Abstract
The “off label” use of rhBMP-2 in the transforaminal lumbar interbody fusion (TLIF) procedure has become increasingly popular. Although several studies have demonstrated the successful use of rhBMP-2 for this indication, uncertainties remain regarding its safety and efficacy. The purpose of this study is to evaluate the clinical and radiographic outcomes of the single-level TLIF procedure using rhBMP-2. Patients who underwent a single-level TLIF between January 2004 and May 2006 with rhBMP-2 were identified. A retrospective evaluation of these patients included operative report(s), pre- and postoperative medical records, and dynamic and static lumbar radiographs. Patient-reported clinical outcome measures were obtained from a telephone questionnaire and included a modification of the Odom’s criteria, a patient satisfaction score, and back and leg pain numeric rating scale scores. Forty-eight patients met the study criteria and were available for follow-up (avg. radiographic and clinical follow-up of 19.4 and 27.4 months, respectively). Radiographic fusion was achieved in 95.8% of patients. Good to excellent results were achieved in 71% of patients. On most recent clinical follow-up, 83% of patients reported improvement in their symptoms and 84% reported satisfaction with their surgery. Twenty-nine patients (60.4%) reported that they still had some back pain, with an average back pain numeric rating score of 2.8. Twenty patients (41.7%) reported that they still had some leg pain, with an average leg pain numeric rating score was 2.4. Thirteen patients (27.1%) had one or more complications, including transient postoperative radiculitis (8/48), vertebral osteolysis (3/48), nonunion (2/48), and symptomatic ectopic bone formation (1/48). The use of rhBMP-2 in the TLIF procedure produces a high rate of fusion, symptomatic improvement and patient satisfaction. Although its use eliminates the risk of harvesting autograft, rhBMP-2 is associated with other complications that raise concern, including a high rate of postoperative radiculitis.
Keywords: Transforaminal, Lumbar, Interbody fusion, TLIF, Bone morphogenetic protein
Introduction
The transforaminal lumbar interbody fusion (TLIF) procedure was originally described by Harms [11, 12] as a means of obtaining a lumbar interbody fusion through a posterior approach. Because preparation of the disc space and placement of the interbody cage is transforaminal, traction on the nerve root and thecal sac is minimized. Iliac crest autograft has traditionally been used in and around the interbody cage with documented clinical success [16, 17, 19]. The use of iliac crest autograft is not without consequence, however, with approximately 2–3% of patients requiring reoperation at the donor-site due to wound complications and/or infection and up to 38% of patients reporting persistent donor-site pain [7, 9, 20].
Because of the morbidity associated with iliac crest harvest, the “off label” use of rhBMP-2 (InFuse; Medtronic Sofamor Danek, Memphis, TN, USA) in the TLIF procedure has become increasingly popular. Although several studies have demonstrated the successful use of rhBMP-2 for this indication, concerns remain regarding the safety of its use, specifically in regards to the development of postoperative radiculitis, ectopic bone formation, and vertebral osteolysis. The purpose of this study is to evaluate the clinical and radiographic outcomes of the single-level open TLIF procedure using rhBMP-2 in and around the interbody cage.
Materials and methods
Following approval from the Institutional Review Board, all patients who underwent a TLIF procedure from January 2004 to May 2006 for the treatment of a degenerative condition were identified by searching a computerized surgical case log database. All surgeries were performed at a single institution by one of the senior authors. Only patients between the ages of 18 and 80 who had single-level TLIF procedure rhBMP-2 were considered eligible for this study. Patients who underwent a multilevel TLIF procedure, who received a bone graft substitute or extender other than rhBMP-2, or who had surgical treatment for conditions not of a degenerative nature (i.e. tumor, infection, or trauma) were excluded from the study. Patients who had degenerative or isthmic spondylolisthesis and/or who had prior lumbar surgery were considered eligible for the study.
A retrospective review of the patients who met the study inclusion criteria was performed, including a review of the operative report(s), pre- and postoperative clinic records, dynamic and static lumbar radiographs, and CT scans (when available). Demographic information, including age, sex, medical and surgical history, and smoking history were obtained from these records. Intraoperative and postoperative complications were documented. The occurrence and duration of postoperative radiculitis, defined by worsening leg pain following surgery in a dermatomal distribution, was recorded from clinic records.
