Abstract
Central cord syndrome is an incomplete spinal cord injury first described by Schneider et al. (J Neurosurg 11:546–547, 1954). The typical neurological deficit is symmetrical incomplete quadriplegia affecting the upper more than the lower limbs; sensory impairment is variable and urinary retention is common. Records of all patients with acute traumatic central cord syndrome admitted to the National Spinal Injuries Unit from 1999 to 2007 were compiled from a prospectively collected computerized spinal database. Data recorded included patient demographic profile, mechanism of injury, initial neurological deficit, treatment instituted, complications of treatment, and final neurological status. A total of 50 patients with acute traumatic central cord syndrome were identified accounting for 6.2% of all admissions to the spinal injuries unit. There were 42 men and 8 women. The mean age was 56.1 years with a range of 41–88 years. We stratified patients according to age at injury to determine the effect of age on clinical outcome. There were 13 patients <50 years of age, 24 aged 50–70, and 13 aged >70 years. The mean follow-up time was 42.2 months. More than one-third of patients were intoxicated with alcohol at the time of the injury. Both upper and lower limb motor scores and total sensory scores improved in all age groups. Absolute and relative improvements were greatest in patients <50 years of age. Sphincter disturbance was documented in 42% of all the patients on admission. At follow up 12% of patients had residual disturbance. At final follow up none of the patients under 70 years of age had residual sphincter disturbance while 60% of those aged >70 years group had. Both of these results reached statistical significance. In conclusion, central cord syndrome is a common cause of acute traumatic neurological deficit, with alcohol intoxication being a common confounder at the time of injury. The clinical outcomes are significantly worse in patients aged 70 years or older.
Keywords: Central cord syndrome, Age, Outcome, Spine
Introduction
Central cord syndrome is an incomplete spinal cord injury first described by Schneider in 1954 [14]. The typical neurological deficit is a symmetrical incomplete quadriplegia affecting the upper more than the lower limbs; sensory impairment is variable and urinary retention is common [6].
Central cord syndrome is classically associated with a traumatic hyperextension injury. In the stiff spondylitic spine altered segmental spinal cord mechanics, buckling of the ligamentum flavum combined with compression from anterior osteophytes results in acute focal spinal cord compression with resultant hemorrhage and spinal cord edema. While this syndrome is more classically seen in the spondylotic elderly spine there have been cases reported of central cord syndrome in patients with normal canal diameter. In these cases extreme cervical hyper flexion may result in a vascular mediated cord injury [8].
The literature is mixed/varied as to recommendations for the treatment of central cord syndrome. No consensus exists as to the role or timing of surgical decompression in the absence of an unstable injury/fracture [11].
This retrospective study examined the clinical characteristics, radiological findings, management, and functional outcome in 50 patients who presented with traumatic central cord syndrome to the National Spinal Injuries Unit, Ireland.
Patients and methods
The National Spinal Injuries Unit is the tertiary referral center for major spinal injury management in Ireland. Records of all the patients admitted to our unit with central cord syndrome between 1999 and 2007 were compiled from a prospective computerized spinal database, which details the initial presentation, mechanism of injury, and the treatment instituted. Patient data was augmented with extracts from the Hospital Inpatient Enquiry System (HIPE). This comprehensive data collection system identified all cases of spinal cord injury as defined by the WHO’s International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic codes 952.x (SCI without evidence of fracture) and 806.x (fracture of vertebral column with SCI) to ensure a complete dataset.
Only patients with an ASIA Impairment Grade of C or D, sacral sensory sparing and those whose motor score was greater in the lower limbs than in the upper limbs were studied. Patients with neurological deficits secondary to tumor, infection, psychogenic paralysis, or any other non-traumatic condition were excluded. Exclusion criteria also included those with multiple injuries, patients with more than one vertebral fracture or those with a documented intracranial injury.
Data collected included patient demographics, mechanism of injury, injury pattern, neurological status, hospital stay, and treatment. Parameters were also recorded on discharge and during rehabilitation to final follow up. Patients were divided into three age categories, <50, 50–70, and >70 years of age.
Descriptive statistics were reported using proportions for the categorical data and means/medians for the continuous data. Trends and relationships over the 8-year study period were examined for strengths of association using Chi-squared tests while differences between study groups were tested using the 2-tailed Student’s t test.
