Abstract
Intervertebral disc (IVD) cells within the annulus fibrosus (AF) and nucleus pulposus (NP) maintain distinct functional extracellular matrices and operate within a potentially noxious and stressful environment. How disc cells respond to stress and whether stress is responsible for triggering degeneration is unknown. Disc cell proliferation and cluster formation are most marked in degenerate IVDs, possibly indicating attempts at matrix repair. In other tissues, stress proteins increase rapidly after stress protecting cell function and, although implicated in degeneration of articular cartilage, have received little attention in degenerative IVD pathologies. We have compared the distribution of stress protein immunolocalization in pathological and control IVDs. Disc tissues were obtained at surgery from 43 patients with degenerative disc disease (DDD) and herniation, and 12 controls at postmortem. Tissues were immunostained with a polyclonal antibody for heat shock factor 1 (HSF-1) and monoclonal antibodies for the heat shock proteins, Hsp27 and Hsp72, using an indirect immunoperoxidase method. Positively stained cells were expressed as a percentage of the total. Cell cluster formation was also assessed. The proportion of cells in clusters was similar in the AF (both 2%) and NP (8 and 9%) of control and DDD samples, whereas in herniated tissues this was increased (AF 12%, NP 14%). Stress antigen staining tended to be more frequent in clustered rather than in single/doublet cells, and this was significant (P < 0.005) in both the AF and NP of herniated discs. Clustered cells, which are most common in herniated discs, may be mounting a protective response to abnormal environmental factors associated with disc degeneration. A better understanding of the stress response in IVD cells may allow its utilization in disc cell therapies.
Keywords: Intervertebral disc, Degeneration, Cell cluster, Stress, Heat shock protein
Introduction
Intervertebral discs (IVDs) are tough, but pliable fibrocartilage structures interposed between bony endplates that separate the vertebral bodies and render the vertebral column flexible. Back pain is frequently associated with IVD degeneration. Young healthy IVDs comprise an outer ligamentous zone of concentric collagen lamellae, the annulus fibrosus (AF) that surrounds the inner gel-like nucleus pulposus (NP), both sparsely populated with cells. IVD degeneration occurs via failure of the disc cells to maintain a functional tissue matrix. Loss of proteoglycan and its associated fluid results in reduction of disc height, which is typical of degenerative disc disease (DDD). This may predispose to disc herniation [27], which begins with the protrusion of the disc into the spinal canal. Sometimes, the AF remains intact, whereas at other times it ruptures and the disc tissue extrudes, gaining access to the epidural space. Complete detachment of extruded material results in a loose body of sequestrated tissue within the spinal canal [1, 28].
At the cellular level in the normal IVD, the slow exchange of nutrients and waste products in the virtually avascular disc may produce a physiologically challenging environment detrimental to disc cell function and survival, possibly explaining why IVD degeneration begins early in life [8]. Disc degeneration is associated with increased vascularization and innervation of peripheral disc tissue [15, 20]. Changes to the composition of disc extracellular matrix (ECM) accompany the appearance of clusters of disc cells [26], which are generally found confined within a pericellular chondron [11, 29]. Clusters, believed to form via disc cell proliferation [14], are commonly found in the inner zones of the AF and NP in regions with the greatest macroscopic evidence of degeneration.
Typically, most cells respond to physiological and environmental challenges by mounting a stress response that involves the production of a range of molecular chaperones with cytoprotective functions that include the regulation of protein folding, cell growth, development and survival [24]. These include the stress-activated heat shock factors (HSFs), which are transcription factors that translocate to the cell nucleus upregulating expression of an array of heat shock proteins (Hsps) that function in proteostasis [3, 39], and in the regulation of signal transduction pathways [24]. Hsp27, a member of the small heat shock protein family, along with members of the highly conserved Hsp70 family of proteins, is present in cells in both constitutive and stress-induced forms. Both Hsp27 and 70 have been identified by immunostaining in the chondrocytes and osteoblasts of rat growth plate cartilage and newly formed bone [25, 35, 37]. Although their precise roles in many tissues remain unclear, certain stress proteins have been identified in connective tissues as having roles in the chaperoning of specific matrix proteins. For example, Hsp47 chaperones the newly synthesized alpha-chains of type I collagen in the endoplasmic reticulum (reviewed in [23]), and Hsp25 (an isoform of the small-Hsp family that includes Hsp27 and Hsp28) is involved in the intracellular trafficking of aggrecan core protein, a major component of cartilage ECM [40]. The presence of Hsp27 has sometimes been associated with DNA fragmentation in chondrocytes within the hypertrophic zone of the growth plate [35]. In human IVD tissues retrieved at autopsy [34], both Hsp27 and Hsp72 were found in AF and NP cells from foetuses, children and adults with increased immunolocalization in discs showing greater levels of degeneration. However, little is known about the functions of stress proteins in IVD tissue or their relationships with disc cell cluster formation in degenerative disc pathologies. Since disc cells exist in a challenging tissue environment [36] and cell cluster formation appears to be associated with degenerative changes in IVD tissues, we hypothesized that their presence would be accompanied by the dynamic expression of cell stress markers. Thus, we examined excised human IVD tissues from patients with symptomatic disc pathologies and also from control tissues obtained at autopsy for the presence of cell clusters and immunolocalization of cellular HSF-1, Hsp27 and stress-inducible Hsp72.
