Abstract
Literature indicates that loss of disc tissue from herniation and/or surgery can accelerate degeneration of the disc. The associated loss of disc height may correspond with recurrent back and/or leg pain. A novel hydrogel has been developed to replace lost nucleus pulposus and potentially restore normal disc biomechanics following herniation and surgery. A single-center, non-randomized, prospective feasibility study was undertaken to investigate the use of NuCore® Injectable Nucleus hydrogel (Spine Wave, Inc., Shelton, CT, USA) as a replacement for nuclear tissue lost to herniation and microdiscectomy. Fourteen patients were enrolled at the authors’ hospital as the initial site in a worldwide multicenter pilot study. Subjects who were entered into the study suffered from radicular pain due to single-level herniated nucleus pulposus and were non-respondent to conservative therapy. Following a standard microdiscectomy procedure, the hydrogel material was injected into the nuclear void to replace what tissue had been lost to the herniation and surgery. Leg and back pain, function and disability scores were monitored pre- and post-operatively through 2 years. Neurologic and physical evaluations, blood and serum analyses, and radiographic evaluations of disc height and implant stability were also performed. Results showed significant improvement for leg and back pain, as well as function scores. No complications or device related adverse events were observed. MR controls confirmed stable position of the implants with no reherniations. Radiographic measurements indicated better maintenance of disc height compared to literature data on microdiscectomy alone. The NuCore® material appears to protect the disc from early collapse following microdiscectomy; and therefore, may have the potential to slow the degenerative cascade of the spinal segment over time.
Keywords: Nucleus replacement, Herniation, Hydrogel, Microdiscectomy, NuCore®
Introduction
Lumbar disc herniation is the most common cause of radicular leg pain in the adult working population. Although the majority of cases will improve with conservative care, surgical intervention is indicated in a significant subgroup of patients. This includes cases with severe neurological compromise, i.e., cauda equina syndrome and progressive motor deficit, as well as the larger group of cases which have failed a minimum of 6 weeks of conservative therapy [1]. If surgical intervention is needed, microdiscectomy and/or sequestrectomy under microscopical vision are recommended treatment options [2, 3].
Unsuccessful surgical results may be associated with two problems: reherniation and disc space narrowing. Reherniation of the operated disc with recurrence of radiculopathy may occur in as many as 27% of cases, particularly when a large posterior annular defect is present [4]. More aggressive removal of disc tissue might reduce the reherniation rate, but also lead to further destabilization of the segment resulting in progressive low back pain. Disc height loss and its associated increase in radial disc bulge occur in proportion to the amount of tissue lost from the disc structure [5]. Patients with lumbar disc herniations show reduced disc height pre-operatively compared to normal, with progressive narrowing over time [6, 7]. Young patients under 35 years of age and cases with advanced disc degeneration before surgery are particularly likely to suffer from accelerated disc deterioration and narrowing over time, resulting not only in discogenic low back pain, but also in spondylotic changes of the facet joints [8]. The reoperation rate after 10 years has been reported to be 25% with a median time to reoperation of 24 months [9]. Therefore, initial surgical treatment of the disc should attempt to preserve as much height and function as possible without increasing the risk for reherniation.
Several attempts have been made to design an efficient implant to replace herniated or surgically removed nucleus tissue in order to restore normal loads and biomechanics at the diseased level. Pre-formed intervertebral spacers have been tested clinically with mixed results, the main problem being migration of the implant and inflammatory reaction of the adjacent endplates [10, 11]. Recently, additional attention has been focused on injectable materials. The flowable nature of these materials allows a void of any size and shape to be filled. Thus, the nucleotomy can be as minimal or aggressive as necessary, and is not dictated by the geometry of the implant.
The present study investigates the safety and performance of the use of a novel injectable protein hydrogel as a nuclear replacement in microdiscectomy cases.
Materials and methods
Material properties
NuCore® Injectable Nucleus is an in situ curing protein polymer hydrogel which mimics the properties of the natural nucleus (Table 1). The polymer chain is made up of silk and elastin components designed for both elasticity and toughness. The polymer (P27K) is mixed with a crosslinking agent at the time of implantation, and is injected as a fluid through the annular defect where it adheres to the surrounding intradiscal tissue as it cures. There is no measurable temperature increase produced during the cure process. The material thus fills the nuclear void and seals the annulotomy. Preclinical testing has shown the material to be extremely resistant to extrusion under biomechanical loading conditions, remaining intact when loaded well above levels experienced in activities of daily living [12]. It has also been shown to have a restorative effect on biomechanical function in benchtop testing [13]. A full battery of pre-clinical biologic safety tests performed in accordance with ISO10993 standards indicated the material is biocompatible.