The American Spinal Injury Association (ASIA) scoring system was used to record the motor and sensory deficit, of upper and lower limbs, respectively [2]. The motor score is based on the examination of 10 key-muscles on each side. For each movement, force is measured and assigned a coefficient from 0 (absence of muscle contraction) to 5 when contraction creates a movement in all the joint amplitude against a complete resistance. The maximal total score is so 100 (50 on the right and 50 on the left). The sensory score is established after studying light touch and pinprick sensitivity on a key point in each of 28 dermatomes on each side. Absence of sensitivity is quoted: 0, the hypo or the hyperesthesia: 1, and normal sensitivity: 2. It is preferable to begin the examination by testing the light touch and the lower part of the body.
Finally, the examination seeks to specify the incomplete or complete status of the cord injury. The deficit may be complete or partial, on the sensory or motor side. The persistence of any sensitivity, even in a very limited area, or any muscle activity, below the level of injury, especially in the sacral area (sensitivity of the anal margin, deep anal sensation, and voluntary contraction of the external sphincter) signs by definition the incomplete status of the neurological injury. Often there is a dissociation between the sensory and motor level, especially, in complete injuries, the sensory level is usually lower than the motor level. The precise study of the sensitivity, the motricity, reflexes under the injury level, as well as of sphincters, is mandatory.
The ASIA Impairment Scale is then applied as shown in Fig. 1.
Fig. 1.
ASIS Impairment Scale
During the entire study period as per national spinal cord injury policy patients received intravenous steroids as per the NASCIC Steroid Protocol [4]. Methylprednisolone was prescribed as a bolus intravenous infusion of 30 mg/kg of body weight over 15 min within 8 h of acute closed spinal cord injury, followed 45 min later by an infusion of 5.4 mg/kg of body weight per hour for 23 h.
Results
Between 1999 and 2007 a total of 50 patients with acute traumatic central cord syndrome were admitted to the National Spinal Injuries Unit, Ireland. This accounted for 6.2% of all admissions to the unit. The mean study follow-up period was 42.2 months. There were 42 men and 8 women. There were 13 patients <50 years of age, 24 aged between 50 and 70 years, and 13 were >70 years of age. The mean age was 56.1 years with a range of 41–88 years. Henceforth, many of the results will be presented according to these age groups.
The average time to transfer from a peripheral hospital to the tertiary spinal unit was 1.5, 1.2, and 2.4 days when stratified according to age. A total of 42 patients received intravenous steroids as per NASCIS protocol, as was the national practice at the time. Steroids were not administered in the remaining 8 patients as consultation with the tertiary unit was >8 h after injury/onset of neurological deficit.
The mechanism of injury differed between age groups, with falls from a standing height being the most common cause in those aged >50 years (65%), while high-speed injuries being more common in those <50 (70%). The mechanism of injury for the three different groups is illustrated in Table 1. The single ‘other’ injury mechanism was from a diving accident. There were no other sports related injuries.
Table 1.
Mechanism of injury
| Group | <50 years | 50–70 years | >70 years |
|---|---|---|---|
| RTA | 8 | 8 | 5 |
| Fall | 4 | 16 | 8 |
| Other | 1 | ||
| Total | 13 | 24 | 13 |
According to Blood alcohol Levels on admission, 36% of all cases were intoxicated at the time of injury. A total of 15 patients (30%) had an associated facial laceration indicative of a head injury.
Tables 2 and 3 represent the axial and cross-sectional imaging of the population of patients. Twenty-four percent of patients had a demonstrable acute traumatic radiological abnormality on plain X-ray. Fifty-two percent had ‘classical” central cord syndrome with generalized spondylotic changes only. On Magnetic Resonance Imaging, all the patients had an area of increased cord signal intensity on the T2-weighted images. Twenty percent had evidence frank intra-medullary hemorrhage.
Table 2.
Plain radiographic findings
| Group | <50 years | 50–70 years | >70 years |
|---|---|---|---|
| Spondylosis | 5 | 12 | 13 |
| Subluxation/dislocation | 2 | 1 | 2 |
| Fracture | 2 | 3 | 2 |
| Nil | 4 | 8 | – |
Table 3.
Level of injury
| Level | Number |
|---|---|
| C2/3 | 3 |
| C3/4 | 24 |
| C4/5 | 7 |
| C5/6 | 11 |
| C6/7 | 5 |
| Total | 50 |
Almost half of the injuries were radiologically identified at the C3C4 level, with corresponding localizing neurological findings.
