Abstract
Symptomatic intervertebral disc degeneration is associated with several spinal diseases, which cause losses of life quality and money. Tissue engineering provides a promising approach to recover the functionality of the degenerative intervertebral disc. Most studies are directed toward nucleus pulposus (NP) tissue engineering because disc degeneration is believed to originate in NP region, and considerable progress has been made in the past decade. Before this important technique is utilized for clinical treatment of disc degeneration, many challenges need to address including in all three principal components of tissue engineering, i.e., seed cells, signals and biomaterial scaffolds. This article briefly gives certain aspects of state of the art in this field, as well as pays a little more attention to our work published in the past 5 years, on growth and differentiation factor-5 (GDF-5), adipose-derived stem cells (ADSCs) and heparin functionalization of scaffold. We suggest that combinatorial application of ADSCs, GDF-5, heparin functionalization and injectable hydrogels will be advantageous in NP tissue engineering.
Keywords: Nucleus pulposus, Intervertebral disc degeneration, Tissue engineering, Review
Introduction
Intervertebral disc (IVD) is anatomically composed of three parts: the annulus fibrosus (AF), the nucleus pulposus (NP) and the endplate (EP). Medical conditions associated with symptomatic IVD degeneration include disc herniation, radiculopathy, myelopathy, spinal stenosis, instability and low back pain, and constitute the vast majority of the diagnoses treated by spine specialists. Musculoskeletal disorders of the spine and low back pain are the leading source of disability in people under 45 years of age and result in national economic losses of over 90 billion dollars per year [47]. Treatment of disc degeneration remains still a great challenge to both clinical physicians and basic researchers. As a biological treatment strategy, tissue engineering approach has received considerable attention during the last decade, and the achievements and challenges in this field have been well documented in several reviews [25, 27, 44, 46].
Unlike most of previous reviews, this article is not dedicated to giving remarks on the biology of disc and the pathology of disc degeneration, but briefly reviewing some data published recently on NP tissue engineering. In addition, our achievements on growth and differentiation factor-5 (GDF-5), adipose-derived stem cells (ADSCs) as well as heparin functionalization of scaffold in this field in the past 5 years, are described.
Disc degeneration and therapy
Disc degeneration usually begins from the second decade of life and becomes severely developed with aging [22]. Both poor nutrient supply [4] and inappropriate mechanical load [1] may result in loss/alteration of cell viability and disc functionality. Matrix destruction results from an imbalance between synthesis and degradation and leads to a diminished water-binding capacity in the NP region. In pathology, the degraded matrix can no longer carry loads effectively, and thus disc degeneration occurs. Concurrently, the well-organized lamellar architecture of the AF begins to deteriorate, eventually developing internal fissures spreading outward to the periphery [14, 46].
Current therapies of disc degeneration involve nonsurgical treatment modalities or surgical intervention. Nonsurgical treatment include lifestyle modifications (e.g., weight reduction, smoking cessation), rehabilitation programs (e.g., exercises, physical modalities such as heat/cold, electrical stimulation, acupuncture, and traction), and pain medications. When pain relief is not achieved with nonsurgical approaches, surgical removal of the diseased disc, e.g., spinal fusion and disc arthroplasty may be performed [44, 52]. However, both nonsurgical and surgical therapies do not deal with the inherent loss of functional native disc tissue, and therefore they fail to regenerate or cure the degenerated, painful disc tissue itself.
NP tissue engineering
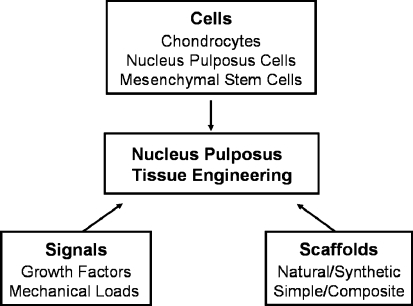
With great progresses in both materials engineering and molecular biology of IVD, tissue engineering has so far been regarded as a promising approach to recover the functionality of the degenerative IVD [44]. Since disc degeneration is believed to originate in the NP region, most studies are currently directed toward NP tissue engineering. For this therapeutic strategy, three principal components of tissue engineering, including cells, signals and scaffolds can be utilized independently or combinatorially (Scheme 1). Signals include biomechanical factor (mechanical load) and biochemical factors (cytokines, enzymes, enzyme inhibitors and growth factors, etc.), among which growth factors have been extensively investigated [44].