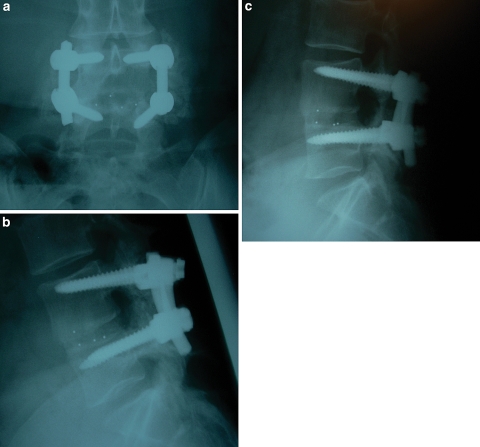
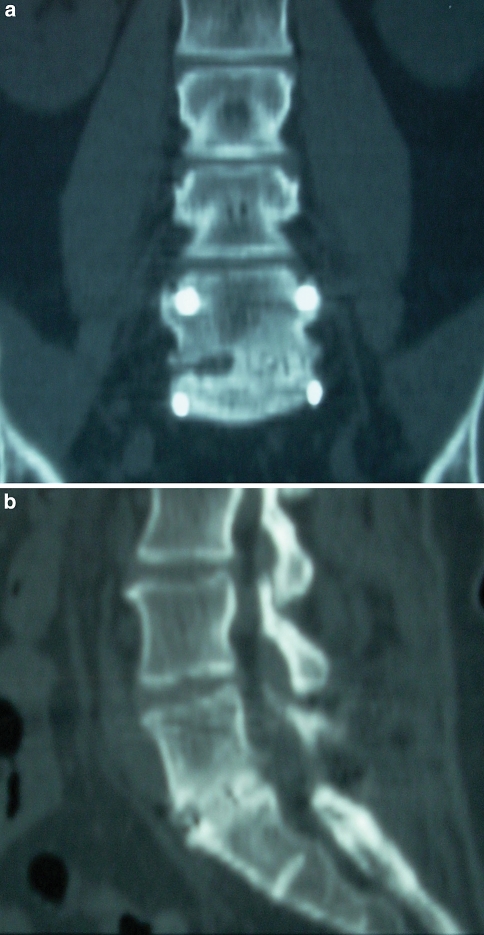
Fusion was assessed on most recent static and dynamic lumbar radiographs. A successful fusion was defined as the presence of bridging trabecular bone connecting the vertebral bodies, the absence of evidence of loosening of the pedicle screws (i.e. a “halo” around the screws), and a translation of less than or equal to 3 mm and angular motion of less than or equal to 5° on flexion/extension lateral lumbar radiographs. Similar definitions of fusion have been used previously in studies evaluating both interbody fusion and posterolateral fusion (Fig. 1a–c) [1, 2, 17, 22]. Radiographs were assessed for fusion by an independent spine surgeon who was not involved with patient care. When available, a lumbar CT scan was reviewed for further evidence of fusion. (Fig. 2a, b).
Fig. 1.
Anteroposterior (a) and lateral flexion (b) and extension (c) lumbar radiographs 16 months status post a L4–L5 TLIF procedure performed with rhBMP-2. Note the bridging interbody bone between the L4 and L5 vertebral bodies and the absence of motion when comparing the flexion and extension views. Because the interbody cage itself is radiolucent, metallic markers are used in the cage to allow visualization of its position on X-ray
Fig. 2.
Coronal (a) and sagittal (b) reconstructions of a lumbar CT scan performed 14 months status post a L5–S1 TLIF procedure performed with rhBMP-2. There is bone bridging the L5 and S1 vertebral bodies that is evident on both the coronal and sagittal images
Patient-reported clinical outcome measures were obtained in the form of a questionnaire completed during a telephone interview with an independent research assistant. The clinical outcomes obtained from this questionnaire included a modification of the Odom’s criteria (Table 1) [18], a patient satisfaction score (Table 2), and back and leg pain numeric rating scale (NRS) scores (range 0–10, with 10 being the worst pain) (Table 3). Similar clinical outcome measures have been used previously in the literature and shown to be reliable and valid patient-reported measures for lumbar fusion procedures [4–6, 8, 13, 18, 23, 25].
Table 1.
Modified Odom’s criteria
| Grade | Definition |
|---|---|
| Excellent | All preoperative symptoms relieved, patient is able to perform daily occupations and activities without impairment |
| Good | Minimal persistence of preoperative symptoms, patient is able to perform daily occupations and activities without significant interference |
| Fair | Relief of some preoperative symptoms, but daily occupations and activities remain significantly limited |
| Poor | Symptoms and signs unchanged or worse |
Table 2.
