 15, 22, 29, 31, 33, 34, 38].
VITOSS® synthetic cancellous bone void filler (Orthovita, Inc., Malvern, PA, USA) represents an advanced formulation of tricalcium phosphate that is composed of nanometer sized pure β-tricalcium phosphate (β-TCP) particles. It has a 90% interconnected porosity with micro-, meso-, and macropores, ranging in size from 1 to 1,000 μm. While its meso- and macroporosity provides space for cell seeding and bone and blood vessel ingrowth, the effective wicking of the hydrophilic micropores facilitates the migration of oxygen, nutrients, and cells via capillary forces. Scaffold resorption is mediated through phagocytosis of the nanometer sized particles as part of the remodeling of the newly formed bone [7, 16, 28].
This prospective randomized pilot study (EBM-Level 1) compares the results of posterior correction and fusion with the use of ultraporous β-TCP as a graft extender versus augmentation with ICBG for the treatment of AIS. In addition, the morbidity associated with ICBG harvesting was evaluated.
Materials and methods
Study design
Between December 2000 and August 2003, 40 consecutive patients with AIS who were scheduled for corrective surgery at our institution were enrolled in this prospective pilot study and randomized to receive either ultraporous β-TCP or autograft from the posterior iliac crest to augment the local bone graft (from here on ‘β-TCP group’ and ‘ICBG group’). All deformities were treated by means of posterior corrective instrumentation using a titanium pedicle screw and rod system and fusion with additional grafting. None of the patients had prior spinal surgery. Written informed consent was obtained from all patients. The protocol was approved by the institutional review board (IRB) of the University.
Patient data
The β-TCP group included 18 females and 2 males, and the ICBG group consisted of 16 females and 4 males. The average age at the time of surgery was 18.5 years in the β-TCP group and 19.5 years in the ICBG group (P = 0.158). Both groups were well matched regarding curve types according to the classification system of King et al. [27]. The baseline clinical characteristics and curve classification of all patients are listed in Table 1. Both groups were also comparable with respect to the magnitude of the preoperative major curve (β-TCP group: average Cobb angle [10] 59.1°; ICBG group: 60.8°; P = 0.168; see Table 3). All patients underwent clinical examination as well as a detailed radiographic analysis preoperatively, postoperatively, and at all follow-up visits (3-, 6-, 12-, 24-, and 48-months). Thirty-eight patients (95%) had an average of 49 months of complete clinical and radiographic follow-up, with a minimum follow-up of 40 months. One patient in the ICBG group who attended the 24-months follow-up died in a car accident 3 years after surgery. One patient in the β-TCP group with a follow-up of 20 months was diagnosed with a pseudarthrosis and underwent revision surgery. The patient’s data collected before the revision were included in the evaluation to follow the ITT (intention to treat) regulations.
Table 1.
Patient characteristics and curve classification
| β-TCP (n = 20) | ICBG (n = 20) | P | |
|---|---|---|---|
| Female | 18 | 16 | |
| Male | 2 | 4 | |
| Age at time of surgery (years) (median; SD; range) | 18.5 (17; ±4.4; 12.9–26.1) | 19.5 (18.5; ±4.3; 14.1–27.8) | 0.158 |
| Follow-up (months) | 46.8 (45.9; ±9; 20–61) | 48.8 (48.2; ±10.2; 24–69) | 0.280 |
| Curve types | |||
| King I | 3 | 2 | |
| King II | 8 | 8 | |
| King III | 5 | 7 | |
| King V | 4 | 1 | |
| Triple curve | 0 | 1 | |
| Thoracolumbar | 0 | 1 |
All values displayed in bold letters are mean values
Table 3.
Radiographic results coronal plane
| β-TCP (n = 20) | ICBG (n = 20) | P | |
|---|---|---|---|
| Cobb angle major curve (°) | |||
| Preoperative (median; SD; range) | 59.1 (58; ±7.2; 48–71) | 60.8 (60; ±5.5; 52–72) | 0.168 |
| Postoperative | 22.9 (21.5; ±7.6; 10–43) | 23.8 (21.5; ±7; 13–36) | 0.352 |
| Follow-up | 25.5 (24.5; ±8.7; 14–47) | 28 (25.5; ±8.1; 17–44) | 0.136 |
| Curve correction (%) | |||
| Postoperative | 61.7 (63.3; ±10.7; 39.4–81.8) | 61.2 (61.6; ±9.1; 47–79.7) | 0.313 |
| Follow-up | 57.2 (61.1; ±12.4; 29.3–72.7) | 54.3 (54.4; ±10.6; 33.3–73.4) | 0.109 |
| Loss of curve correction (°) | 2.6 (2; ±2.2; –2–7) | 4.2 (3.5; ±2.3; 1–9) | 0.033 |
All values displayed in bold letters are mean values
Perioperative parameters
The perioperative parameters that were collected and evaluated included operative time, estimated blood loss (EBL) intraoperatively, blood retransfusion by cell saver, blood transfusion requirements, blood drained, number of motion segments fused, weight of harvested bone, intra- and postoperative complications.