Scheme 1.
Principal components of nucleus pulposus tissue engineering
Growth factors
Disc cell metabolism is modulated by a variety of growth factors, which act in a paracrine and/or autocrine fashion [46]. These factors could involve in increasing the synthesis of certain extracellular matrix components such as proteoglycans, blocking their catabolic breakdown, or a combination of increased synthesis and decreased catabolism. Growth factors can be applied in NP tissue regeneration via delivery of the “naked” or “embedded” proteins as well as prolonged supplement by vector- or cell-based gene therapy [46]. Previous studies have documented the beneficial effects of growth factors, such as transforming growth factor-β (TGF-β) [37, 55], insulin-like growth factor 1 (IGF-1) [21, 45], platelet-derived growth factor (PDGF) [21], osteogenic protein-1/bone morphogenetic protein-7 (OP-1/BMP-7) [28, 36, 63], bone morphogenetic protein-2 and 12 (BMP-2 and BMP-12) [20, 24], fibroblast growth factor-2/basic fibroblast growth factor (FGF-2/bFGF) [60] and GDF-5 [9, 10, 13, 31, 62].
GDF-5, also known as cartilage-derived morphogenetic protein-1 (CDMP-1) and BMP-14, is a member of the GDF family closely related to the bone morphogenetic proteins. Among the known effects of GDF-5 its crucial role is found in stimulating the production of matrix and proliferation of disc cells. Walsh et al. [62] induced degeneration in murine caudal disc by static compression, and then investigated in vivo treatment of various exogeneous growth factors. A single injection of GDF-5 stimulated a significant increase in disc height, but IGF-1, TGF-β, or bFGF did not. Masuda and co-workers [9] found that rhGDF-5 enhanced cell proliferation and matrix synthesis in both bovine NP and AF cells, with a greater response by NP cells than by AF cells. They also reported that in a rabbit disc degeneration model, injection of rhGDF-5 into the NP area induced restoration of disc height and ameliorations determined by histological and magnetic resonance imaging (MRI) analysis. These results, along with ours described below [10, 31] indicated a potential use of GDF-5 in cell-based gene therapy.
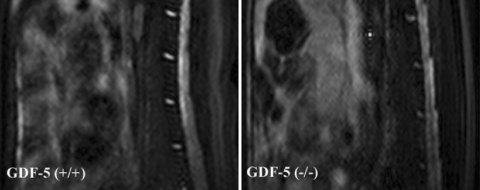
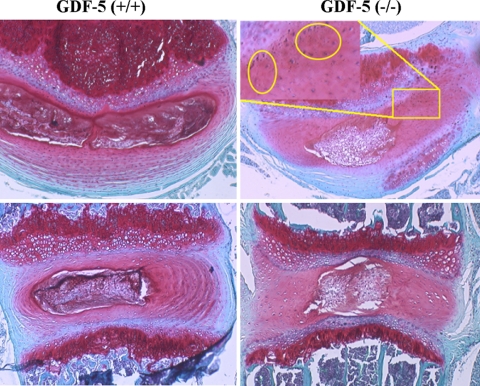
Using a GDF-5 deficient mouse model, we explored the effect of GDF-5 deficiency and identified for the first time that the deficiency led to abnormalities of disc tissues [31]. Baseline MRIs of GDF-5 deficient and wild-type mouse, lumbar spines were engaged to investigate signal differences between the two strains. A lower signal intensity was found in the GDF-5 deficient mouse discs on T2-weighted sequences, indicating a loss of water within discs, a hallmark of disc degeneration (Fig. 1). Histological analysis showed that GDF-5 (+/+) lumbar disc was composed of an outer AF with prominent concentric fibrous lamellas and an inner NP with an amorphous matrix and a heterogeneous cell population. In a normal disc, Safranin-O stained both regions in a deep red color as a result of the presence of proteoglycans. In contrast, staining of the AF from GDF-5 (−/−) mice demonstrated loss of the normal lamellar organization and replacement with chondroid tissue resembling fibrocartilage, and the discs from GDF-5 (−/−) animals apparently had a smaller, less cellular NP region with a disorganized matrix (Fig. 2).
Fig. 1.