Table 1.
| Property | NuCore injectable nucleus | Natural nucleus pulposus |
|---|---|---|
| Protein content | 19.2% | 13.6–21.9% |
| Water content | 79.1% | 74–81% |
| pH | 7.1 | 6.7–7.1 |
| Complex modulus (G*) | 26 kPa | 7–21 kPa |
Study protocol
Inclusion/exclusion criteria
Patients enrolled in this study were between the ages of 18 and 60 and suffered from a single-level symptomatic herniated nucleus pulposus between L1 and S1 with predominant leg pain that had been non-respondent to at least 6 weeks of conservative therapy. In addition, a minimum central disc height of 5 mm was required. Patients were specifically excluded if any of the following conditions were present:
Disc protrusion only with intact annulus.
Prior invasive surgery at treatment level or previous fusion at adjacent level(s).
Clinically significant facet joint degeneration at affected level based on radiologic review and physical examination.
Lytic or degenerative spondylolisthesis (>Grade 1).
Obesity (BMI > 30).
Ethics committee approval was obtained from the responsible authorities, and each patient was required to sign an informed consent prior to being enrolled in the study.
Follow-up/data collection
Data were collected pre-operatively, post-operatively (within 48 h after surgery), at 6 and 12 weeks, as well as 6, 12, and 24 months.
The clinical data at each time point included a neurological exam as well as an assessment of leg and back pain, disability, and function using the Visual Analog Scale (VAS), Oswestry Disability Index (ODI), and The SF-36® Health Survey (SF-36) patient questionnaire.
At each time-point, standing lateral X-rays with a marker attached were taken. Disc height analysis was performed by an independent analysis group (Medical Metrics Inc.) using a validated measurement method utilizing landmark recognition software to consistently and accurately locate anatomic landmarks. Magnetic resonance (MR) images obtained pre-operatively, post-operatively and at 6, 12, and 24 months were qualitatively reviewed with regard to the signal in the disc, potential material extrusions, and any endplate changes.
Standard blood samples were analyzed and a serum titer was assayed to determine any presence of antibodies to the P27K protein.
Patients
Per the above criteria, 14 patients were enrolled in the study over a period of 17 months (8 male, 6 female). There were no dropouts over the follow-up period of 2 years. The average age of the patients was 37 years with a range from 25 to 52 years. Of the 14 levels treated, 10 were at L5/S1 and 4 were at L4/5.
Surgical technique
All patients were operated under general anesthesia in prone position with their hips and knees flexed at least 90° (Andrews or knee–chest position). After localizing the appropriate level under fluoroscopy, a 2.5–3.0 cm median skin incision was performed, fascia incised, and soft tissues and muscles retracted to expose the interlaminar window. Under microscopic control, the spinal canal was opened by partial resection of the ligamentum flavum and a minimal undercutting of the cranial lamina. The dural sleeve and nerve root were mobilized and retracted medially to expose the intervertebral space. The herniated tissue was removed and its volume measured. The disc was further cleared of loose tissue by several washouts and gentle use of rongeurs through the existing annular defect. The extent of these partial nucleotomies was defined by the firmness of the remaining tissue. The total volume of the removed tissue was then measured. Finally the remaining discal void was dried with the help of suction and filled with NuCore® material. For the first seven cases a standard ball-tipped cannula was used to fill the defect from its bottom to the level of the inner annulus under direct microscopic vision. The next seven cases of the series were injected with a sealed vented needle, allowing for some pressure whilst injecting. The goal of all injections was to replace the tissue lost to herniation and discectomy. Therefore, the previously measured tissue volume was used as the target for the injection of Nucore® material. The pressure was held for 5 min to allow the material to cure. After removal of the needle, the spinal canal was explored with a nerve hook to locate any material which may have leaked past the seal during injection. The wound was closed in layers and no drain was used.
Patients were allowed to mobilize according to back school principles as soon as they recovered from anesthesia. No brace was used, but patients were reminded to restrict their activities for 6 weeks.