Radiographic analysis
In all patients, standardized long-cassette posteroanterior and lateral radiographs in standing posture (together with preoperative side bending radiographs) were obtained before surgery, after postoperative mobilization, and at all follow-up visits. Postoperative radiographs were closely examined for implant failure in terms of breakage, dislocation, and loosening, as well as for defects in the fusion mass as indicators of nonunion. Furthermore, curve correction and potential loss of correction (using the same vertebral segments as preoperative) were carefully assessed. Following the established criteria for possible and definite pseudarthrosis, the presence of a definite pseudarthrosis was proven by radiographically visible implant failure or by direct evidence of a defect in the fusion mass at the time of surgical exploration [6]. A possible pseudarthrosis was considered if one of the following situations was encountered: (1) loss of curve correction greater than 10°, (2) persistent midline back pain of moderate to severe intensity, or (3) radiographically visible defect in the fusion mass.
Assessment of pain and analgesic use
Overall pain and pain specific to the back and donor site were assessed using a visual analog scale (VAS; range 0–10) preoperatively, at hospital discharge, and at all follow-up visits. The use of any analgesic during the study was also recorded.
Data analysis and statistics
Following summation of the presented data, mean values (together with median and standard deviation) were calculated for age at the time of surgery, follow-up time, perioperative parameters, preoperative, postoperative, and latest Cobb angle of the major curve, initial and final proportional curve correction, loss of curve correction, and VAS pain scores. All values were analyzed using the Mann–Whitney-U-test. Statistical significance was determined at the P < 0.05 level.
Surgical procedure
All 40 patients underwent corrective instrumentation through a posterior procedure using a multisegmented titanium pedicle screw and rod system (Micomed Posterior Dual Rod System, micomed Ortho AG, Unteraegeri, Switzerland). Patients were randomized in a 1:1 ratio to undergo either a standard bone graft harvesting procedure of the posterior iliac crest or to receive β-TCP: cancellous ICBG or 15 cc of β-TCP in single major curves and corticocancellous ICBG or 30 cc of β-TCP in double major curves. Treatment assignments were generated through the use of a blocked randomization scheme and provided in sequentially numbered envelopes. All operations were performed by the two surgeons (V.B., U.L.) using a standard technique. After exposure of the spine and release of the facet joints, pedicle screws were placed at the strategic vertebrae. In high thoracic levels where it was not possible to place screws, hooks were used instead. A pre-bent rod was then inserted, and correction was accomplished by a combination of the cantilever and rod rotation technique with slight concave distraction and convex compression. Thereafter, a wake-up test was conducted to evaluate the neurologic status. The spinous processes were then resected and the laminae decorticated. In the ICBG group, the morselized local bone procured from the removal of the spinous processes was placed together with either pure cancellous (n = 11) or corticocancellous ICBG (n = 9), respectively, on the decorticated posterior vertebral elements. In the experimental arm, the local bone graft was mixed with β-TCP and blood oozing from the decorticated laminae. The resulting composite was allowed to gel for at least 15 min before application. In every case, the surgeon was blinded as to which group the patient was assigned to until it was time for the application of the graft, at which time the envelope with the treatment assignment was opened. To address prominent rib cage deformity, thoracoplasty was performed. In both groups, the resected rib bone was added to the graft volume.
Postoperative management
No postoperative bracing was administered. All patients were restricted from bending or lifting greater than 5 kg for 6 months and from sport activities for 1 year, except for swimming and cycling which were allowed after 3 and 6 months, respectively.