T2-weighted magnetic resonance imaging analysis of GDF-5 (+/+) and (−/−) lumbar spines. A lower signal was shown in the intervertebral disc of the GDF-5 (−/−) mouse than in the GDF-5 (+/+) control
Fig. 2.
Photomicrographs of horizontal and vertical sections of GDF-5 (−/−) and (+/+) discs. The GDF-5 (−/−) discs have a smaller nucleus pulposus region and less lamellar architecture in the annulus than the GDF-5 (+/+) controls. The area within the square is shown in the inset at a higher magnification, with the chondroid tissues indicated within the yellow circles in the horizontal section of GDF-5 (−/−) disc
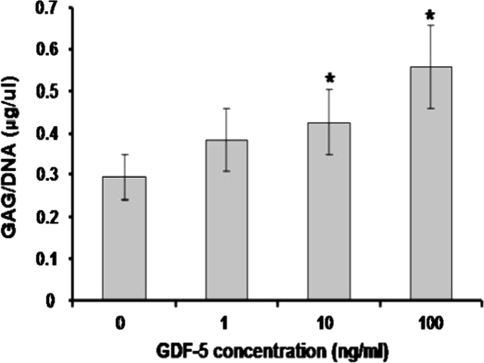
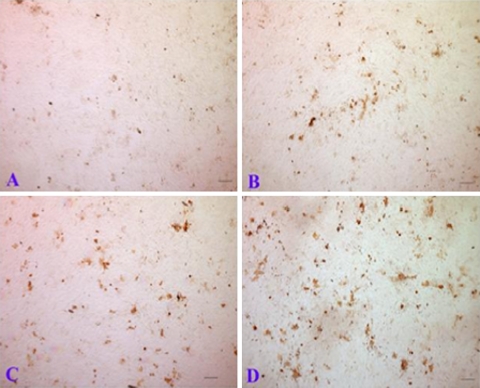
In a recent study [10], we provided evidences that GDF-5 treatment was capable of improving chondrogenic features of NP cells. Lumbar discs were obtained from six balb/c mice and a cut was made through the middle of the annulus with a #15 scalpel blade under a 40× dissecting microscope. The exposed NP were scooped out with a 30G needle and then the sample was digested with 0.01% collagenase. NP cells were cultured in 24-well plates and treated with different concentrations of GDF-5. DNA and glycosaminoglycan (GAG) assays, gene expression, and immunostaining were performed to investigate the effects of GDF-5 on NP cells. The DNA content after treatment with 10 and 100 ng/mL GDF-5 was significantly increased to 158 and 169% of the control, respectively. The amount of GAG/DNA in the presence of 10 or 100 ng/mL GDF-5 was significantly higher than control cells (P < 0.01). At 10 ng/mL GDF-5, GAG accumulation in NP cells was approximately 1.4-fold higher than the control and it increased to 190% of control in the presence of 100 ng/mL GDF-5 (Fig. 3). After 7 days in culture, numerous differently sized clusters with positive staining of collagen type II were found in NP cells with GDF-5. More and larger three-dimensional aggregates were observed in cells treated with the higher concentration of GDF-5, while the most significant effect was observed at 100 ng/mL (Fig. 4).
Fig. 3.
GDF-5 protein increased GAG production of mouse NP cells in alginate culture. NP cells were cultured in alginate beads for 7 days in the absence of GDF-5 or in the presence of three different doses of GDF-5. *P < 0.05 compared with control
Fig. 4.
GDF-5 promoted chondrogenic nodule formation of NP cells. NP cells were cultured on cover slips for 7 days in the absence of GDF-5 (a), or in the presence of on of three doses of GDF-5 (b 1 ng/mL; c 10 ng/mL; and d 100 ng/mL). Chondrogenic nodules were identified by type II collagen immunostaining. The scale bar in all images is 100 μm
Cells
NP region of the disc is anatomically encapsulated by the dense AF tissue and surrounding extracellular matrix as well as the cartilaginous EPs, in which an established matrix-blood barrier protects the nucleus from immune cellular exposure. Nomura et al. [43] used CD4 and CD5 monoclonal antibodies to detect T cells and B cells, and found no immune reaction after injecting allogeneic NP cells into rabbit intervertebral discs to retard the degenerative process of discs. Furthermore, the disc, like other specialized tissues such as the eye, constitutively expresses Fas ligand (FasL), which plays a major role in maintaining the immunoprivileged status of these organs [57]. Recently Hiyama et al. [23] concluded that mesenchymal stem cell (MSC) transplantation elicited a positive effect on preservation of immune privilege in a canine model of disc degeneration, possibly by differentiation of transplanted MSCs into cells expressing FasL. These data suggest that the NP region may tolerate MSCs from other areas of the body or even other individuals.