Patient satisfaction score questionnaire
| Question | Score |
|---|---|
| How satisfied are you with the results of your surgery? | |
| Very satisfied | 5 |
| Somewhat satisfied | 4 |
| Neither satisfied nor dissatisfied | 3 |
| Somewhat dissatisfied | 2 |
| Very dissatisfied | 1 |
| Knowing what you know now, how likely is it that you would still choose to have surgery for your initial problem? | |
| Very likely | 5 |
| Somewhat likely | 4 |
| Neither likely nor unlikely | 3 |
| Somewhat unlikely | 2 |
| Very unlikely | 1 |
| How likely is it that you would recommend a family member or close friend that they undergo the procedure that you had for a similar problem? | |
| Very likely | 5 |
| Somewhat likely | 4 |
| Neither likely nor unlikely | 3 |
| Somewhat unlikely | 2 |
| Very unlikely | 1 |
| Total patient satisfaction score | 3–15 |
Table 3.
Numeric pain rating (NPR) scale
| Numeric pain rating (NPR) scale | Question |
|---|---|
| Back | “If 0 is no pain and 10 is the worst possible pain, please give me a number that indicates the amount of back pain you are having now” |
| Leg | “If 0 is no pain and 10 is the worst possible pain, please give me a number that indicates the amount of leg pain you are having now” |
All data were entered into a computerized database and statistical analyses were performed using SPSS v12.0.1 (SPSS Inc., Chicago IL, USA). Descriptive statistics were calculated, including frequencies for categorical and ordinal variables and means, standard deviations, and ranges for continuous variables. Univariate analyses were performed using independent t tests for the continuous outcome measures and chi-squared tests for categorical outcome measures. Statistical significance was set at a p value of <0.05.
Surgical procedure
After adequate exposure of the posterior elements of the appropriate spinal levels, an osteotomy was made through the lamina and pars of the cephalad vertebrae of interest (e.g. In an L4–5 TLIF, the osteotomy is made trough the lamina and pars of L4). The osteotomized lamina, pars, and inferior articular process was removed. In patients with an isthmic spondylolisthesis, a pars defect is already present, and therefore an osteotomy is not required. In patients with concomitant lumbar stenosis, a complete laminectomy in addition to removal of the ipsilateral pars and inferior articular process was performed at the level of interest. The thecal sac and traversing nerve root were protected medially. An anulotomy was made in the involved disk space and the intervertebral disc was debrided and the endplates prepared using curettes, pituitary rongeurs, and endplate chondrotomes of various designs and angles. Following adequate disc space preparation, serial dilators were placed in the disc space until an appropriate size was reached. A trial cage was then placed to ensure an adequate fit.
A medium size package of rhBMP-2 (InFuse; Medtronic Sofamor Danek, Memphis, TN, USA) was used. One of the two rhBMP-2/collagen sponges was cut in half. One piece of the cut sponge was placed in the anterior most portion of the intervertebral disc space. The additional piece of the rhBMP-2/collagen sponge was placed within an interbody cage (Leopard System; Depuy Spine, Raynham, MA, USA). The interbody cage was then impacted into the anterior two-thirds of the disc space.
Posterior pedicle screw and rod fixation (Expedium or Monarch 5.5 Spine system; Depuy Spine, Raynham, MA, USA) and posterolateral fusion was then performed in standard fashion. Local autograft bone and the remaining rhBMP-2/collagen sponge was used posterolaterally between the transverse processes following adequate decortication. On the side ipsilateral to the TLIF, local bone alone without rhBMP-2 was used to minimize the risk of ectopic bone formation around the exposed nerve root.
Results
Patient data
A total of 53 consecutive patients underwent a single-level TLIF procedure using rhBMP-2 between January 2004 and May 2006 and met the study inclusion criteria. All surgeries where performed by one of the senior authors at a single institution. Of the 53 patients, 48 patients were available for follow-up, with an average radiographic follow-up of 19.4 months and an average clinical follow-up of 27.4 months. The demographics (i.e. age, sex, smoking history, radiographic and clinical follow-up) and initial lumbar pathology (i.e. diagnosis, prior surgery, level of TLIF) of the study patients can be seen in Table 4.
Table 4.