Results
Perioperative parameters
Perioperative data are presented in Table 2. Operation time averaged 212.3 min in the β-TCP group and 222.5 min in the ICBG group (P = 0.167). Mean estimated blood loss (EBL) was 1124 ml for the β-TCP-treated patients, including one patient with an extraordinary high EBL of 3,800 ml due to a bleeding disorder. In the ICBG group, EBL averaged 1,020 ml (P = 0.403). If the patient with the bleeding disorder was excluded from the analysis, mean EBL at 983 ml was slightly lower in the β-TCP group (P = 0.296). Mean fusion length was 9.9 segments in the β-TCP group and 9.2 segments in the ICBG group (P = 0.137). Weight of the harvested local bone per each motion segment fused averaged 3 g in the β-TCP group and 2.6 g in the ICBG group (P = 0.023). In the experimental group, the eight patients with a single major curve received 15 cc and the 12 patients with a double major curve 30 cc of β-TCP. In the ICBG group, the local bone graft was supplemented with on average 13.2 g of cancellous ICBG in the 11 patients with a single major curve and with on average 23.3 g of corticocancellous ICBG in the nine patients with a double major curve. In every patient with corticocancellous and in one patient with cancellous bone grafting, a drain was inserted at the end of the procedure. Average amount of blood drained was 169 ml. The remaining ten patients had their donor site incisions closed without a drainage device. Nine patients in the β-TCP group and eight patients in the ICBG group underwent thoracoplasty. In both groups, the harvested rib bone was added to the graft volume. Weight of the rib graft averaged 7.9 g for both study arms.
Table 2.
Perioperative parameters
| β-TCP (n = 20) | ICBG (n = 20) | P | |
|---|---|---|---|
| Operative time (min) (median; SD; range) | 212.3 (205; ±37; 150–270) | 222.5 (227.5; ±34; 160–300) | 0.167 |
| EBL (ml) | 1124a (950; ±816, 400–3800) | 1020 (850; ±456; 450–2400) | 0.403 |
| Cell saver retransfusion (ml) | 358a (248; ±330; 0–1300) | 289 (245; ±228; 0–783) | |
| Blood requirements (no.) | |||
| Intraoperative | 0.6 (0; ±1; 0–3) | 0.4 (0; ±0.7; 0–2) | |
| Postoperative | 0.7 (0; ±1; 0–3) | 0.9 (0; ±1.1; 0–3) | |
| Iliac crest drain (no.) | – | 10 | |
| Blood drained (ml) | – | 169 (155; ±118; 10–400) | |
| Segments fused (no.) | 9.9 (10.5; ±1.9; 7–13) | 9.2 (9.5; ±2.3; 6–13) | 0.137 |
| Local bone graft per segment fused (g) | 3 (2.8; ±0.6; 1.4–3.9) | 2.6 (2.6; ±0.7; 1.7–4.0) | 0.023 |
| 15 cc β-TCP (no.) | 8 | – | |
| 30 cc β-TCP (no.) | 12 | ||
| Cancellous ICBG (no.) | – | 11 | |
| Weight (g) | 13.2 (11; ±5.6; 6–22) | ||
| Corticocancellous ICBG (no.) | – | 9 | |
| Weight (g) | 23.3 (23; ±4.2; 18–32) | ||
| Thoracoplasty (no.) | 9 | 8 | |
| Rib graft (g) | 7.9 (8; ±2.3; 4–10) | 7.9 (8; ±2; 5–10) |
All values displayed in bold letters are mean values
EBL Estimated blood loss
aIncludes one patient with a bleeding disorder who lost 3,800 ml
Adverse events
Intraoperative complications in the β-TCP group included five patients with a prolonged wake-up test (>30 min) including one patient with an extensive bleeding diathesis with a blood loss of 3,800 ml. There were no complications during surgery in the ICBG group. Postoperative complications in the β-TCP group were: pleural effusion without need for pleuracentesis (n = 2), subcutaneous seroma requiring puncture (n = 1), revision of one screw which was placed in close proximity to the aorta (n = 1), and a pseudarthrosis which was revised (see below). None of these complications was considered related to the study material (β-TCP). Complications in the ICBG group were: pleural effusion (n = 3) with need for pleuracentesis in one patient, subcutaneous seroma requiring puncture (n = 1), and pneumonia (n = 1).
Curve correction and loss of correction
The complete radiographic data are listed in Table 3. In the β-TCP group, Cobb angle of the major curve averaged 59.1° before surgery, 22.9° immediately after surgery (correction of 61.7%), and finally 25.5° (correction of 57.2%). In the ICBG group, Cobb angle measured on average 60.8° preoperatively, 23.8° postoperatively (correction of 61.2%), and 28° at follow-up (correction of 54.3%). Initial and follow-up proportional correction were not statistically different between the groups (P = 0.313 and P = 0.109, respectively). Comparing the ICBG group and the subgroups β-TCP with and without rib graft also revealed no significant differences. Loss of curve correction averaged 2.6° in the β-TCP group and 4.2° in the ICBG group (P = 0.033).