Results
Clinical
All surgeries were successfully completed, no complications occurred and recovery was uneventful in all cases. All patients were discharged from the hospital within expected timeframes following surgery. Eleven patients presented pre-operatively with mild numbness, primarily in the calf and foot. Five of these returned to normal, while the rest showed some improvement during the course of follow-up. Six patients presented with mild Grade 4 functional weakness which recovered to normal. One additional patient presented with severe Grade 1 weakness which improved to mild Grade 4 by 3 months post-operatively. None of the patients required revision surgery within the follow-up period.
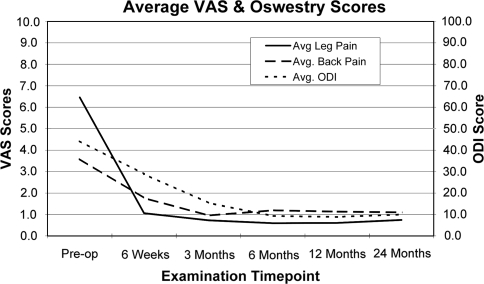
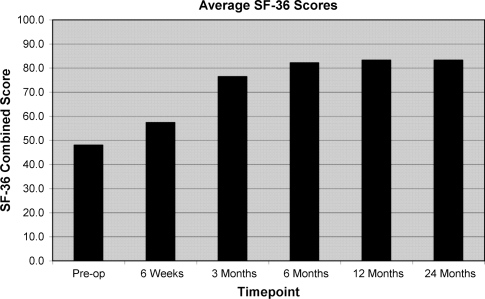
Mean leg pain reduced from 6.5 (range 1.8–9.6) at pre-operatively to 0.7 (range 0.0–3.5) at 2 years post-operatively (Fig. 1). Mean back pain reduced from 3.6 (range 0.7–7.8) at pre-operatively to 1.1 (range 0.0–4.5) at 2 years post-operatively. Mean ODI scores reduced from 43 (range 20–82) at pre-operatively to 10 (range 2–36) at 2 years post-operatively. All components of the SF-36 score improved over time (Fig. 2).
Fig. 1.
Mean VAS pain scores and mean ODI scores over course of follow-up
Fig. 2.
Mean SF-36 function scores over course of follow-up
The amount of nuclear tissue removed averaged 1.7 ml with a range of 0.4–3.3 ml. On average, 52% of the removed tissue was sequestrum with the remainder from inside the disc.
The mean implanted volume of NuCore® Injectable Nucleus was 1.2 ml with a range from 0.3 to 1.9 ml. Overall, the average volume of material injected was 75% of the total volume of tissue lost to herniation and discectomy (range 31–150%). In the last seven cases of this study, modifications were made to improve the injection technique to increase the volume of NuCore® material delivered and thus replace most of the nuclear tissue lost. These improvements in the last seven cases led to markedly increased injection volumes and an average of nearly 100% replacement of the lost nuclear tissue. The difference in injection volumes in the two groups (Group A = early group and Group B = later group) was statistically significant (50% [SD = 24%] replacement vs. 100% [SD = 33%] replacement, P = 0.007). However, differences were not significant when pain and function scores were compared between the two groups.
No device-related adverse events were reported in these first 14 patients. One patient required oral steroid treatment for 7 days at 6 weeks post-operatively for a mild recurrence of numbness in the toes of the affected leg. An MR performed at that time revealed no radiographic abnormalities and no recurrent herniation. This event had resolved at the next follow-up.
Results of all blood, serum, and urine tests showed no clinically relevant abnormalities at any time point. Five of the 14 patients showed a mild transient increase in anti-P27K antibody level relative to existing pre-operative level, all of which resolved over the course of follow-up. There was no clinical evidence of any immunologic reaction to the NuCore® material.
Radiological
Disc height maintenance
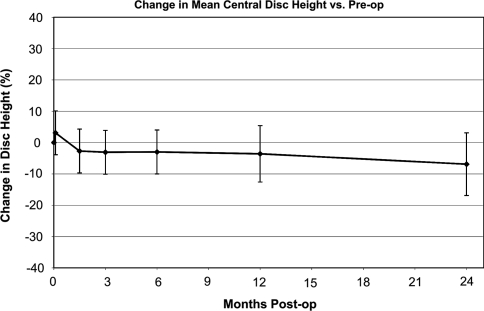
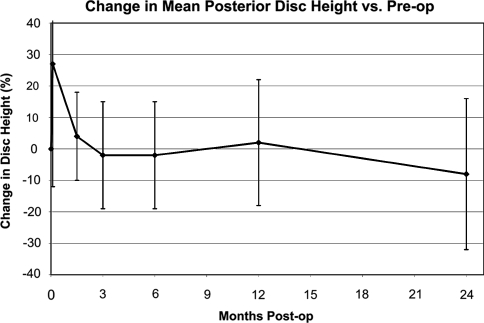
Central disc height showed a stable trend after 6 weeks, holding 93% of the original pre-operative value at 24 months post-operatively (Fig. 3). Posterior disc height is likewise maintained after 6 weeks, holding 92% of the original pre-operative height at 24 months post-operatively (Fig. 4). Both figures show an initial settling period in the acute post-operative timeframe, stabilizing thereafter.