Materials and methods
Tissue samples
Samples of IVD from the lumbo-sacral spine were obtained from 55 subjects either following surgery or at autopsy. A total of 43 disc samples were obtained from patients undergoing surgery for the management of disc disorders. Of these, 16 were from patients with DDD (age 45 ± 11years, range 23–60 years) and 27 from patients with herniated discs (42 ± 14, 17–81 years). Of the herniated group, 14 patients had protruded (39 ± 17, 17–81 years), 6 extruded (45 ± 11, 32–59 years) and 7 sequestrated (46 ± 8, 36–58 years) discs. Cadaveric material from 12 individuals (62 ± 18, 22–90 years) with no previously known spinal pathology was collected at autopsy between 10 and 96 h postmortem. Disc tissues were snap frozen in liquid nitrogen-cooled hexane and stored in liquid nitrogen.
On removal at surgery, the orientation of the disc specimens with respect to macroscopic in vivo disc anatomy (whether AF or NP) was noted by the surgeon and confirmed later by its microscopic appearance under polarized light. From the collected disc tissues, we identified 12 AF and 5 NP samples from control discs, 14 AF and 2 NP in the DDD group, 11 AF and 10 NP in the protruded group, 6 AF and 3 NP in the extruded group and 4 AF and 5 NP from the sequestrated group.
Detection of stress antigens in IVD tissues by immunohistochemistry and Western immunoblotting
Frozen tissues were sectioned (8 μm) with a cryostat, collected onto a polylysine-coated slide and stored at −20°C. Before staining, slides were brought to room temperature and fixed in acetone for 10 min at 4°C. Immunostaining was carried out at room temperature and PBS used for each wash step. Sections were incubated for 1 h with the following antibodies (Ab): polyclonal Ab, raised in rabbits against human HSF-1 (SPA-901C, StressGen, Victoria, Canada, 1:2,000 dilution) and monoclonal Abs raised in mice against Hsp27 (SPA-800D, StressGen, 1:1,000 dilution) and Hsp72 (SPA-810D, StressGen, 1:1,000 dilution). Negative control sections were incubated appropriately with rabbit normal serum and mouse IgG (Dako Ltd, Cambridge, UK). The sections were then incubated with either secondary biotinylated goat anti-rabbit IgG or horse anti-mouse IgG (Vectastain Elite ABC, Vector Laboratories, Peterborough, UK) for 30 min. Sections were incubated in 0.3% hydrogen peroxide in methanol for 30 min to block endogenous peroxidase activity, and immunolabelling visualized using the streptavidin–biotin detection system with diaminobenzidine tetrachloride (Sigma Ltd, Poole, UK) as substrate. Some sections were counterstained with Mayer’s haemotoxylin to reveal general cellular morphology. All sections were dehydrated using serial isopropyl alcohol solutions (70% × 1; 90% × 1; 100% × 2), washed twice with xylene, mounted in Pertex and assessed using a Leitz Diaplan Bright-Light microscope with digital image capture (Optivision, Ossett, West Yorkshire, UK).
Since control tissues were collected over a wide time span, ranging from 10–96 h postmortem, the effects of storage conditions and time on stress antigen immunoreactivity were assessed. Two disc samples, freshly collected at surgery, were dissected into three pieces and stored at 4°C for times ranging from 0 (fresh) to 120 h, after which they were processed as described above.
For Western immunoblotting, stress protein antigens were extracted from the IVD tissue of three representative patients (two herniated and one DDD). Lyophilized tissue extracts were re-dissolved (2 mg dry weight/ml) overnight in 0.1 M Tris 30 mM acetate buffer (pH 6.5) at 4°C, combined to make a representative tissue extract sample, electrophoresed and blotted using previously described methods [19].