A total of 13 patients (26%) had surgical intervention within 24 h of the admission; 6 had posterior cervical decompression and instrumented fusion performed for deteriorating neurological function. None of these patients had any demonstrable acute radiological spinal column injury, thus representing 20% of all the patients with “classical” spondylotic central cord syndrome. A total of 4 patients had an emergent anterior cervical discectomy for acute traumatic disk herniation with spinal cord compression, while 3 underwent anterior C4 corpectomy and reconstruction to treat unstable cervical spine fractures with evidence of cord compression. None of the patients in this series had delayed surgery for either neurological plateau or deterioration.
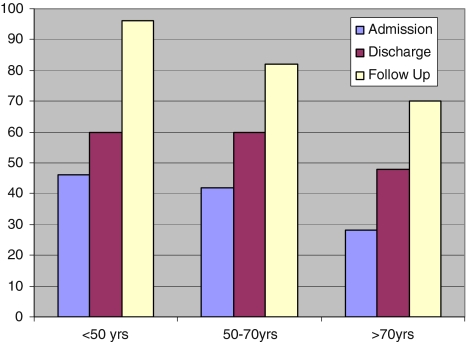
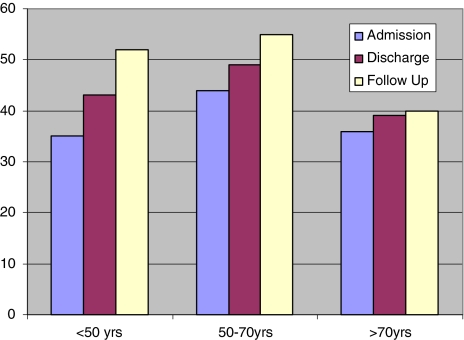
Both upper and lower limb motor scores and total sensory scores improved in all the age groups. Absolute and relative improvements were greatest in patients <50 years of age, P < 0.0001 (Figs. 2, 3). Sphincter disturbance was documented in 42% of all the patients on admission. At follow up 12% had residual disturbance. At final follow up none of the patients <70 years of age had residual sphincter disturbance while 60% of those aged >70 years had (P < 0.001).
Fig. 2.
Total motor score
Fig. 3.
Total sensory score
Complications were more common in the older age group (Table 4). There was a 100% major complication rate in patients >70 years of age, while there were only four major complications in 3 patients (23%) <50 years of age.
Table 4.
Complications
| Group | <50 years | 50–70 years | >70 years |
|---|---|---|---|
| Respiratory failure | 3 | 3 | 7 |
| PE | 0 | 0 | 3 |
| RTI | 1 | 3 | 3 |
| SIADH | 0 | 0 | 3 |
| Renal failure | 0 | 1 | 2 |
The length of time spent in the acute spinal injuries unit prior to transfer to rehabilitation increased significantly with age (Table 5). The mean LOS for patients >70 years was more than thrice that of patients <50.
Table 5.
Length of stay in days
| Group | <50 years | 50–70 years | >70 years |
|---|---|---|---|
| Spinal unit stay | 13.7 | 19.8 | 43.7 |
| Range | 4–35 | 3–60 | 30–70 |
Patients were transferred to the National Rehabilitation Hospital on discharge where their progress was followed. Mean follow up from the time of injury was 42 months. Only 6/13 (46%) of patients in the >70 age category were alive at final follow up.
Discussion
Central cord syndrome is the most common of the spinal cord injury syndromes, accounting for approximately 9%, and 6.2% in our study, of all traumatic spinal cord injuries [5].
The two most common etiologies are following a simple fall and motor vehicle accidents [5]. In our study the mechanism of injury differed between age groups, with falls from a standing height being the most common cause in those aged >50 years (65%), while high-speed injuries being more common in those <50 (70%).
Central cord syndrome generally has a favorable prognosis for functional recovery. Penrod et al. [13] noted that older patients with central cord syndrome have a worse prognosis for ambulation (97 vs. 41%) relative to younger patients. Aito [1] showed patients <65 years had better outcomes with less neuropathic pain. Patients with traumatic central cord syndrome are generally older than the traumatic spinal cord injured patient population but often show improvement after discharge. Improvement in subjects <65 years was significantly better than for those >65 years in Aito’s study [1].
In this study, both upper and lower limb motor scores and total sensory scores improved in all the age groups. Absolute and relative improvements were greatest in patients <50 years of age. Bowel and Bladder sphincter disturbance was documented in 42% of all the patients on admission. At final follow up none of the patients <70 years of age had residual sphincter disturbance while 60% of those aged >70 year group had.