To repair the matrix of degenerative NP tissue, transplanted cells must produce proteoglycans (e.g., aggrecan), collagens and other matrix proteins in large quantities [53]. Chondrocytes and cells from the central regions of the disc normally produce these matrix proteins, making them candidates for cell-based disc repair. Moreover, development of stem cell research in the past decade provides an attractive prospect of adult MSCs for NP tissue engineering [23, 25, 27, 44, 49, 50, 54, 56, 61, 64].The study of MSCs was initiated by Friedenstein and colleagues [15] more than 40 years ago. So far it has been well recognized that MSCs have the ability to differentiate along various lineages of mesenchymal origin, including chondrocyte, osteoblast, and adipocyte lineages, depending upon the biological environment, and can be obtained from multiple adult tissues such as bone marrow, trabecular bone, articular cartilage, muscle, and adipose [6].
Although relatively new in stem cell research field, ADSC has attracted intensive attention as a cell source in bone and cartilage repair [59]. Particularly, application of ADSCs in NP tissue regeneration is greatly inspired by recent studies [19, 33, 34, 58]. Lu et al. [33] reported that co-culture of human ADSCs and NP cells in a micromass-cultured way resulted in differentiation of ADSCs into a NP cell-like phenotype. Tapp et al. [58] revealed that either treatment of TGF-β or co-culture of human disc cells could significantly stimulate expression of proteoglycan and type I collagen in 3D-cultured sand rat ADSCs. Gaetani et al. [19] presented data indicating that co-culture of human NP and ADSCs improved the quality of the in vitro reconstructed tissue in term of matrix production and 3D cell organization. On the other hand, cell expansion is fundamentally required in order to achieve sufficient cell amounts for transplantation, and the conventional approach of culture in monolayer has aroused several concerns including cell dedifferentiation, senescence and genetical mutagenesis [27]. Retrieval of adipose tissue involves a minimally invasive procedure that can be easily performed in outpatient clinics, and yields of adherent ADSCs can reach up to 25,000/g of tissue. Therefore, adipose tissue might be regarded as a suitbale source of stem cells for a clinical use, both in terms of ease of procedure and number of cells obtained.
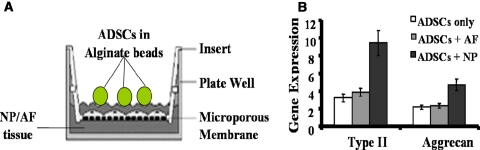
A couple of papers have been published by us with regards to the use of ADSCs as seed cells in NP tissue engineering [13, 32]. In the first paper, Li et al. [32] co-cultured rabbit ADSCs with NP tissues through a specifically designed device (Fig. 5a), and concluded that ADSCs were able to respond to soluble mediators from NP tissues. In the experiments, alginate beads containing ADSCs isolated from inguinal fad pads of NZW rabbits were co-cultured with NP or AF tissues. Real time RT-PCR analysis showed NP tissues could significantly stimulate expression of type II collagen and aggrecan genes in ADSCs, while AF tissues could not (Fig. 5b).
Fig. 5.