The blots were initially blocked for 1 h in 0.1 M Tris–HCl (pH 7.4) containing 8% liquid skimmed milk (blocking buffer), and then primary antibodies (diluted 1 in 200) in blocking buffer were added for 1 h [Stressgen, Hsp27 (SPA-800, Mse mAb); Hsp72 (SPA-810, Mse mAb); HSF-1 (SPA-901, Rab pAb)]. After washing in blocking buffer, the appropriate biotinylated secondary antibody was added (Vectastain ABC-AP, Rabbit IgG and Mouse IgG Kits) and the blots developed and detected (Vector BCIP/NBT Kit) as per manufacturer’s instructions. Appropriately diluted mouse and rabbit IgG were included as negative controls for each primary antibody. No false positive bands were observed.
Data collection and analysis
As many as 200 cells were counted per section where possible (in 84% of samples) at high magnification (×500) from representative, but random, fields of view (more than 5); for the remaining 16% of samples, the total number of cells counted ranged between 10 and 190 (mean 111 ± 46). Absolute cell counts are presented in Tables 1 and 2. Cells were assessed for positive antigen staining, in addition to noting their presence within cell clusters (defined as aggregates of three or more cells within a common territorial matrix). Cells in associated disc structures, such as endplate or blood vessels, were strictly excluded. Groups were compared using the Mann–Whitney U test. Differences in stress antigen staining between single/doublet and cell clusters were determined by Pearson χ2 statistic for categorical variables (Fisher’s exact test). For this, test data are reported as the fold difference and P value; in all cases, the value of the degrees of freedom (df) was 1. Statistical analyses were performed using SPSS v16.0 (SPSS Inc., IL, USA). Statistical significance was taken at a P value of ≤0.05.
Table 1.
Summary data of disc cell cluster prevalence, proportion of cells found in cell clusters and stress protein staining in the annulus fibrosus of control and pathological groups
| Annulus fibrosus | Age Mean ± SD (range) |
Percentage of disc sections with cell clusters | Percentage of total cells counted found in clusters | Percentage of positive cells [median (range)] Absolute no. of cells counted in “n” specimens |
||
|---|---|---|---|---|---|---|
| HSF-1 | HSP27 | HSP72 | ||||
| Control n = 12 |
62 ± 18 (22–90) |
58 | 2 | 10 (0–51) 2,400, n = 12 |
54 (20–82) 2,400, n = 12 |
46 (28–88) 2,300, n = 12 |
| DDD n = 14 |
46 ± 10† (33–60) |
36 | 2 | 2 (0–29) 2,660, n = 14 |
43 (3–79) 2,390, n = 14 |
29† (1–75) 2,560, n = 13 |
| Herniated n = 21 |
43 ± 13† (21–81) |
86 | 12 | 9 (0–70) 3,051, n = 17 |
52 (8–80) 3,608, n = 19 |
42 (5–90) 3,382, n = 19 |
| Protruded n = 11 |
41 ± 16† (21–81) |
73 | 5 | 9 (0–23) 1,280, n = 7 |
52 (8–80) 1,646, n = 9 |
37 (5–90) 1,547, n = 9 |
| Extruded n = 6 |
45 ± 11† (32–59) |
100 | 13 | 14 (1–34) 1,050, n = 6 |
59 (35–78) 1,202, n = 6 |
46 (20–72) 1,050, n = 6 |
| Sequestrated n = 4 |
45 ± 9 (36–58) |
100 | 24 | 16 (1–70) 721, n = 4 |
44 (38–58) 760, n = 4 |
52 (17–69) 785, n = 4 |
†A statistically significant difference (P < 0.05) between pathological and control groups
Table 2.