Fig. 3.
Mean central disc height over course of follow-up
Fig. 4.
Mean posterior disc height over course of follow-up
Comparing the two injection technique groups, central disc height was maintained better in Group B with 97.0% (SD 11.0) and 88.6% (SD 8.4) in Group A. This difference was statistically not significant.
MR findings
Since the NuCore® material is approximately 80% water, it presents a hyperintense image on T2 MRI similar to healthy nucleus pulposus. All treated discs showed an increased signal on T2 images post-operatively. Immediate post-operatively images showed a brighter signal which usually decreased over time. These changes became stable by the 6 month images (Fig. 5), indicating that the initial changes were partly caused by post-operative effects, such as edema and hematoma. There was no evidence in any patient of migration of the material, reherniation, nor subsidence into the endplates.
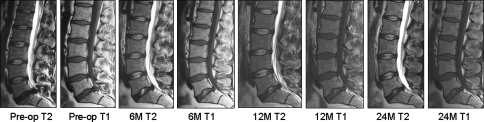
Fig. 5.
A 25-year-old male with herniation at L5/S1: 2.0 ml nucleus removed, 2.0 ml NuCore® Injectable Nucleus implanted. No Modic changes are visible on any of the films. Twenty-four months central disc height was maintained compared to pre-operative one
In five patients, no Modic endplate changes were detectable on any of the MRs (Fig. 5). Six cases presented with changes on the pre-operative MR, four of them being classified as Type 2 and one with a mixed Type 1/2. At the 24 month follow-up, five of these cases showed stable or improved Modic changes. However, in two cases a transient progression of changes was observed, mostly at the 6 month follow-up (Fig. 6). Three patients had no changes present pre-operatively, but developed some at follow-up. All these changes were classified as Type 1 and were first seen at the 6 month follow-up, then improved again at 2 years.
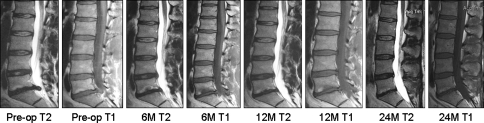
Fig. 6.
A 43-year-old male with herniation at L5/S1: 2.0 ml nucleus removed, 0.8 ml NuCore® Injectable Nucleus implanted. Pre-operative: Modic Type 2 at L5 endplate. Six months: enlargement with possible conversion to Type 2. A total of 12 and 24 months: decrease in size and intensity. Twenty-four month central disc height 4.6% less than pre-operative one
Discussion
Although microdiscectomy of herniated lumbar discs show high success rates in relieving radicular leg pain in the short term, results deteriorate over time in a considerable proportion of patients [14]. Cases with initially maintained disc height and preserved disc function may have a favorable prognosis, but their risk of reherniation is higher [8]. Discs with greater loss of material and volume either due to the hernia itself or the surgical intervention may be prone to accelerated loss in disc height resulting in spondylotic changes of the spinal segment associated with increased low back pain. Therefore, the appropriate aggressiveness in removing disc tissue during surgery is a matter of debate [4, 15]. However, ideally the lost material should be replaced to preserve the biomechanical capacity of the disc and at least slow the degenerative cascade.
The idea of augmenting the discal nucleus is not new. Intervertebral spacers in the form of a memory coiling spiral have been suggested [10], and since 1996 the PDN device consisting of a hydrogel core with a woven polyethylene cover has been implanted for nucleus augmentation [16]. However, despite advances in defining the surgical indications, adjustments to the design of the device and improvement of the surgical techniques, migration remains the primary source of complications [11]. Changes of the endplate over time are also of concern. Reasons for this might include differences in the biochemical composition and biomechanical properties between the device and the remaining disc as well as interface reactions due to the defined shape and stiffness of the implant. More recently, favorable results have been reported on a nucleus replacement using a balloon-contained, injectable polyurethane device (DASCOR® device) [17]. However, the implantation of this device requires an anterior or lateral approach to the spine and intends to treat patients suffering from degenerative disc disease (DDD).