Demographics and lumbar pathology
| rhBMP-2 (n = 48) | |
|---|---|
| Age (years) | 48.3 |
| Sex (% male) | 52.1 |
| Smoker (%) | 34.5 |
| Diagnosis (%) | |
| DDD | 12.5 |
| DDD/HNP | 6.3 |
| RHNP | 29.2 |
| IS | 35 |
| DS | 15 |
| Failed lami fusion | 2.1 |
| Radiographic f/u (mo.) | 19.4 |
| Clinical f/u (mo.) | 27.4 |
| Previous surgery (%) | 43.9 |
| TLIF level (%) | |
| L2–3 | 0 |
| L3–4 | 6.3 |
| L4–5 | 52.1 |
| L5–S1 | 41.7 |
DDD degenerative disc disease, HNP herniated nucleus pulposus, RHNP recurrent herniated nucleus pulposus, IS isthmic spondylolisthesis, DS degenerative spondylolisthesis
Clinical and radiographic outcomes
The fusion rate was 95.8% on most recent radiographic follow-up. In regards to the Odom’s criteria, 71% of patients achieved good to excellent results at most recent clinical follow-up (Table 5). The group demonstrated a high degree of patient satisfaction, with 84% of patients stating they were somewhat or very satisfied with surgery, and 84% of patients stating that they would be very likely to undergo surgery again for a similar problem. The mean patient satisfaction score was 13.5. Eighty-three percent of patients stated that their symptoms improved since the surgery. When asked about back pain, 60.4% of the rhBMP-2 patients reported that they did still have some back pain. Overall, the average back pain numeric rating score was 2.8. When asked about leg pain, 41.7% of the rhBMP-2 patients reported that they still have some leg pain. Overall, the average leg pain numeric rating score was 2.4 (Table 5). This is not to be confused with the percentage of patients who developed a postoperative radiculitis, as the majority of the patients reporting some residual leg pain did not satisfy the definition of postoperative radiculitis, i.e. worsening leg pain following surgery in a dermatomal distribution.
Table 5.
Patient-reported clinical outcomes
| rhBMP-2 (n = 48) | |
|---|---|
| Odom’s criteria (% good/excellent) | 71 |
| Patient satisfaction score | 13.5 |
| % with back pain | 60.4 |
| Back pain NRS score | 2.8 |
| % with leg pain | 41.7 |
| Leg pain NRS score | 2.4 |
NRS numeric rating scale score, with a range of 1–10, 10 being the worst pain
Complications
Overall, 27.1% of patients had one or more complications related to the surgical procedure. A summary of the complications can be seen in Table 6. The overall reoperation rate was 10.4% (5/48). Reasons for reoperation included lumbar wound infection/osteomyelitis (n = 1), lumbar hematoma (n = 1), lumbar seroma/wound drainage (n = 1), malpositioned screw with radiculitis (n = 1), and ectopic bone formation within the neuroforamen with radiculitis (n = 1).
Table 6.
Complications in TLIF patients
| Complicationa | rhBMP-2 (n = 48) (%) |
|---|---|
| Lumbar infection | 1 (2.1) |
| Iliac crest infection | N/A |
| Lumbar hematoma | 1 (2.1) |
| Lumbar seroma | 1 (2.1) |
| Radiculitis | 8 (16.7) |
| Ectopic bone formation | 1 (2.1) |
| Vertebral osteolysis | 3 (6.3) |
| Dural tear | 1 (2.1) |
| Nonunion | 2 (4.2) |
| Malpositioned instrumentation | 1 (2.1) |
aSome patients had more than one complication
Three out of the 48 patients (6.3%) developed vertebral osteolysis diagnosed by CT scan. The diagnosis was made between 1.5 and 4 months following surgery. All three patients presented with increased low back pain. One patient went on to fuse without complication and with resolution of back pain at 1 year following surgery. One patient had persistent back pain and evidence of worsening osteolysis. This patient was diagnosed with vertebral osteomyelitis and nonunion and required an anterior/posterior debridement and reconstruction as well as long-term intravenous antibiotics. The third patient with vertebral osteolysis was diagnosed with a nonunion, but at the time of this study, was refusing reoperation.