Summary data of disc cell cluster prevalence, proportion of cells found in cell clusters and stress protein staining in the nucleus pulposus of control and pathological groups
| Nucleus pulposus | Age Mean ± SD (range) |
Percentage of disc sections with cell clusters | Percentage of total cells counted found in clusters | Percentage of positive cells [median (range)] Absolute no. of cells counted in “n” specimens |
||
|---|---|---|---|---|---|---|
| HSF-1 | HSP27 | HSP72 | ||||
| Control n = 5 |
69 ± 7 (61–78) |
80 | 8 | 4 (3–14) 942, n = 5 |
44 (5–66) 1,000, n = 5 |
36 (4–62) 855, n = 5 |
| DDD n = 2 |
32 ± 13 (23–41) |
100 | 9 | 0.3 (0–1) 400, n = 2 |
40 (30–51) 400, n = 2 |
19 (11–26) 400, n = 2 |
| Herniated n = 18 |
43 ± 15† (17–81) |
94 | 14 | 11 (0–61) 3,050, n = 17 |
47 (6–82) 3,212, n = 18 |
35 (0–77) 3,070, n = 17 |
| Protruded n = 10 |
41 ± 19† (17–81) |
100 | 13 | 6 (1–25) 1,800, n = 9 |
44 (6–81) 1,952, n = 10 |
32 (0–77) 2,000, n = 10 |
| Extruded n = 3 |
45 ± 14† (32–59) |
100 | 6 | 23† (18–31) 450, n = 3 |
45 (26–62) 510, n = 3 |
30 (26–42) 450, n = 3 |
| Sequestrated n = 5 |
44 ± 7† (36–51) |
80 | 23 | 5 (0–61) 800, n = 5 |
57 (34–82) 750, n = 5 |
41 (21–61) 620, n = 4 |
†A statistically significant difference (P < 0.05) between pathological and control groups
Results
Overall, the cadaveric controls were significantly older than the patients with disc pathology (P < 0.05); however, there were no meaningful associations between age of the sample donors and stress protein staining. The number of cells stained positively for HSF-1, Hsp27 and Hsp72 in the AF and NP of control and pathological discs was variable (Tables 1, 2) and staining localized to the disc cells rather than the pericellular and ECM (Fig. 1). With the exception of lower Hsp72 staining in the AF of the DDD group (P = 0.006) and increased HSF-1 in the NP of the extruded group (P = 0.025), both compared with controls, no other statistically significant differences were found.
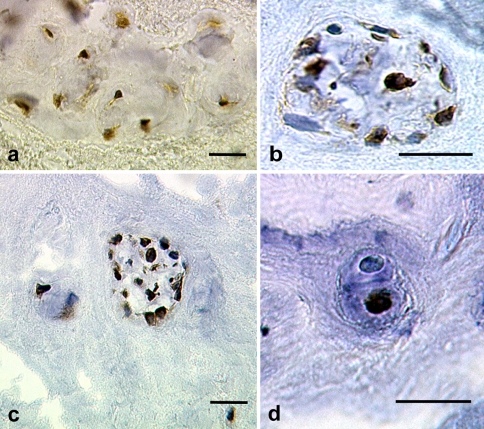
Fig. 1.
a HSF-1 immunostaining in a cluster of disc cells within the AF tissue surgically excised from a patient (24 years, female) with protruded disc at L5–S1. Hsp27 (b) and Hsp72 (c) immunostaining in a cluster of disc cells within the NP tissue surgically excised from a patient (51 years, female) with a sequestrated disc at L2–L3. d Hsp72 immunostaining in a disc cell doublet within the NP tissue surgically excised from a patient (51 years, female) with a sequestrated disc at L2–L3. Note that the lower cell shows positive staining localized to the cell nucleus, whereas the upper cell of the doublet does not show staining. Original magnification ×500; bar = 20 μm
Frequency of cell clusters in IVDs
Data are summarized in Tables 1 and 2. Proportionately fewer AF tissues from the control and DDD groups contained clusters compared with the herniated group. In the NP, cell clusters were commonly found in all groups. The proportion of cells within clusters in the control group was similar to that in the DDD group for both AF and NP. In contrast, the proportion of cells in clusters in the herniated group was increased in the AF and NP. In the AF of herniated discs, the proportion of cells associated with clusters increased with the degree of disc disruption and was greatest for both AF and NP in sequestrated discs.
Immunolocalization of stress proteins in the disc matrix
Data are summarized in Table 3. Comparisons relate to differences in incidence of stress antigen staining between single/doublet cells and disc cell clusters.
Table 3.