In addition to these “pre-formed” nucleus augments, injectable biomaterials have been considered as augments to discectomy. As early as in the 1960s, Nachemson [18] suggested the injection of room temperature vulcanizing silicone into a degenerated disc using an ordinary syringe. Since then and increasingly within the last 10 years, a number of injectable biomaterials have been investigated for potential nucleus augmentation including: cross-linkable silk–elastin copolymer, polyurethane-filled balloons, collagen–PEG, chitosan, recombinant bioelastic materials, and multicomponent polymer systems [19–24]. Several commercial groups are actively pursuing the development of these injectable nucleus replacement devices [19, 25]. However, most of these implants are still under pre-clinical evaluation and clinical data is sparse.
The clinical results from this patient group suggest that the NuCore® material can be applied safely and without any surgical or clinical follow-up complications. The adverse events reported were all unrelated to the device, and there was no evidence of any local or general reaction to the material. Blood, serum, and urine tests were within expected ranges. All but three patients showed a measurable anti-P27K antibody level prior to surgery, and some showed a transient increase in antibody levels post-operatively; but over time antibody levels approximated the pre-operative level in all patients. The presence of antibody and transient changes are in line with those reported in the use of other protein-based products, e.g., collagen and serum albumin [26, 27]. In all cases, including the present study, the presence of an antibody response has had no correlation with clinical outcome or response.
All function scores indicated substantial benefit and recovery from the surgery. These results were consistent with the successful short term results reported on standard lumbar microdiscectomy alone [8, 28]. As in other studies of lumbar microdiscectomy, our patients responded to surgery with a large improvement in leg pain and to a lesser, but notable extent in back pain [29]. In our initial fourteen patients treated with the NuCore® Injectable Nucleus, no migration of the material within the disc or extrusion into the spinal canal was observed. This was consistent with the pre-clinical test data showing stability of the implant and its resistance to extrusion [12].
The relevance of Modic endplate changes is difficult to interpret. Recent papers have shown that Modic changes show a high prevalence in patients with degenerative disc disease and are seen in 23% of non-operatively treated sciatica patients [30], with a high rate of increased changes following microdiscectomy [6]. Some authors associate Modic Type 1 changes with low back pain, although conversions to “more benign” Type 2 changes seem possible [31, 32]. However, in our small group of fourteen patients, we could not correlate the Modic changes to low back pain. Intuitively, better distribution of intradiscal stresses across the endplates due to the presence of the nucleus replacement material might lead to the improvements seen in the endplate changes in this series of patients, but much more data and a control series would be necessary to conclude this.
Our data indicates that disc height was maintained better with NuCore® augmentation compared to the literature data on microdiscectomy alone. After 2 years, our treated discs showed an average central height loss of 7%, whereas literature indicates that height losses of 20% or more even after 6 months are not uncommon following microdiscectomy alone [7, 33, 34]. Our seven cases where the injection and discal fill was performed under pressure with a vented needle showed even better disc height maintenance compared to the initial seven cases with a simple unsealed fill of the defect without pressure. However, the difference was not yet statistically significant given the small patient population.
Conclusion
The results of this pilot study indicate that NuCore® Injectable Nucleus can be applied safely into lumbar discs following microdiscectomy. No complications or adverse events related to the material occurred. The material did not migrate from the disc and no extrusion was observed. Additionally, no reherniations were seen. Radiographic data suggest a better maintenance of disc height after 2 years compared to microdiscectomy alone. NuCore® hydrogel seems to protect the disc from early collapse following microdiscectomy and therefore could carry the potential of slowing the degenerative cascade of the spinal segment over time. If so, this treatment may also lead to reduced treatment costs in the long term for these relatively young patients. In light of the results of this study, the authors believe this material merits further investigation of its safety and efficacy in expanded control studies.
Acknowledgments
Spine Wave Inc. provided implants and support for this study. Approval for this study was obtained through the Bern Ethics Committee and SwissMedic.