Fusion assessment: possible and definite pseudarthrosis
At the most recent follow-up, all patients in the ICBG group and all but one patient in the β-TCP group were considered fused as assessed by conventional radiography, showing a continuous and mature fusion mass in the residual curves’ concavity without any evidence of implant failure (see Figs. 1, 2). Generally, the β-TCP morsels could not be observed or were only slightly visible up to the 6-months follow-up.
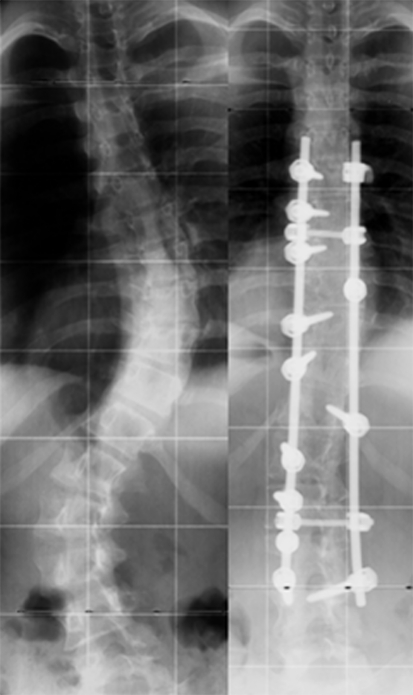
Fig. 1.
Posteroanterior radiographs of a 16-year-old girl (at the time of surgery) out of the β-TCP group (without thoracoplasty) showing a King I scoliosis with a thoracic curve of 54° from T6 to T12 and a lumbar curve of 58° from T12 to L4 and the instrumentation from T6 to L4 at last follow-up, 52 months after surgery
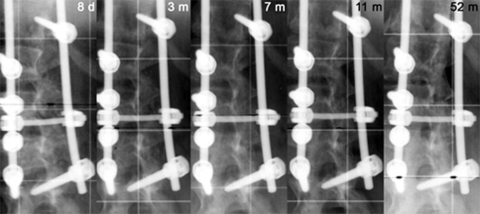
Fig. 2.
Postoperative and follow-up posteroanterior radiographs (3-, 7-, 11-, and 52-months) of the patient presented in Fig. 1 with focus on the lumbar curve demonstrating a progressive resorption of the β-TCP morsels and the formation and maturation of a continuous bony fusion mass visible at the curve’s concavity
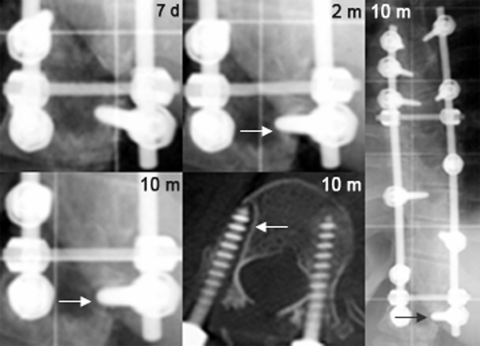
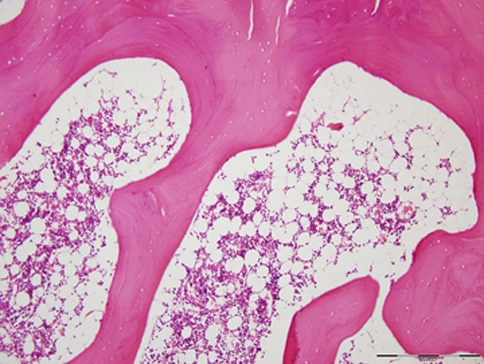
One patient in the β-TCP group with persistent midline back pain of 8/10 on the VAS presented a progressive lucency around one screw at the caudal end of the instrumentation at T12, which was obvious on radiographs as early as 2 months postoperatively (see Fig. 3), and was considered as definite pseudarthrosis. Revision surgery 20 months after the index surgery confirmed the pseudarthrosis at T11/12 without any further defect in the entire fusion mass above. Biopsies taken from T10/11 showed solid bone formation without evidence of β-TCP (see Fig. 4). The patient underwent complete hardware removal and local re-instrumentation with fusion using ICBG.
Fig. 3.
Postoperative radiographs of a 24-year-old girl (at the time of surgery) out of the TCP group with a pseudarthrosis at the caudal end of the instrumentation at T12: close-up view 1 week after surgery, 2 months postoperatively showing a slight lucency around the right pedicle screw (arrow), and 10 months after surgery with progression of the lucency confirmed by computed tomography
Fig. 4.
Biopsy taken from the fusion mass at T10/11 in the patient with the pseudarthrosis at T11/12 presented in Fig. 3 showing solid bone formation without evidence of β-TCP
There were no patients with a loss of curve correction ≥10°, another criterion for possible pseudarthrosis.