Summary of HSF-1, Hsp27 and Hsp72 immunostaining in single/doublet cells and in cells associated with clusters in the AF and NP regions of control and pathologic IVDs
| Proportion of cells stained for stress antigen | ||||||
|---|---|---|---|---|---|---|
| Annulus fibrosus | Nucleus pulposus | |||||
| n | Single/doublet cells | Cells in clusters | n | Single/doublet cells | Cells in clusters | |
| HSF-1 | ||||||
| Control | 12 | 15 | 7 | 5 | 6 | 7 |
| DDD | 14 | 6 | 11 | 2 | 0 | 0 |
| Herniated | 17 | 11 | 34† | 17 | 12 | 18† |
| Protruded | 7 | 8 | 20† | 9 | 8 | 19† |
| Extruded | 6 | 11 | 34† | 3 | 25 | 35 |
| Sequestrated | 4 | 14 | 41† | 5 | 13 | 13 |
| Hsp27 | ||||||
| Control | 12 | 49 | 56 | 5 | 38 | 68† |
| DDD | 14 | 40 | 57† | 2 | 39 | 59 |
| Herniated | 19 | 50 | 59† | 18 | 46 | 66† |
| Protruded | 9 | 48 | 48 | 10 | 42 | 66† |
| Extruded | 6 | 56 | 66† | 3 | 42 | 86† |
| Sequestrated | 4 | 43 | 56† | 5 | 59 | 65 |
| Hsp72 | ||||||
| Control | 12 | 49 | 54 | 5 | 39 | 78† |
| DDD | 13 | 27 | 26 | 2 | 17 | 34† |
| Herniated | 19 | 40 | 71† | 17 | 31 | 56† |
| Protruded | 9 | 39 | 40 | 10 | 31 | 59† |
| Extruded | 6 | 42 | 84† | 3 | 32 | 90† |
| Sequestrated | 4 | 37 | 74† | 4 | 32 | 48† |
†A statistically significant difference (P < 0.05) between staining in cell clusters compared with single/doublet cells using the χ2-test, and n the number of tissue samples. From a maximum of 200 cells counted for each sample, the number of positively stained cells observed as either single/doublet or within clusters was noted and the relative percentages calculated and averaged
In controls HSF-1 staining was most prevalent in single/doublet cells of the AF region, whereas in pathological discs HSF-1 was predominantly found in cell clusters. In clusters in herniated discs, HSF-1 was increased threefold (P < 0.001), with increased incidence in all three of the herniated sub-groups (protruded: 2.5-fold, P = 0.001, extruded: 3.1-fold, P < 0.001, sequestrated: 2.9-fold, P < 0.001). There was a similar tendency for HSF-1 to be localized to cell clusters in the NP of all groups and was again evident in herniated discs (1.5-fold, P = 0.001).
Hsp27 staining in the AF tended to be more localized to cells in clusters and was significantly greater than in single/doublets in both DDD (1.5-fold, P = 0.002) and herniated groups (1.2-fold, P = 0.001), particularly for extruded (1.2-fold, P = 0.02) and sequestrated (1.3-fold, P = 0.004) discs. Again in the NP, Hsp27 was predominantly localized to clustered cells, and this was greater in control and herniated groups (1.8-fold, P < 0.001 and approximately 1.4-fold, P < 0.001, respectively). In the herniated sub-groups, both protruded and extruded revealed significantly greater Hsp27 staining in clusters than single/doublet cells (1.6-fold, P < 0.001 and 2-fold, P < 0.001, respectively).
Hsp72 staining in the AF of all groups also tended to be localized to cells in clusters. In herniated discs, Hsp72 was 1.8-fold (P < 0.001) greater in cell clusters than single/doublet cells with significantly greater staining in cell clusters in both extruded and sequestrated discs (2-fold, P < 0.001 and 2-fold, P < 0.001, respectively). In the NP, Hsp72 was significantly greater in cell clusters than single/doublet cells in controls (2-fold, P < 0.001), DDD (2-fold, P = 0.01) and herniated (1.8-fold, P < 0.001) groups, and similarly in all of the herniated sub-groups (protrusions: 1.9-fold, P < 0.001, extrusions: 2.8-fold, P < 0.001 and sequestrations: 1.5-fold, P < 0.001).
In summary, staining for all three stress antigens was more prevalent in disc cells associated with cell clusters. In herniated discs, cell clusters were more prevalent and associated with increased stress antigen staining. Western immunoblotting of IVD tissue extracts confirmed the presence of HSF-1 and Hsp27 immunoreactive bands (at 38 and 49, and 28 kD, respectively), but the stress-inducible Hsp72 antigen was not detected in these samples.
Stress protein staining in stored disc tissues
Stress antigen staining for Hsp27 and Hsp72 in the two surgical disc samples stored at 4°C for various times was variable, and no consistent pattern (i.e. no increase or decrease) was observed. However, there was a trend for HSF-1 immunogenicity to decrease during the initial 48 h in storage. In one sample at times 0, 48 and 120 h, the percentage of positive cells was 40, 2.5 and 2.4%, respectively, and in the other sample at times 24, 48 and 96 h the percentage of positive cells was 6.5, 1.5 and 1.5%, respectively.