References
- 1.McCulloch JA. Focus issue on lumbar disc herniation: macro- and microdiscectomy. Spine. 1996;21:45S–56S. doi: 10.1097/00007632-199601010-00010. [DOI] [PubMed] [Google Scholar]
- 2.Koebbe CJ, Maroon JC, Abla A, El-Kadi H, Bost J. Lumbar microdiscectomy: a historical perspective and current technical considerations. Neurosurg Focus. 2002;13:1–6. doi: 10.3171/foc.2002.13.2.4. [DOI] [PubMed] [Google Scholar]
- 3.Wenger M, Mariani L, Kalbarczyk A, Gröger U. Long-term outcome of 104 patients after lumbar sequestrectomy according to Williams. Neurosurgery. 2001;49:329–334. doi: 10.1097/00006123-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Carragee EJ, Spinnickie AO, Alamin TF, Paragioudakis S. A prospective controlled study of limited versus subtotal posterior discectomy: short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior annular defect. Spine. 2006;31:653–657. doi: 10.1097/01.brs.0000203714.76250.68. [DOI] [PubMed] [Google Scholar]
- 5.Brinckmann P, Grootenboer H. Change of disc height, radial disc bulge, and intradiscal pressure from discectomy: an in vitro investigation on human lumbar discs. Spine. 1991;16:641–646. doi: 10.1097/00007632-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Thome C, Barth MB, Diepers M, Schmiedek P. A prospective randomized comparison of clinical and radiological outcome after lumbar sequestrectomy versus microdiscectomy. Eur Spine J. 2006;15:S477–S478. [Google Scholar]
- 7.Tibrewal SB, Pearcy MJ. Lumbar intervertebral disc heights in normal subjects and patients with disc herniation. Spine. 1985;10:452–454. doi: 10.1097/00007632-198506000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Yorimitsu E, Chiba K, Toyama Y, Hirabayashi K. Long-term outcomes of standard discectomy for lumbar disc herniation. Spine. 2001;26:652–657. doi: 10.1097/00007632-200103150-00019. [DOI] [PubMed] [Google Scholar]
- 9.Atlas SJ, Keller RB, Wu YA, Deyo R, Singer D. Long-term outcome of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Main lumbar spine study. Spine. 2005;30:927–935. doi: 10.1097/01.brs.0000158954.68522.2a. [DOI] [PubMed] [Google Scholar]
- 10.Korge A, Nydegger T, Polard JL, Mayer H, Husson J. A spiral implant as nucleus prosthesis in the lumbar spine. Eur Spine J. 2002;11:S149–S153. doi: 10.1007/s00586-002-0444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertagnoli R, Schönmayr R. Surgical and clinical results with the PDN® prosthetic disc-nucleus device. Eur Spine J. 2002;11:S143–S148. doi: 10.1007/s00586-002-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walkenhorst J, Lee D, Spenciner D (2004) Extrusion resistance of an injectable nucleus replacement in the human cadaver spine. Poster IMAST
- 13.Kitchel S, Capello J (2003) Injectable biomaterials for augmentation of the nucleus pulposus. Poster IMAST
- 14.Dewing CB, Provencher MT, Riffenburgh RH, Kerr S, Manos RE. The outcomes of lumbar microdiscectomy in a young, active population: correlation by herniation type and level. Spine. 2008;33:33–38. doi: 10.1097/BRS.0b013e31815e3a42. [DOI] [PubMed] [Google Scholar]
- 15.Fountas KN, Kapsalaki EZ, Feltes CH, Smisson HF, Johnston KW, Vogel RL, et al. Correlation of the amount of disc removed in a lumbar microdiscectomy with long-term outcome. Spine. 2004;29:2521–2524. doi: 10.1097/01.brs.0000145413.79277.d0. [DOI] [PubMed] [Google Scholar]
- 16.Ray CD. The PDN® prosthetic disc-nucleus device. Eur Spine J. 2002;11:S137–S142. doi: 10.1007/s005860100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahrens M, Donkersloot P, Martens F, Sherman J, Le Huec JC, Tsantrizos A, et al. Nucleus replacement using an in situ cured, balloon contained, injectable polyurethane device: clinical results from two prospective multicenter European studies. Spine J. 2007;7:S103. doi: 10.1016/j.spinee.2007.07.235. [DOI] [PubMed] [Google Scholar]
- 18.Nachemson A. Some mechanical properties of the lumbar intervertebral disc. Bull Hosp Joint Dis. 1962;23:130–132. [PubMed] [Google Scholar]