According to Blood alcohol Levels on admission, 36% of all our cases were intoxicated at the time of injury. To our knowledge ours is the first report containing any information on the incidence of alcohol intoxication in a population of patients with acute traumatic central cord syndrome. These results are in keeping with previous reports, which have demonstrated that alcohol plays a major role in acute adult SCI [15].
Neurological and functional recovery was observed on discharge from rehabilitation, which continued in the period following discharge. No difference in outcome was found as a result of spine surgery.
Favorable prognostic factors that have been previously documented include evidence of early motor recovery, young age, absence of lower extremity neurologic motor impairment at rehabilitation admission, and presence of documented upper and lower extremity strength improvement during rehabilitation [12].
Central cord syndrome is classically associated with a traumatic hyperextension injury. In the stiff spondylitic spine altered segmental spinal cord mechanics, buckling of the ligamentum flavum combined with compression from anterior osteophytes results in acute focal spinal cord compression with resultant hemorrhage and spinal cord edema [16, 17].
Pathological analysis reveals, in most cases, a diffuse, dorsal lateral white matter injury. This correlates well with ante mortem and postmortem MRI, suggesting that buckling of hypertrophied ligament flavum known to occur in hyperextension creates a shearing injury to the underlying cord tissue [13].
In our study, 24% of patients had a demonstrable acute traumatic radiological abnormality on plain X-ray. Fifty-two percent had ‘classical” central cord syndrome with generalized spondylotic changes only. On Magnetic Resonance Imaging, all the patients had an area of increased cord signal intensity on the T2-weighted images.
Evidence from clinical-pathologic magnetic resonance imaging (MRI) correlation studies reveals that this pattern may not be based on the anatomical locations of the arm and leg fibers within the corticospinal tract, but rather that the corticospinal tract sub serves mainly distal limb musculature, and thus, the functional deficit would be more pronounced in the hands when the tract is the primary site of damage [10].
There are currently no standards regarding the role and timing of decompression in acute spinal cord injury. We undertake urgent decompression in unstable fractures and facet subluxations in patients with incomplete neurology or in patients with spinal cord injury with neurologic deterioration. Urgent decompression in acute cervical spinal cord injury remains a reasonable practice option and can be performed safely. There is emerging evidence that surgery within 24 h may reduce length of intensive care unit stay and reduce post-injury medical complications [7].
Several studies have tried to determine if operative intervention might lead to better patient outcomes compared with that of conservative treatment. Bose et al. compared motor function recovery in patients treated surgically versus conservatively after acute traumatic central cord syndrome. They found that although both groups showed significant motor recovery using a modified ASIA scoring, a greater degree of motor recovery was found in the operative group [3].
Guest et al. [9] compared early and late surgical intervention after acute traumatic central cord syndrome and found excellent motor recovery rates, with mean ASIA motor scores of 90 for the early (<24 h) operative group and 85.3 for the late (>24 h) operative group.
In our series a total of 13 patients (26%) had surgical intervention within 24 h of the injury; 6 had posterior cervical decompression and instrumented fusion performed for deteriorating neurological function; 4 had an emergent anterior cervical discectomy for acute traumatic disk herniation with spinal cord compression, while 3 underwent anterior C4 corpectomy and reconstruction to treat unstable cervical spine fractures with evidence of cord compression. None of the patients in this series had delayed surgery for either neurological plateau or deterioration.
Neurological recovery after central cord syndrome has been extensively studied and shown to occur in a definitive pattern. Many authors have contributed this pattern of recovery to the regression of spinal cord edema. They postulate that as the edema subsides, motor function follows a definite pattern, with the lower extremities recovering first, followed by bladder recovery and finally movement of the upper extremities, with finger movements recovering last [16].
Limitations of this study include its retrospective character with its well-known limitations and that the limited number of patients with central cord syndrome and the small sample size of geriatric patients with only 46% of patients in the 70 years or older category being alive at final follow-up investigation may result in a limited statistical power. Each individual statistical comparison was tested using the Student’s t test. This allowed univariate comparison to determine if there was a direct link between advanced ages and “absolute and relative neurological improvements”, “residual sphincter disturbance” and “major complication rate”. Following consultation with the departmental statistician it was determined that the study groups were too small in number to allow meaningful multivariate or regression analysis. Thus, descriptive statistics alone were utilized.