Discussion
The factors responsible for triggering the degenerative cascade in the IVD have yet to be elucidated. Throughout life disc cells are constantly challenged by mechanical and environmental stress, which may contribute to ECM breakdown resulting in IVD degeneration and loss of function. This study assessed cell cluster prevalence and compared the immunolocalization of the stress sensing transcription factor HSF-1, the small heat shock protein Hsp27 and the stress-induced Hsp72 in the AF and NP of pathological and control human IVDs. Overall, we found that the proportion of cells in clusters was greater in pathological discs in both the AF and NP. Stress antigen staining was confined to the cell and was not evident within the pericellular or ECM and, moreover, was more prevalent in disc cells associated with clusters than in cells occurring singly or as doublets.
Cell clusters form in degenerate IVDs as a consequence of the proliferation of a single or small group of disc cells, possibly in an effort to repair a damaged or dysfunctional ECM [14]. However, after proliferation disc cells may become senescent [6, 12, 18], a common finding in herniated discs [31]. Both Hsp27 and Hsp72 have been implicated in the regulation of cell proliferation, apoptosis and senescence [for reviews see 16 and 33]. In particular, a member of the Hsp70 family has been detected in cultures of proliferating chondrocytes [25] and supports our finding of Hsp72 in disc cell clusters, which may indicate recent disc cell proliferation. IVD tissue, even in health, presents a harsh and challenging environment for cell survival. Within the inner regions of the avascular disc, exchange of nutrients and waste products is slow and in degenerating tissues calcification of the bony vertebral endplates may further disrupt diffusion [32]. In disc cells, activation of the stress sensing HSF-1 complex could be triggered by several mechanisms, which result in Hsp expression [21]. ECM degeneration may lead to an environmental trigger such as exposure of the disc cells, particularly in extruded or sequestrated states, to environmental conditions (including hydrostatic loading or oxygen tensions) different from those experienced by cells in intact discs [5, 7]. Alternatively, a stress response could also be initiated by a proteotoxic stress: in this case, the presence of abnormal ECM components in the cytoplasm, such as incorrectly folded or denatured proteins. However, we cannot exclude the possibility that a combination of degenerative changes within the ECM, cell proliferation, cellular senescence and environmental factors, all contribute to the trigger of a stress response in the IVD. What is evident from our data is that progressive degenerative changes to the disc are associated with an increased prevalence of cell clusters composed of cells commonly synthesizing stress antigens, indicative of a cellular stress response. This is consistent with our hypothesis that cell cluster formation is accompanied by a stress response.
The roles of the various stress antigens are diverse and have not been thoroughly investigated in connective tissues. From the proposed functions of the stress antigens investigated, we can speculate on their roles in the pathophysiology of disc degeneration. In non-stressed cells, HSF-1 monomers exist in the cytoplasm as an Hsf heterocomplex together with other chaperones and co-chaperones [39]. Stress releases the HSF-1 monomers, which localize to the nucleus where, as trimers, they act as transcription factors binding to the HSP genes and promoting gene transcription [21, 39]. Both Hsp27 and stress-inducible Hsp72 can function as molecular chaperones to protect labile cytosolic proteins from stress-induced denaturation. In other cell types, both are induced by a range of challenges [13]. Hsp27 forms large oligomeric complexes that store misfolded proteins awaiting either refolding or degradation, whereas small oligomers modulate F-actin cytoskeletal integrity in stressed cells and also possess anti-apoptotic actions [2, 38]. Similarly, Hsp70 family members hold partially or unfolded proteins in intermediate states preventing irreversible aggregation and are also capable of refolding denatured proteins in energy-dependent reactions modulated by co-chaperones [reviewed in 22, 10]. Recent evidence has implicated Hsps in several pathological conditions caused either by the expression of mutant proteins that undergo aberrant folding or conformationally challenged proteins that result in cellular dysfunction [4]. In disc cells, it is likely that these stress antigens are expressed as a result of the disc degeneration cascade (e.g. following protease action [9, 17, 30] resulting in loss of proteoglycan, altered osmolality and loading). These, as in other tissues, are likely to have a protective effect, which, if better understood, could possibly be harnessed and controlled to limit matrix damage or even to facilitate repair. Further work that identifies specific functions of stress antigens in disc cells is warranted.