Postoperative radiculitis, defined by worsening leg pain following surgery in a dermatomal distribution, developed ipsilateral to the side on which the TLIF was performed. No patients developed contralateral radiculitis. The rate of postoperative radiculitis was 16.7% (8/48). All patients who had postoperative radiculitis had postoperative CT scans available for review. Three patients had identifiable reasons for the postoperative radiculitis. One patient was noted on CT scan to have ectopic bone formation in the L5–S1 neuroforamen. This patient was taken back to the operating room for decompression of the nerve root at this level with subsequent improvement in the radiculitis. A second patient was noted to have a malpositioned pedicle screw on postoperative CT scan. This patient’s radiculitis did not improve, however, after removal of the malpositioned screw. A third patient had radiculitis which was felt to be related to the development of a postoperative infection which required reoperation and long-term intravenous antibiotics. The radiculitis persisted in this patient despite successful treatment of the infection. A reason for the radiculitis could not be identified in the other five patients. The onset of the radiculitis was within the first 2 weeks following surgery for all affected patients, except for the patient with the infection, in which case the onset of the radiculitis occurred 5 weeks following surgery. Four patients had resolution of the radiculitis at an average of 10 months following surgery (range 6–12 months). The remaining four patients had a persistent radiculitis at the time of most recent clinical follow-up (average 35 months, range 23–48 months) despite appropriate treatment (i.e. removal of nerve compression, treatment of infection, pharmaceuticals, and/or selective nerve root injections).
Discussion
The TLIF procedure is becoming a popular choice among spine surgeons for achieving a lumbar interbody and posterolateral fusion without having the morbidity associated with an anterior surgical approach. In order to avoid the morbidity associated with iliac crest autograft, the “off label” use of rhBMP-2 in the TLIF procedure is becoming increasingly popular. Studies to date have shown that rhBMP-2 is an effective and safe alternative to iliac crest autograft when used in the TLIF procedure [17, 22].
In 2004, Mummaneni et al. [17] reported their preliminary experience with TLIF using rhBMP-2. In this study one rhBMP-2 soaked absorbable collagen sponge was placed anterior to the interbody cage, and a second rhBMP-2 soaked sponge was placed within the interbody cage. Either iliac crest autograft (n = 12) or local autograft bone (n = 9) was then placed posterior to the interbody cage after its insertion. Fusion was assessed using static and dynamic lumbar radiographs. One patient in the rhBMP-2/local bone group was diagnosed with nonunion. All patients in the rhBMP-2/autograft group had successful fusion. No patients developed symptomatic ectopic bone formation. The authors concluded that rhBMP-2 is a safe and effective alternative to autograft iliac crest for use in the TLIF procedure. This study was limited, however, in that there were relatively few patients in each of the study groups and was a relatively short follow-up period of an average of 9 months.
Villavicencio et al. [22] published their experience with the use of rhBMP-2 in both minimally invasive and open single- and multi-level TLIF procedures. Seventy-four patients were included in the study with an average follow-up of 20.6 months. The rhBMP-2 was applied on an absorbable collagen sponge and was combined with autologous local bone and/or allograft bone. Fusion was assessed by plain radiographs and CT scan. These authors reported a 100% fusion rate within 10 months of surgery, with a mean time to fusion of approximately 4 months. Two patients in this series developed persistent, postoperative radiculitis. No ectopic bone formation was noted to occur throughout the duration of the study.
Prior to this study, the use of BMP, particularly in TLIF and posterior lumbar interbody fusion (PLIF) procedures, has been implicated in the development of a severe postoperative radiculitis with onset days after the surgery and without any evidence of neural compression. Although it remains unclear, the cause of this radiculitis is felt to be a BMP-related pro-inflammatory reaction and/or ectopic bone formation in the vicinity of the nerve root and neuroforamen. Two patients in the series of Villavicencio et al. [22] developed persistent postoperative radiculitis with no identifiable cause documented in the report (i.e. ectopic bone formation, malpositioned instrumentation). In our study, three out of the eight patients who developed postoperative radiculitis had reasons for developing such symptoms (i.e. infection, malpositioned pedicle screw, and ectopic bone formation in the neuroforamen). The remaining five patients, however, had no identifiable cause. Of those who did develop postoperative radiculitis, 63% had symptoms that persisted at most recent follow-up. Future research is needed to identify the nature of BMP-related radiculitis and a possible means of preventing this complication.