Real-time RT-PCR analysis of rabbit adipose-derived stem cells (ADSCs) in alginate beads co-cultured with annulus fibrosus (AF) or nucleus pulposus (NP) tissues isolated from lumbar discs. a The co-culture system. b Gene expression of type II collagen and aggrecan in alginate beads with ADSCs co-cultured with AF or NP tissues
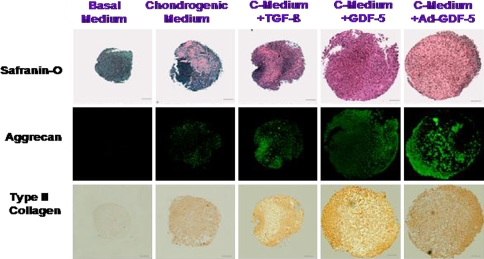
In the second paper, Feng et al. [13] investigated the chondrogenic effect of GDF-5 on rat ADSCs. 2.0 × 105 cells were gently centrifuged for 5 min at 500g in a 15-mL polypropylene tube. Without disturbing the resulting pellet, cells were treated as follows: (a) basal media control (BM), (b) chondrogenic medium (CM) in which 10 nM dexamethasone and 1% ITS-Premix was added, (c) CM containing 10 ng/mL TGF β1, (d) CM containing 100 ng/mL GDF-5, (e) Cells transduced with an adenovirus vector containing the cDNA encoding GDF5 (Ad-GDF-5) and cultured in CM. Safranin-O staining showed that TGF β1, GDF-5 and Ad-GDF-5 all increased deposition of proteoglycans compared with basal media or chondrogenic media alone. Immunostaining technique revealed in the presence of TGF β1, GDF-5 and Ad-GDF-5 was found robust staining for collagen II and aggrecan (Fig. 6). These results implied an activity of GDF-5 to augment chondrogenesis of ADSCs and a possible application of Ad-GDF-5 transduced ADSCs in NP tissue engineering.
Fig. 6.
Safranin-O staining (top panel), aggrecan immunofluorescence (middle panel), and type II collagen immunostaining (bottom panel) of rat ADSCs in a pellet culture with various treatment (see the text)
Scaffolds
Scaffolds in tissue engineering can help to retain cells in the desired location and provide appropriate mechanical properties and/or biochemical signals. To date, a variety of biomaterials have been used for fabricating scaffolds in NP tissue engineering, such as chitosan/hydroxybutyl chitosan, alginate, collagen/atelocollagen, gelatin, hyaluronan, calcium polyphosphate, poly-d, l-lactide (PDLA), demineralized bone matrix (DBM) and small intestine submucosa (SIS) [44].
Injectable scaffolds have been used to agument NP tissues after surgical treatment for many years because of easy and minimally invasive operation. Since silicone elastomer was first applied for disc replacement in 1974, a lot of injectable biomaterials have been delevoped, such as hyaluronic acid, fibrin, elastin-like polypeptides, type I collagen, alginate and chitosan [5, 8]. Among them, chitosan, derived from partial depolymerisation and deacetylation of chitin which is a main component of the shells of crustaceans, has outstanding biological properties like biocompatibility, biodegradability and antibacterial activity. These properties, along with its unique physical and chemical properties, make it a versatile biopolymer for orthopedic tissue engineering [11]. Especially, in the presence of a gelling agent, it can form a hydrogel through a shift of either pH or temperature. It has been shown that a combination of chitosan with glycerophosphate or ammonium hydrogen phosphate (AHP) produces a thermo-sensitive gel, i.e., soluble at room temperature and solidified at physiological temperature [7, 40]. This property affords chitosan-based hydrogel for intradiscal injection. Hydrogels have been proposed as ideal candidates of scaffolds for NP replacement because of their similarity in mechanical properties to native tissues [2]. In fact, a couple of studies have provided supporting evidences that using chitosan/glycerophosphate hydrogel as a scaffold can keep viability and functionality of encapsulated bovine NP cells [51] or induce differentiation of human MSCs into NP-like cells even in the absence of a differentiating medium [49].
Functionalization is an effective way to improve the performance of a scaffold. Due to its binding ability toward growth factors such as BMPs, bFGF and vascular endothelial growth factor (VEGF), heparin functionalization of scaffolds to sequester and protect these growth factors becomes a concept with great appeal [26, 41]. So far a large number of methods have been established for heparin functionalization [38], and published results have inspired the versatility of heparin-functionalized scaffolds in tissue engineering [3, 12, 18, 26, 30, 35, 42]. For bone repair, a multifunctional hydrogel, constructed by dual functionalization with heparin and fluvastatin, has been reported to promote osteogenic differentiation of human MSCs [3]. In another study, BMP-2 was immobilized onto heparin-functionalized surface of resorbable polymers, inducing a greatly improved attachment and proliferation of murine MSCs [12]. In addition, a chitosan/periodate-oxidized (IO4)-heparin hydrogel has been fabricated and proven capable of stimulating the controlled release of biologically active FGF-2 [16]. Since growth factors like BMP-2 and FGF-2 are benefical to NP regeneration, it is highlighted that heparin-functionalized scaffolds will find a potential use in NP tissue engineering.