Interestingly, the total number of cells apparently producing stress proteins in cadaveric controls was comparable with pathological specimens. Reasons for this are unclear, although a feasible explanation is that a stress response might be triggered by events occurring in the disc tissue in the hours after death. Viable disc cells with intact cell membranes have been found up to 7 days postmortem [29]. Our limited time course study suggested a decline in HSF-1 immunoreactivity after 24 h storage at 4°C, but reasonably constant Hsp27 and Hsp72 staining over the duration of the experiment up to 120 h. It is possible that HSF-1 immunoreactivity is more labile than that of the Hsps and declines after 24 h postmortem. From our limited data set, we suggest that autopsy disc material may need to be collected within a defined time frame after death to minimize variability and that its usefulness as a control for this type of comparison may require further validation.
A single previous study has described stress protein expression in human disc tissues [34]. These authors investigated Hsp27 and Hsp72 immunostaining in stored disc tissues taken during autopsy at unspecified times after death, from the lumbar spines of foetuses, infants and adults. Notably, staining for both Hsp27 and Hsp72 were increased in degenerate discs. Our data extend these findings and suggest a relationship between IVD degeneration, cell cluster formation and stress antigen expression. Furthermore, our data indicate that the severity of IVD disruption was associated with greater numbers of cells within clusters and an increase in the proportion of these cells staining for the stress antigens HSF-1, Hsp27 and Hsp72.
The present study has several limitations. First, only a limited number of stress proteins were investigated, of which Hsp27 and Hsp72 had previously been studied in discs [34]. Examination of other stress antigens, such as Hsp73, the constitutively expressed isoform of Hsp70, and those of the Hsp60 and Hsp90 families may, in future, shed light on other aspects of the stress response in disc cells. Second, the cadaveric control samples were not collected under standardized conditions and this may have led to postmortem changes in the stress proteins. However, our check on the influence of time of the postmortem appeared not to be a significant factor.
In conclusion, previous work has shown that stress proteins could be detected in cadaveric human IVD tissues. In the present study, we have confirmed these earlier findings and report for the first time a link between increased immunolocalization of stress antigens in the cells present in cell clusters and the increased prevalence of cell clusters in degenerate IVDs, especially in herniated discs. The relationships between disc cell cluster formation and pathology-related cell stress requires further investigation; it may be instrumental in elucidating aspects of the pathophysiology of disc degenerative disease or perhaps utilized in regenerative or biological repair therapies.
Acknowledgments
We wish to acknowledge the patients who kindly donated their disc tissue, the surgeons (Professor Stephen Eisenstein, Mr David Jaffray and Mr Jayesh Trivedi) for collecting the clinical material and Dr Naomi Rowlands and Mrs Janis Menage for their help throughout the study. The study was conducted in accordance with the necessary ethical permission and informed consent, as approved by the local research ethics committee. This study was supported by the Charles Wolfson Charitable Trust.
References
- 1.Adams M, Bogduk N, Burton K, Dolan P. The biomechanics of back pain. Edinburgh: Elsevier Churchill Livingstone; 2006. [Google Scholar]
- 2.Arrigo A-P, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P. Hsp27 (HspB1) and αB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 4.Barral JM, Braodley SA, Schaffer G, Hartl FU. Roles of molecular chaperones in protein misfolding diseases. Semin Cell Dev Biol. 2004;15:17–29. doi: 10.1016/j.semcdb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Bartels EM, Fairbank JCT, Winlove CP, Urban JPG. Oxygen and lactate concentrations in vivo in the intervertebral disc of patients with scoliosis and back pain. Spine. 1998;23:1–7. doi: 10.1097/00007632-199801010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Porath I, Weinberg RA. When cells get stressed: an integrated view of cellular senescence. J Clin Invest. 2004;113:8–13. doi: 10.1172/JCI200420663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Crean JKG, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22:2877–2884. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 10.Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 11.Gruber HE, Hanley EN. Analysis of aging and degeneration of the human intervertebral disc. Spine. 1998;23:751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Gruber HE, Ingram JA, Norton HJ, Hanley EN. Senescence in cells of the aging and degenerating intervertebral disc: immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine. 2007;32:321–327. doi: 10.1097/01.brs.0000253960.57051.de. [DOI] [PubMed] [Google Scholar]