Two patients in the β-TCP group who had underwent thoracoplasty complained of moderate to severe back pain of 5 and 8/10, respectively, on the VAS (2 and 6/10 preoperatively) at final follow-up (41 and 61 months postoperatively). The patient with intense back pain had been diagnosed with a chronic pain syndrome already before surgery. In the ICBG group, three patients with thoracoplasty in one case reported back pain ranging from 5 to 6/10 on the VAS (1, 2, and 5/10 preoperatively) at last follow-up (49, 51, and 56 months postoperatively). All these patients complained of paravertebral back pain, and no midline pain. Radiographically, there was no evidence for nonunion in these cases, and the presence of an infection was excluded as well.
VAS Pain scores and analgesic use
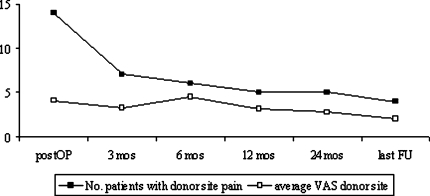
Pain scores as assessed by VAS are presented in detail in Table 4. VAS scores were reported for 39 of the 40 patients. One patient in the ICBG group did not fill out the VAS. At hospital discharge, the VAS score for overall pain was statistically higher in the ICBG group than in the β-TCP group (P = 0.036) but was not statistically different between before surgery and at the most recent follow-up. Figure 5 displays the course of donor site pain. Four patients still complained of slight pain at the harvest site of on average 2/10 on the VAS at follow-up. No patients in either group reported use of an analgesic preoperatively. At hospital discharge, nine patients in the β-TCP group and 12 patients in the ICBG group needed an analgesic. Finally, four patients in the β-TCP group and two patients in the ICBG group were using an over the counter analgesic occasionally, predominantly because of painful muscular tension, especially in the neck and shoulder region.
Table 4.
Pain scores (VAS)
| β-TCP (n = 20) | ICBG (n = 19)a | P | |
|---|---|---|---|
| VAS overall | |||
| Preoperative (median; SD; range) | 2.6 (2; ±2.7; 0–7) | 2.8 (3; ±2.6; 0–7) | 0.375 |
| At hospital discharge | 2.9 (3; ±1.9; 0–8) | 3.9 (4; ±2.2; 0–8) | 0.036 |
| Follow-up | 1.9 (1; ±2.6; 0–8) | 2.3 (2; ±2.1;0–6) | 0.173 |
| VAS back | |||
| Preoperative | = VAS overall | = VAS overall | |
| At hospital discharge | = VAS overall | 3.5 (4; ±2.3; 0–8) | 0.169 |
| Follow-up | = VAS overall | 1.9 (1; ±2.1; 0–6) | 0.368 |
| VAS iliac crest | |||
| At hospital discharge | – | 3 (3; ±2.5; 0–8) | |
| Follow-up | – | 0.4 (0; ±1; 0–3) | |
All values displayed in bold letters are mean values
an = 19, one patient did not fill out the VAS
Fig. 5.
Number of patients who experienced donor site pain after ICBG harvesting and average pain intensity evaluated by a visual analogue scale (0–10) during follow-up
Discussion
This prospective randomized pilot study with a mean follow-up of 4 years demonstrates that the use of ultraporous β-TCP as a graft extender provides results comparable to those of ICBG in attaining spinal fusion using transpedicular instrumentation in the posterior correction of AIS. This supports other reports presenting β-TCP as an effective graft extender in posterior spinal fusion procedures [15, 22, 33, 34].
In the current study, the two treatment groups were well matched in terms of age at the time of surgery, gender ratio, distribution of curve types, magnitude of the preoperative major curve, and thus length of instrumentation. At final follow-up, both groups had virtually identical outcomes, except for four patients in the ICBG group who still complained of donor site pain.
Persistent pain at the donor site is the most common complication of harvesting procedures [2, 4, 18, 21, 39, 41, 43, 44, 46]. Goulet et al. and Sasso et al. reported 19 respective 31% of patients still experiencing pain 2 years after harvesting of pelvic bone [21, 41]. Skaggs et al. reported that even 4 years after posterior iliac crest bone grafting in children 15% of patients suffered from pain severe enough to limit daily activity [43]. In this study, the VAS score for overall pain at hospital discharge was statistically higher in the ICBG group than in the β-TCP group whereas the VAS scores for back pain were not statistically different. The maximum donor site pain was found after 6 months, which matches the results of Robertson et al. [39]. At this time, patients normally are no longer restricted from bending or lifting as well as from swimming and cycling which strains the affected ilium. At the most recent follow-up, four patients in the ICBG group still experienced slight pain or discomfort at the donor site. Even though the differences in VAS pain scores were marginal in this study, and no major complications were observed, the potential morbidity of harvesting procedures can be completely avoided using bone substitutes like β-TCP. In addition, the cosmetic aspect has to be considered. Most female patients in the ICBG group with a second incision at the ilium were unhappy with the scar, which frequently was more prominent than the midline scar.