The common strategy for heparin functionalization is to covalently link heparin to the scaffold. However, we recently developed a new strategy [30], by which heparin-carrying microcapsules were grafted onto the surface of a scaffold by a specifically designed route. The heparin-carrying microcapsules were assembled with poly allylamine hydrochloride (PAH) and heparin, alternately coated on the Ca2+-cross-linked alginate microspheres via the layer-by-layer (LbL) technique. Then these microcapsules were grafted to poly (lactic acid) (PLA) films, by –CONH– linkage between carbohydroxyl groups on surface PLA molecules and primary amino groups on the outer PAH layer of microcapsules, in the presence of catalysts of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The advantage of our strategy is to keep immobilized heparin molecules intact, and thus induce minimal loss of their bioactivity. We suggest that this strategy could also be applied to graft heparin-carrying microcapsules onto chitosan hydrogels, which will be potentially used in NP tissue engineering.
Characterization of NP cells
A successfully tissue-engineered NP graft should have similar functionality to the native tissue. With the lack of current sufficient knowledge about their biology, characterization of NP cells is still under being defined. Certain cell markers such as CD24, hypoxia inducing factor-1 (HIF-1), glucose transporter-1 (GLUT-1), matrix metalloproteinase-2 (MMP-2), glypican 3 (GPC3) and keratin 19 (K19) are regarded as useful references [17, 29, 48, 50].
Because the phenotype of extracellular matrix in NP tissue is similar to that in articular cartilage, expression of several chondrogenic markers including SOX-9, aggrecan and type II collagens, as well as the ratio of proteoglycans and collagens have also been used as references for verifying NP cells [49]. It is reported that in NP tissue the ratio of proteoglycans and collagens is around 27:1, in comparison with 2:1 in articular cartilage [39].
Summary and perspectives
NP tissue engineering provides promising prospects for treatment of disc degeneration. However, its clinical application is still far away. So far most of in vivo experimental data have been obtained from adult rabbits and rodents because of the ease of handling and availability [44]. In these animals, notochordal cells that have potential to develop into NP cells are present in the discs throughout their lives, however, in human discs notochordal cells exist only during the embryonic development and disappear shortly after birth [27]. Apparently this difference should be considered seriously as a limiting factor when these experimental data are transferred to humans. Another obvious obstacle comes from the bad nutrient supply in the NP area of the degenerated disc due to its avascularity. The nutrient shortage will undoubtedly prevent the cellularity and functionality of the implanted NP graft, and bring a specific challenge of nutrient supplementation in NP tissue engineering, which is currently far from being solved [27]. Other challenges in this field involve all aspects of a general tissue engineering approach, i.e., cells, signals and scaffolds. In these regards, ADSCs, GDF-5, heparin functionalization and injectable hydrogels have advantages towards NP tissue engineering, and designed combination of these beneficial factors is expected to have synergentic effects. Specifically, NP tissue engineering based on combination of heparin-functionalized chitosan hydrogel with GDF-5 genetically modified ADSCs, deserves desirable prospects for NP regeneration.