In summary, we present 50 cases of acute traumatic central cord syndrome treated at a single national institution over 8 years. Much of our demographic data is in keeping with previously reported series. The unique results of our study highlight the different surgical pathologies involved in the etiology of this devastating injury. We identify alcohol intoxication as a common association and a fall from a standing height is the primary cause in this age group. We strongly recommend “falls prevention strategies” to ameliorate this problem. Particularly in the light of the poorer prognosis for neurological recovery in patients >70 years.
References
- 1.Aito S, D’Andrea M, Werhagen L, Farsetti L, Cappelli S, Bandini B, Di Donna V. Neurological and functional outcome in traumatic central cord syndrome. Spinal Cord. 2007;45:292–297. doi: 10.1038/sj.sc.3102018. [DOI] [PubMed] [Google Scholar]
- 2.Standards for neurological classification of spinal injury patients. Chicago: ASIA; 1984. [Google Scholar]
- 3.Bose B, Northrup BE, Osterholm JL, Cotler KM, Ditunno JF. Reanalysis of central cervical cord injury management. Neurosurgery. 1984;15:367–372. doi: 10.1097/00006123-198409000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 5.Bosch A, Stauffer ES, Nickel VL. Incomplete traumatic quadriplegia: a ten-year review. JAMA. 1971;216:473–478. doi: 10.1001/jama.216.3.473. [DOI] [PubMed] [Google Scholar]
- 6.Chen TY, Lee ST, Lui TN, Wong CW, Yeh YS, Tzaan WC, Hung SY. Efficacy of surgical treatment in traumatic central cord syndrome. Surg Neurol. 1997;48:435–440. doi: 10.1016/S0090-3019(97)00037-2. [DOI] [PubMed] [Google Scholar]
- 7.Fehlings MG, Perrin RG. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine. 2006;31:S28–S35. doi: 10.1097/01.brs.0000217973.11402.7f. [DOI] [PubMed] [Google Scholar]
- 8.Gros C, Vlahovitch B, Mohasseb G. Traumatic tetraplegia with predominantly brachial involvement. Neurochirurgia. 1960;3:92–112. doi: 10.1055/s-0028-1095483. [DOI] [PubMed] [Google Scholar]
- 9.Guest JD, Eleraky MA, Apostolides PJ, Dickman CA, Sonntag VKH. Traumatic central cord syndrome: results of surgical management. J Neurosurg Spine. 2002;97:25–32. doi: 10.3171/spi.2002.97.1.0025. [DOI] [PubMed] [Google Scholar]
- 10.Levi AD, Tator CH, Bunge RP. Clinical syndromes associated with disproportionate weakness of the upper versus the lower extremities after cervical spinal cord injury. Neurosurgery. 1996;38:179–185. doi: 10.1097/00006123-199601000-00039. [DOI] [PubMed] [Google Scholar]
- 11.Maroon JC, Abla AA, Wilberger JI, Bailes JE, Sternau LL. Central cord syndrome. Clin Neurosurg. 1991;37:612–621. [PubMed] [Google Scholar]
- 12.McKinley W, Santos K, Meade M, Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007;30:215–224. doi: 10.1080/10790268.2007.11753929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penrod LE, Hegde SK, Ditunno JF., Jr Age effect on prognosis for functional recovery in acute, traumatic central cord syndrome. Arch Phys Med Rehabil. 1990;71:963–968. [PubMed] [Google Scholar]
- 14.Schneider RC, Cherry G, Pantek H. The syndrome of acute central cervical spinal cord injury; with special reference to the mechanisms involved in hyperextension injuries of cervical spine. J Neurosurg. 1954;11:546–547. doi: 10.3171/jns.1954.11.6.0546. [DOI] [PubMed] [Google Scholar]
- 15.Tator CH, Duncan EG, Edmonds VE, Lapczak LI, Andrews DF. Changes in epidemiology of acute spinal cord injury from 1947 to 1981. Surg Neurol. 1993;40:207–215. doi: 10.1016/0090-3019(93)90069-D. [DOI] [PubMed] [Google Scholar]
- 16.Uribe J, Green B, Vanni S, Moza K, Guest J, Levi A. Acute traumatic central cord syndrome—experience using surgical decompression with open-door expansile cervical laminoplasty. Surg Neurol. 2005;63:505–510. doi: 10.1016/j.surneu.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Waters R, Adkins R, Sie IH, Yakura JS. Motor recovery following spinal cord injury associated with cervical spondylosis: a collaborative study. Spinal Cord. 1996;34:711–715. doi: 10.1038/sc.1996.129. [DOI] [PubMed] [Google Scholar]