A number of authors reported significant lower blood loss and reduced operative time using synthetic bone graft substitutes or allograft instead of ICBG [3, 12, 17]. Compared to the ICBG group, operative time in the β-TCP group was slightly reduced by 10 min though not statistically significant. However, it has to be considered that five patients in the β-TCP group had a prolonged wake-up test (>30 min), and that one more patient in this group underwent thoracoplasty. Due to these differences and one patient in the β-TCP group with a bleeding diathesis and thus an extraordinary high blood loss mean intraoperative blood loss was slightly higher than in the ICBG group.
There was one patient in the β-TCP group who experienced pseudarthrosis at the caudal end of the instrumentation at T12. Given that in this case screw loosening became radiographically evident as early as two months after surgery and was accompanied by continued back pain, this failure might be the result from insufficient instrumentation and localized mechanical stresses, rather than a failure of the graft extender per se. Normally, a foundation of a pair of bilateral pedicle screws is placed at the two most distal vertebrae. However, in this case a pair of pedicle screws was placed only at the convexity while at the concavity T12 was instrumented with T10 being the next neighboring vertebra instrumented. This assumption appears to be supported by the fact that during revision surgery the graft material directly above the pseudarthrosis had matured into solid bone with no histological evidence of remaining β-TCP in the biopsy taken.
A number of recent studies recommended the use of bone substitutes in combination with BMA [5, 7, 16, 19, 28, 32, 35, 36]. In this pilot study, β-TCP was mixed with local bone resected as part of the procedure and blood oozing from the decorticated laminae thus adding some osteoinductive and osteogenic components as well. An efficient technique to obtain abundant BMA, which was not followed in the present study but is currently our clinical practice, is to take BMA directly from vertebral bodies during instrumentation: after the pedicle is opened with an awl and the vertebral body perforated with a probe, a syringe is applied to aspirate the bone marrow. In this regard, McLain et al. reported that aspirates of vertebral marrow showed equivalent or even greater concentrations of progenitor cells compared with matched controls from the iliac crest [32].
Limitations of the study
From a methodological perspective, it would have been better to discard the harvested rib bone in the patients who underwent thoracoplasty since this provides additional autogenous bone graft material which by itself might provide sufficient graft augmentation. However, discarding the rib graft was considered to be unethical.
Conclusions
This prospective randomized pilot study compares the use of β-TCP as a graft extender versus autogenous bone from the iliac crest in 40 patients with AIS who underwent posterior correction and fusion of their deformity. After an average follow-up of 48 months, both materials provided equivalent results. Thus β-TCP appears to be an effective bone substitute which makes it possible to avoid bone harvesting from the iliac crest and its related morbidity in these patients.
Acknowledgments
The authors thank Randal R. Betz, MD, Shriners Hospitals for Children, Philadelphia, for his valuable help and contribution in reviewing this manuscript.