References
- 1.Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25:1625–1636. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bader RA, Rochefort WE. Rheological characterization of photopolymerized poly(vinyl alcohol) hydrogels for potential use in nucleus pulposus replacement. J Biomed Mater Res A. 2008;86:494–501. doi: 10.1002/jbm.a.31637. [DOI] [PubMed] [Google Scholar]
- 3.Benoit DS, Collins SD, Anseth KS. Multifunctional hydrogels that promote osteogenic hMSC differentiation through stimulation and sequestering of BMP2. Adv Funct Mater. 2007;17:2085–2093. doi: 10.1002/adfm.200700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd LM, Carter AJ. Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc. Eur Spine J. 2006;15(Suppl 3):S414–S421. doi: 10.1007/s00586-006-0172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthr Res Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho J, Heuzey MC, Bégin A, Carreau PJ. Physical gelation of chitosan in the presence of beta-glycerophosphate: the effect of temperature. Biomacromolecules. 2005;6:3267–3275. doi: 10.1021/bm050313s. [DOI] [PubMed] [Google Scholar]
- 8.Chou AI, Nicoll SB (2008) Characterization of photocrosslinked alginate hydrogels for nucleus pulposus cell encapsulation. J Biomed Mater Res A [Epub ahead of print] [DOI] [PubMed]
- 9.Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, Andersson G, Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc—in vitro bovine study and in vivo rabbit disc degeneration model study. Spine. 2006;31:2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 10.Cui M, Wan Y, Anderson DG, Shen FH, Leo BM, Laurencin CT, Balian G, Li X. Mouse growth and differentiation factor-5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. Spine J. 2008;8:287–295. doi: 10.1016/j.spinee.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Edlund U, Dånmark S, Albertsson AC. A strategy for the covalent functionalization of resorbable polymers with heparin and osteoinductive growth factor. Biomacromolecules. 2008;9:901–905. doi: 10.1021/bm701267u. [DOI] [PubMed] [Google Scholar]
- 13.Feng G, Wan Y, Balian G, Laurencin CT, Li X. Adenovirus-mediated expression of growth and differentiation factor-5 promotes chondrogenesis of adipose stem cells. Growth Factors. 2008;26:132–142. doi: 10.1080/08977190802105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 15.Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV (1966) Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 16:381-390 [PubMed]
- 16.Fujita M, Ishihara M, Simizu M, Obara K, Ishizuka T, Saito Y, Yura H, Morimoto Y, Takase B, Matsui T, Kikuchi M, Maehara T. Vascularization in vivo caused by the controlled release of fibroblast growth factor-2 from an injectable chitosan/non-anticoagulant heparin hydrogel. Biomaterials. 2004;25:699–706. doi: 10.1016/S0142-9612(03)00557-X. [DOI] [PubMed] [Google Scholar]
- 17.Fujita N, Miyamoto T, Imai J, Hosogane N, Suzuki T, Yagi M, Morita K, Ninomiya K, Miyamoto K, Takaishi H, Matsumoto M, Morioka H, Yabe H, Chiba K, Watanabe S, Toyama Y, Suda T. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem Biophys Res Commun. 2005;338:1890–1896. doi: 10.1016/j.bbrc.2005.10.166. [DOI] [PubMed] [Google Scholar]
- 18.Furue MK, Na J, Jackson JP, Okamoto T, Jones M, Baker D, Hata R, Moore HD, Sato JD, Andrews PW. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc Natl Acad Sci USA. 2008;105:13409–13414. doi: 10.1073/pnas.0806136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaetani P, Torre ML, Klinger M, Faustini M, Crovato F, Bucco M, Marazzi M, Chlapanidas T, Levi D, Tancioni F, Vigo D, Rodriguez y Baena R. Adipose-derived stem cell therapy for intervertebral disc regeneration: an in vitro reconstructed tissue in alginate capsules. Tissue Eng Part A. 2008;14:1415–1423. doi: 10.1089/ten.tea.2007.0330. [DOI] [PubMed] [Google Scholar]
- 20.Gilbertson L, Ahn SH, Teng PN, Studer RK, Niyibizi C, Kang JD. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8:449–456. doi: 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Gruber HE, Norton HJ, Hanley EN., Jr Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine. 2000;25:2153–2157. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Haefeli M, Kalberer F, Saegesser D, Nerlich AG, Boos N, Paesold G. The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine. 2006;31:1522–1531. doi: 10.1097/01.brs.0000222032.52336.8e. [DOI] [PubMed] [Google Scholar]
- 23.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, Tamura F, Sakai D. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26:589–600. doi: 10.1002/jor.20584. [DOI] [PubMed] [Google Scholar]
- 24.Huang KY, Yan JJ, Hsieh CC, Chang MS, Lin RM. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: an animal experiment. Spine. 2007;32:1174–1180. doi: 10.1097/01.brs.0000263369.95182.19. [DOI] [PubMed] [Google Scholar]