- 13.Henderson B, Pockely AG, editors. Molecular chaperones and cell signalling. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- 14.Johnson WEB, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res. 2001;42:197–207. doi: 10.3109/03008200109005650. [DOI] [PubMed] [Google Scholar]
- 15.Kauppila LI. Ingrowth of blood vessels in disc degeneration: angiographic and histological studies of cadaveric spines. J Bone Joint Surg Am. 1995;77:26–31. doi: 10.2106/00004623-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12:743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Maitre CL, Freemont AJ, Hoyland JA. Human disc degeneration is associated with increased MMP 7 expression. Biotech Histochem. 2006;81:125–131. doi: 10.1080/10520290601005298. [DOI] [PubMed] [Google Scholar]
- 18.Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9:R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melrose J, Fuller ES, Roughley PJ, Smith MM, Kerr B, Hughes CE, Caterson B, Little CB. Fragmentation of decorin, biglycan, lumican and keratocan is elevated in degenerate human meniscus, knee and hip articular cartilages compared with age-matched macroscopically normal and control tissues. Arthritis Res Ther. 2008;10:R79. doi: 10.1186/ar2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore RJ, Vernon-Roberts B, Fraser RD, Osti OL, Schembri M. The origin and fate of herniated lumbar intervertebral disc tissue. Spine. 1996;21:2149–2155. doi: 10.1097/00007632-199609150-00018. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto R. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 22.Morishima N. Control of cell fate by Hsp70: more than an evanescent meeting. J Biochem. 2005;137:449–453. doi: 10.1093/jb/mvi057. [DOI] [PubMed] [Google Scholar]
- 23.Nagata K. Hsp47: a collagen-specific molecular chaperone. Trends Biochem Sci. 1996;21:23–26. doi: 10.1016/0968-0004(96)80881-4. [DOI] [PubMed] [Google Scholar]
- 24.Nollen EAA, Morimoto RI. Chaperoning signalling pathways: molecular chaperones as stress-sensing “heat shock” proteins. J Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- 25.Otsuka G, Kubo T, Imanishi J, Hirasawa Y. Expression of heat-shock proteins in the differentiation process of chondrocytes. Nippon Geka Hokan. 1996;65:39–48. [PubMed] [Google Scholar]
- 26.Pritzker KPH. Ageing and degeneration in the lumbar intervertebral disc. Orthop Clin North Am. 1977;8:65–77. [PubMed] [Google Scholar]
- 27.Rannou F, Lee T-S, Zhou R-H, Chin J, Lotz JC, Mayoux-Benhamou M-A, Barbet JP, Chevrot A, Shyy JY-J. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164:915–924. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts S. Disc morphology in health and disease. Biochem Soc Trans. 2002;30:864–869. doi: 10.1042/BST0300864. [DOI] [PubMed] [Google Scholar]
- 29.Roberts S, Menage J, Duance VC, Wotton S, Ayad S. Collagen types around the cells of the intervertebral disc and cartilage end-plate: an immunolocalisation study. Spine. 1991;16:1030–1038. doi: 10.1097/00007632-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 31.Roberts S, Evans EH, Kletsas D, Jaffray DC, Eisenstein SM. Senescence in human intervertebral discs. Eur Spine J. 2006;15(Suppl. 3):S312–S316. doi: 10.1007/s00586-006-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl. 2):10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 33.Sharman MY, Gabai V, O’Callaghan C, Yaglom J. Molecular chaperones regulate p53 and suppress senescence programs. FEBS Lett. 2007;581:3711–3715. doi: 10.1016/j.febslet.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takao T, Iwaki T. A comparative study of localization of heat shock protein 27 and heat shock protein 72 in the developmental and degenerative intervertebral discs. Spine. 2002;27:361–367. doi: 10.1097/00007632-200202150-00007. [DOI] [PubMed] [Google Scholar]
- 35.Tiffee JC, Griffin JP, Cooper LF. Immunolocalisation of stress proteins and extracellular matrix proteins in the rat tibia. Tissue Cell. 2000;32:141–147. doi: 10.1054/tice.2000.0097. [DOI] [PubMed] [Google Scholar]
- 36.Urban J, Roberts S. Cells of the intervertebral disc: making the best of a bad environment. Biochemist. 2003;25(5):15–17. [Google Scholar]
- 37.Vanmuylder N, Evrard L, Dourov N. Strong expression of heat shock proteins in growth plate cartilage, an immunohistochemical study of HSP28, HSP70 and HSP110. Anat Embryol (Berl) 1997;195:359–362. doi: 10.1007/s004290050056. [DOI] [PubMed] [Google Scholar]
- 38.Vayssier M, Polla BS. Heat shock proteins chaperoning life and death. Cell Stress Chaperones. 1998;3:221–227. doi: 10.1379/1466-1268(1998)003<0221:HSPCLA>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng J, Luo W, Tanzers ML. Aggrecan synthesis and secretion: a paradigm for molecular and cellular coordination of multiglobular protein folding and intracellular trafficking. J Biol Chem. 1998;273:12999–13006. doi: 10.1074/jbc.273.21.12999. [DOI] [PubMed] [Google Scholar]