References
- 1.An HS, Lynch K, Toth J. Prospective comparison of autograft vs. allograft for adult posterolateral lumbar spine fusion: differences among freeze-dried, frozen, and mixed grafts. J Spinal Disord. 1995;8:131–135. doi: 10.1097/00002517-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Arrington ED, Smith WJ, Chambers HG, et al. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 3.Aurori BF, Weierman RJ, Lowell HA, et al. Pseudarthrosis after spinal fusion for scoliosis. A comparison of autogeneic and allogeneic bone grafts. Clin Orthop Relat Res. 1985;199:153–153. [PubMed] [Google Scholar]
- 4.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Betz RR. Limitations of autograft and allograft: new synthetic solutions. Orthopedics. 2002;25:s561–s570. doi: 10.3928/0147-7447-20020502-04. [DOI] [PubMed] [Google Scholar]
- 6.Betz RR, Petrizzo AM, Kerner PJ, et al. Allograft versus no graft with a posterior multisegmented hook system for the treatment of idiopathic scoliosis. Spine. 2006;31:121–127. doi: 10.1097/01.brs.0000194771.49774.77. [DOI] [PubMed] [Google Scholar]
- 7.Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002;395:44–52. doi: 10.1097/00003086-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Cavagna R, Daculsi G, Bouler JM. Macroporous calcium phosphate ceramic: a prospective study of 106 cases in lumbar spinal fusion. J Long Term Eff Med Implants. 1999;9:403–412. [PubMed] [Google Scholar]
- 9.Chen WJ, Tsai TT, Chen LH, et al. The fusion rate of calcium sulfate with local autograft bone compared with autologous iliac bone graft for instrumented short-segment spinal fusion. Spine. 2005;30:2293–2297. doi: 10.1097/01.brs.0000182087.35335.05. [DOI] [PubMed] [Google Scholar]
- 10.Cobb J (1948) Outline for the study of scoliosis. In: Instructional course letters, vol 5. American Academy of Orthopaedic Surgeons, Ann Arbor
- 11.Delecrin J, Takahashi S, Gouin F, et al. A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: a prospective, randomized study. Spine. 2000;25:563–569. doi: 10.1097/00007632-200003010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Dodd CA, Fergusson CM, Freedman L, et al. Allograft versus autograft bone in scoliosis surgery. J Bone Joint Surg Br. 1988;70:431–434. doi: 10.1302/0301-620X.70B3.3286656. [DOI] [PubMed] [Google Scholar]
- 13.Eastlund T. Bacterial infection transmitted by human tissue allograft transplantation. Cell Tissue Bank. 2006;7:147–166. doi: 10.1007/s10561-006-0003-z. [DOI] [PubMed] [Google Scholar]
- 14.Ehrler DM, Vaccaro AR. The use of allograft bone in lumbar spine surgery. Clin Orthop Relat Res. 2000;371:38–45. doi: 10.1097/00003086-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Epstein NE. A preliminary study of the efficacy of beta tricalcium phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006;19:424–429. doi: 10.1097/00024720-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Erbe EM, Marx JG, Clineff TD, et al. Potential of an ultraporous beta-tricalcium phosphate synthetic cancellous bone void filler and bone marrow aspirate composite graft. Eur Spine J. 2001;10(Suppl 2):S141–S146. doi: 10.1007/s005860100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabry G. Allograft versus autograft bone in idiopathic scoliosis surgery: a multivariate statistical analysis. J Pediatr Orthop. 1991;11:465–468. doi: 10.1097/01241398-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Fernyhough JC, Schimandle JJ, Weigel MC, et al. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine. 1992;17:1474–1480. doi: 10.1097/00007632-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Fleming JE, Jr, Cornell CN, Muschler GF. Bone cells and matrices in orthopedic tissue engineering. Orthop Clin North Am. 2000;31:357–374. doi: 10.1016/S0030-5898(05)70156-5. [DOI] [PubMed] [Google Scholar]
- 20.Gibson S, McLeod I, Wardlaw D, et al. Allograft versus autograft in instrumented posterolateral lumbar spinal fusion: a randomized control trial. Spine. 2002;27:1599–1603. doi: 10.1097/00007632-200208010-00002. [DOI] [PubMed] [Google Scholar]
- 21.Goulet JA, Senunas LE, DeSilva GL, et al. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Gunzburg R, Szpalski M. Use of a novel beta-tricalcium phosphate-based bone void filler as a graft extender in spinal fusion surgeries. Orthopedics. 2002;25:s591–s595. doi: 10.3928/0147-7447-20020502-08. [DOI] [PubMed] [Google Scholar]
- 23.Henman P, Finlayson D. Ordering allograft by weight: suggestions for the efficient use of frozen bone-graft for impaction grafting. J Arthroplasty. 2000;15:368–371. doi: 10.1016/S0883-5403(00)90785-X. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim T, Stafford H, Esler CN, et al. Cadaveric allograft microbiology. Int Orthop. 2004;28:315–318. doi: 10.1007/s00264-004-0579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorgenson SS, Lowe TG, France J, et al. A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patient. A minimum of 1-year follow-up in 144 patients. Spine. 1994;19:2048–2053. doi: 10.1097/00007632-199409150-00005. [DOI] [PubMed] [Google Scholar]