Ectopic bone formation in the face of BMP remains a concern in lumbar spine surgery, particularly when performing an interbody fusion from a posterolateral or transforaminal approach, where the tract of insertion of BMP is within the spinal canal and in the vicinity of the nerve roots [10, 14, 24] The risks of developing ectopic bone formation when using BMP remain unknown. It is thought to be due, in part, to hematoma from endplate bleeding that tracts through the anulotomy into the spinal canal and serves as a conduit osteoprogenitor cells. A study that evaluated the use of BMP in the PLIF procedure demonstrated a high rate of ectopic bone formation in the spinal canal [10]. No clinical symptoms, however, developed secondary to this ectopic bone formation. Previous clinical studies evaluating the use of BMP in the TLIF procedure have not reported postoperative problems related to ectopic bone formation [17, 21, 22]. In our study, symptomatic ectopic bone formation was identified in one patient. The ectopic bone formed within the neuroforamen and resulted in a significant radiculitis that did improve following a reoperation to decompress the affected nerve root. Placement of the rhBMP-2/collagen sponge anterior to and within the interbody cage and avoiding placement of the rhBMP-2/collagen sponge in posterior half of the interbody space is felt to minimize the risk of ectopic bone formation in the spinal canal and/or intervertebral foramen.
Transient vertebral osteolysis has been documented following the use of BMP in interbody fusion [3, 15]. The pathophysiology and significance of these findings, however, remain unknown. Burkus et al. [3] reported an 18% incidence of a “transient, localized area of bone remodeling within the vertebral body” following anterior lumbar interbody fusion using rhBMP-2 within an allograft bone dial. All cases of this transient vertebral osteolysis resolved by the 24 month follow-up visit and went onto successful fusion. The development of transient vertebral osteolysis had no effect on clinical outcome [3]. Lewandrowski et al. [15] reported on 68 patients who underwent a TLIF procedure using rhBMP-2 at the L5-S1 level. Five of the patients (7.4%) in this series developed vertebral osteolysis within 4 months of the procedure. All five of these patients had improvement in their back and leg pain after the surgery. They then reported increased back and leg pain with onset between 4 and 12 weeks postoperatively. Imaging studies revealed vertebral osteolysis, usually involving the L5 vertebral body. Over time, the osteolytic defects resolved spontaneously in all five patients, with eventual resolution of the symptoms. The authors hypothesized that the osteolytic defects were due to endplate violation during disc space preparation. In our series, three patients did have identifiable reasons for developing radiculitis. Therefore, although some cases of vertebral osteolysis may be due to a BMP-related, self-limiting process, it is important to rule out other such possible causes, such as infection, and to follow these patients to ensure that they go onto develop a solid fusion.
This study is limited in that it is a retrospective study and therefore is subjected to confounding factors. Additionally, not all eligible study patients were included in the study. Only 90% of patients who were determined to be eligible for this study were available for follow-up. The remaining 10% of patients either could not be contacted or refused to participate in the study. The results of this study are not necessarily reflective of this group of patients that were not included. Furthermore, because we studied the results of single-level TLIF performed with rhBMP-2, the results of this study cannot be generalized to patients who undergo multi-level TLIF procedures or who undergo a TLIF procedure in which a bone graft extender or substitute other than rhBMP-2 is used. Due to the retrospective nature of this study, there were not preoperative patient-reported clinical outcome measures available for comparison. CT scan data was limited in this study to patients who underwent CT scan postoperatively because of persistent back and/or leg pain. Future prospective studies would greatly benefit from postoperative CT scan data on all study patients, which may further our understanding of ectopic bone formation and the development of postoperative radiculitis following the use of rhBMP-2 in this “off label” use.
The results of this study suggest that adequate clinical and radiographic outcomes can be obtained by using rhBMP-2 in the TLIF procedure. Even though using rhBMP-2 eliminates the complications related to the harvesting of iliac crest autograft, complications of a different nature are observed with the use of rhBMP-2. The pathophysiology of and risk factors for developing BMP-related radiculitis, ectopic bone formation, and vertebral osteolysis remain unclear. Further studies are needed to clarify these issues and identify ways to minimize the risk of developing of such complications.
Acknowledgments
No sources of funding were used to perform this study.