- 19.Bao QB, Yuan HA. New technologies in Spine: nucleus replacement. Spine. 2002;27:1245–1247. doi: 10.1097/00007632-200206010-00020. [DOI] [PubMed] [Google Scholar]
- 20.Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoemann CD, et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155–2161. doi: 10.1016/S0142-9612(00)00116-2. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari FA, Richardson C, Chambers J, Causey S, Pollock T, Cappello J et al (2002) Peptides comprising repetitive units of amino acids and DNA sequences encoding the same. US Patent 6,355,776
- 22.Hubbell JA, Wetering Pvd, Cowling DSP (2002) Novel polymer compounds. US Patent 2002/0177680
- 23.Olsen DR, Chang R, McMullin H, Hitzeman R, Chisholm G (2002) Recombinant gelatin and full-length triple helical collagen. US Patent 6,413,742
- 24.Urry DW (1991) Polynanopeptide bioelastomers having an increased elastic modulus. US Patent 5,064,430
- 25.Di Martino A, Vaccaro AR, Lee JY, Denaro V, Lim M. Nucleus pulposus replacement: basic science and indications for clinical use. Spine. 2005;30:S16–S22. doi: 10.1097/01.brs.0000174530.88585.32. [DOI] [PubMed] [Google Scholar]
- 26.DeLustro F, Dasch J, Keefe J, Ellingsworth L. Immune response to allogeneic and xenogeneic implants of collagen and collagen derivatives. Clin Orthop. 1990;260:263–279. [PubMed] [Google Scholar]
- 27.Mogues T, Junzhi L, Coburn J, Kuter D. IgG antibodies against bovine serum albumin in humans–their prevalence and response to exposure to bovine serum albumin. J Immunol Methods. 2005;300:1–11. doi: 10.1016/j.jim.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Findlay GF, Hall BI, Musa BS, Oliveira MD, Fear SC. A 10-year follow-up of the outcome of lumbar microdiscectomy. Spine. 1998;23:1168–1171. doi: 10.1097/00007632-199805150-00019. [DOI] [PubMed] [Google Scholar]
- 29.Pearson AM, Blood EA, Frymoyer JW, Herkowitz H, Abdu W, Woodward R, et al. SPORT lumbar intervertebral disk herniation and back pain—does treatment, location, or morphology matter? Spine. 2008;33:428–435. doi: 10.1097/BRS.0b013e31816469de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuisma M, Karppinen J, Niinimäki J, Kurunlahti M, Haapea M, Vanharanta H, et al. A 3-year follow-up of lumbar spine endplate (Modic) changes. Spine. 2006;31:1714–1718. doi: 10.1097/01.brs.0000224167.18483.14. [DOI] [PubMed] [Google Scholar]
- 31.Mitra D, Cassar-Pullicino VN, McCall IW. Longitudinal study of vertebral type-1 endplate changes on MR of the lumbar spine. Eur Radiol. 2004;14:1574–1581. doi: 10.1007/s00330-004-2314-4. [DOI] [PubMed] [Google Scholar]
- 32.Toyone T, Takahashi K, Kitahara H, Yamagata M, Murakami M, Moriya H. Vertebral bone-marrow changes in degenerative lumbar disc disease: an MRI study of 74 patients with low back pain. J Bone Joint Surg Br. 1994;76B:757–764. [PubMed] [Google Scholar]
- 33.Mochida J, Toh E, Nomura T, Nishimura K. The risks and benefits of percutaneous nucleotomy for lumbar disc herniation: a 10-year longitudinal study. J Bone Joint Surg Br. 2001;83B:501–505. doi: 10.1302/0301-620X.83B4.11508. [DOI] [PubMed] [Google Scholar]
- 34.Gorensek M, Vilendecic M, Eustacchio S, Trummer M, Eskinja N, Ledic D et al (2007) Early clinical results with the intrinsic therapeutics Barricaid® annular repair device—comparison to a prospective control group. Proceedings of the 7th annual global symposium on motion preservation technology, Berlin
- 35.Kitano T, Zerwekh JE, Usui Y, Edwards M, Flicker P, Mooney V. Biochemical changes associated with the symptomatic human intervertebral disk. Clin Orthop. 1993;293:372–377. [PubMed] [Google Scholar]
- 36.Iatridis JC, Weidenbaum M, Setton LA, Mow VC. Is the nucleus pulposus a solid or a fluid? Mechanical behaviors of the nucleus pulposus of the human intervertebral disc. Spine. 1996;21:1174–1184. doi: 10.1097/00007632-199605150-00009. [DOI] [PubMed] [Google Scholar]