- 26.Journeaux SF, Johnson N, Bryce SL, et al. Bacterial contamination rates during bone allograft retrieval. J Arthroplasty. 1999;14:677–681. doi: 10.1016/S0883-5403(99)90222-X. [DOI] [PubMed] [Google Scholar]
- 27.King HA, Moe JH, Bradford DS, et al. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am. 1983;65:1302–1313. [PubMed] [Google Scholar]
- 28.Kwon B, Jenis LG. Carrier materials for spinal fusion. Spine J. 2005;5:224–230. doi: 10.1016/j.spinee.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Le Huec JC, Lesprit E, Delavigne C, et al. Tri-calcium phosphate ceramics and allografts as bone substitutes for spinal fusion in idiopathic scoliosis as bone substitutes for spinal fusion in idiopathic scoliosis: comparative clinical results at four years. Acta Orthop Belg. 1997;63:202–211. [PubMed] [Google Scholar]
- 30.Liljenqvist U, Lepsien U, Hackenberg L, et al. Comparative analysis of pedicle screw and hook instrumentation in posterior correction and fusion of idiopathic thoracic scoliosis. Eur Spine J. 2002;11:336–343. doi: 10.1007/s00586-002-0415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linovitz RJ, Peppers TA. Use of an advanced formulation of beta-tricalcium phosphate as a bone extender in interbody lumbar fusion. Orthopedics. 2002;25:s585–s589. doi: 10.3928/0147-7447-20020502-07. [DOI] [PubMed] [Google Scholar]
- 32.McLain RF, Fleming JE, Boehm CA, et al. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005;87:2655–2661. doi: 10.2106/JBJS.E.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meadows GR. Adjunctive use of ultraporous beta-tricalcium phosphate bone void filler in spinal arthrodesis. Orthopedics. 2002;25:s579–s584. doi: 10.3928/0147-7447-20020502-06. [DOI] [PubMed] [Google Scholar]
- 34.Muschik M, Ludwig R, Halbhubner S, et al. Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J. 2001;10(Suppl 2):S178–S184. doi: 10.1007/s005860100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muschler GF, Matsukura Y, Nitto H, et al. Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Relat Res. 2005;432:242–251. doi: 10.1097/01.blo.0000149812.32857.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res. 2002;395:66–80. doi: 10.1097/00003086-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Price CT, Connolly JF, Carantzas AC, et al. Comparison of bone grafts for posterior spinal fusion in adolescent idiopathic scoliosis. Spine. 2003;28:793–798. doi: 10.1097/00007632-200304150-00012. [DOI] [PubMed] [Google Scholar]
- 38.Ransford AO, Morley T, Edgar MA, et al. Synthetic porous ceramic compared with autograft in scoliosis surgery. A prospective, randomized study of 341 patients. J Bone Joint Surg Br. 1998;80:13–18. doi: 10.1302/0301-620X.80B1.7276. [DOI] [PubMed] [Google Scholar]
- 39.Robertson PA, Wray AC. Natural history of posterior iliac crest bone graft donation for spinal surgery: a prospective analysis of morbidity. Spine. 2001;26:1473–1476. doi: 10.1097/00007632-200107010-00018. [DOI] [PubMed] [Google Scholar]
- 40.Sandhu HS, Grewal HS, Parvataneni H. Bone grafting for spinal fusion. Orthop Clin North Am. 1999;30:685–698. doi: 10.1016/S0030-5898(05)70120-6. [DOI] [PubMed] [Google Scholar]
- 41.Sasso RC, LeHuec JC, Shaffrey C. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(Suppl):S77–S81. doi: 10.1097/01.bsd.0000112045.36255.83. [DOI] [PubMed] [Google Scholar]
- 42.Sengupta DK, Truumees E, Patel CK, et al. Outcome of local bone versus autogenous iliac crest bone graft in the instrumented posterolateral fusion of the lumbar spine. Spine. 2006;31:985–991. doi: 10.1097/01.brs.0000215048.51237.3c. [DOI] [PubMed] [Google Scholar]
- 43.Skaggs DL, Samuelson MA, Hale JM, et al. Complications of posterior iliac crest bone grafting in spine surgery in children. Spine. 2000;25:2400–2402. doi: 10.1097/00007632-200009150-00021. [DOI] [PubMed] [Google Scholar]
- 44.Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 45.Violas P, Chapuis M, Bracq H. Local autograft bone in the surgical management of adolescent idiopathic scoliosis. Spine. 2004;29:189–192. doi: 10.1097/01.BRS.0000105536.65164.B1. [DOI] [PubMed] [Google Scholar]
- 46.Wippermann BW, Schratt HE, Steeg S et al (1997) Complications of spongiosa harvesting of the ilial crest. A retrospective analysis of 1,191 cases. Chirurg 68:1286–1291. doi:10.1007/s001040050361 [DOI] [PubMed]
- 47.Woolf SK, Gross RH. Perceptions of allograft safety and efficacy among spinal deformity surgeons. J Pediatr Orthop. 2001;21:767–771. doi: 10.1097/00004694-200111000-00013. [DOI] [PubMed] [Google Scholar]