References
- 1.Boden SD, Kang J, Sandhu H, et al. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27:2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. 2003;28:372–377. doi: 10.1097/00007632-200302150-00012. [DOI] [PubMed] [Google Scholar]
- 3.Burkus JK, Sandhu HS, Gornet MF. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine. 2006;31:775–781. doi: 10.1097/01.brs.0000206357.88287.5a. [DOI] [PubMed] [Google Scholar]
- 4.Copay AG, Glassman SD, Subach BR, et al. The minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry disability index, Medical Outcomes Study questionnaire Short Form 36, and Pain Scales. Spine J. 2008;8(6):968–974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Epstein NE. A preliminary study of the efficacy of beta tricalcium phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006;19:424–429. doi: 10.1097/00024720-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Epstein NE. An analysis of noninstrumented posterolateral lumbar fusions performed in predominantly geriatric patients using lamina autograft and beta tricalcium phosphate. Spine J. 2008;8(6):882–887. doi: 10.1016/j.spinee.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Fernyhough JC, Schimandle JJ, Weigel MC, et al. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine. 1992;17:1474–1480. doi: 10.1097/00007632-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Glassman SD, Dimar JR, 3rd, Burkus K, et al. The efficacy of rhBMP-2 for posterolateral lumbar fusion in smokers. Spine. 2007;32:1693–1698. doi: 10.1097/BRS.0b013e318074c366. [DOI] [PubMed] [Google Scholar]
- 9.Goulet JA, Senunas LE, DeSilva GL, et al. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;1997:76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Haid RW, Jr, Branch CL, Jr, Alexander JT, et al. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4:527–538. doi: 10.1016/j.spinee.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Harms JG, Jeszenszky D. Oper Orthop Traumatol. 1998;10:90–102. doi: 10.1007/s00064-006-0112-7. [DOI] [PubMed] [Google Scholar]
- 12.Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author’s transl) Z Orthop Ihre Grenzgeb. 1982;120:343–347. doi: 10.1055/s-2008-1051624. [DOI] [PubMed] [Google Scholar]
- 13.Hudak PL, Wright JG. The characteristics of patient satisfaction measures. Spine. 2000;25:3167–3177. doi: 10.1097/00007632-200012150-00012. [DOI] [PubMed] [Google Scholar]
- 14.Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32:2885–2890. doi: 10.1097/BRS.0b013e31815b7596. [DOI] [PubMed] [Google Scholar]
- 15.Lewandrowski KU, Nanson C, Calderon R. Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: a report of five cases. Spine J. 2007;7:609–614. doi: 10.1016/j.spinee.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Lowe TG, Tahernia AD, O’Brien MF, et al. Unilateral transforaminal posterior lumbar interbody fusion (TLIF): indications, technique, and 2-year results. J Spinal Disord Tech. 2002;15:31–38. doi: 10.1097/00024720-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Mummaneni PV, Pan J, Haid RW, et al. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:19–23. doi: 10.3171/spi.2004.1.1.0019. [DOI] [PubMed] [Google Scholar]
- 18.Odom GL, Finney W, Woodhall B. Cervical disk lesions. J Am Med Assoc. 1958;166:23–28. doi: 10.1001/jama.1958.02990010025006. [DOI] [PubMed] [Google Scholar]
- 19.Potter BK, Freedman BA, Verwiebe EG, et al. Transforaminal lumbar interbody fusion: clinical and radiographic results and complications in 100 consecutive patients. J Spinal Disord Tech. 2005;18(4):337–346. doi: 10.1097/01.bsd.0000166642.69189.45. [DOI] [PubMed] [Google Scholar]
- 20.Sawin PD, Traynelis VC, Menezes AH. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88:255–265. doi: 10.3171/jns.1998.88.2.0255. [DOI] [PubMed] [Google Scholar]
- 21.Schwender JD, Holly LT, Rouben DP, et al. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18:S1–S6. doi: 10.1097/01.bsd.0000132291.50455.d0. [DOI] [PubMed] [Google Scholar]
- 22.Villavicencio AT, Burneikiene S, Nelson EL, et al. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3:436–443. doi: 10.3171/spi.2005.3.6.0436. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Hays RD. Methods for measuring patient satisfaction with specific medical encounters. Med Care. 1988;26:393–402. doi: 10.1097/00005650-198804000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Wong DA, Kumar A, Jatana S, et al. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2) Spine J. 2008;8(6):1011–1018. doi: 10.1016/j.spinee.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita K, Ohzono K, Hiroshima K. Patient satisfaction as an outcome measure after surgical treatment for lumbar spinal stenosis: testing the validity and discriminative ability in terms of symptoms and functional status. Spine. 2006;31:2602–2608. doi: 10.1097/01.brs.0000240717.25787.7d. [DOI] [PubMed] [Google Scholar]