- 25.Jandial R, Aryan HE, Park J, Taylor WT, Snyder EY. Stem cell-mediated regeneration of the intervertebral disc: cellular and molecular challenge. Neurosurg Focus. 2008;24:E21. doi: 10.3171/FOC/2008/24/3-4/E20. [DOI] [PubMed] [Google Scholar]
- 26.Joung YK, Bae JW, Park KD. Controlled release of heparin-binding growth factors using heparin-containing particulate systems for tissue regeneration. Expert Opin Drug Deliv. 2008;5:1173–1184. doi: 10.1517/17425240802431811. [DOI] [PubMed] [Google Scholar]
- 27.Kandel R, Roberts S, Urban JP. Tissue engineering and the intervertebral disc: the challenges. Eur Spine J. 2008;17(Suppl 4):480–491. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Chubinskaya S, Yoshida M. Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine. 2005;30:1933–1939. doi: 10.1097/01.brs.0000176319.78887.64. [DOI] [PubMed] [Google Scholar]
- 29.Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, Grad S. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16:2174–2185. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Zhu B, Shao Y, Liu X, Yang X, Yu Q. Construction of anticoagulant poly (lactic acid) films via surface covalent graft of heparin-carrying microcapsules. Colloids Surf B Biointerfaces. 2009;70:15–19. doi: 10.1016/j.colsurfb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Leo BM, Beck G, Balian G, Anderson GD. Collagen and proteoglycan abnormalities in the GDF-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine. 2004;29:2229–2234. doi: 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Lee JP, Balian G, Greg Anderson D. Modulation of chondrocytic properties of fat-derived mesenchymal cells in co-cultures with nucleus pulposus. Connect Tissue Res. 2005;46:75–82. doi: 10.1080/03008200590954104. [DOI] [PubMed] [Google Scholar]
- 33.Lu ZF, Doulabi BZ, Wuisman PI, Bank RA, Helder MN. Differentiation of adipose stem cells by nucleus pulposus cells: configuration effect. Biochem Biophys Res Commun. 2007;359:991–996. doi: 10.1016/j.bbrc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Lu ZF, Doulabi BZ, Wuisman PI, Bank RA, Helder MN. Influence of collagen type II and nucleus pulposus cells on aggregation and differentiation of adipose tissue-derived stem cells. J Cell Mol Med. 2008;12:2812–2822. doi: 10.1111/j.1582-4934.2008.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo X, Qiu D, He B, Wang LJ, Luo J. Biodegradable heparin-loaded microspheres: carrier molecular composition and microsphere structure. Macromol Biosci. 2006;6:373–381. doi: 10.1002/mabi.200500236. [DOI] [PubMed] [Google Scholar]
- 36.Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, Thonar E, Andersson G, An HS. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine. 2006;31:742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 37.Matsunaga S, Nagano S, Onishi T, Morimoto N, Suzuki S, Komiya S. Age-related changes in expression of transforming growth factor-beta and receptors in cells of intervertebral discs. J Neurosurg. 2003;98:63–67. doi: 10.3171/spi.2003.98.1.0063. [DOI] [PubMed] [Google Scholar]
- 38.Murugesan S, Xie J, Linhardt RJ. Immobilization of heparin: approaches and applications. Curr Top Med Chem. 2008;8:80–100. doi: 10.2174/156802608783378891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–63. doi: 10.22203/ecm.v008a06. [DOI] [PubMed] [Google Scholar]
- 40.Nair LS, Starnes T, Ko JW, Laurencin CT. Development of injectable thermogelling chitosan-inorganic phosphate solutions for biomedical applications. Biomacromolecules. 2007;8:3779–3785. doi: 10.1021/bm7006967. [DOI] [PubMed] [Google Scholar]
- 41.Nie H, Wang CH. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. J Control Release. 2007;120:111–121. doi: 10.1016/j.jconrel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Nie T, Akins RE, Jr, Kiick KL. Production of heparin-containing hydrogels for modulating cell responses. Acta Biomater. 2009;5:865–875. doi: 10.1016/j.actbio.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Relat Res. 2001;389:94–101. doi: 10.1097/00003086-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 44.O’Halloran DM, Pandit AS. Tissue-engineering approach to regenerating the intervertebral disc. Tissue Eng. 2007;13:1927–1954. doi: 10.1089/ten.2005.0608. [DOI] [PubMed] [Google Scholar]
- 45.Okuda S, Myoui A, Ariga K, Nakase T, Yonenobu K, Yoshikawa H. Mechanisms of age-related decline in insulin-like growth factor-I dependent proteoglycan synthesis in rat intervertebral disc cells. Spine. 2001;26:2421–2426. doi: 10.1097/00007632-200111150-00005. [DOI] [PubMed] [Google Scholar]
- 46.Paesold G, Nerlich AG, Boos N. Biological treatment strategies for disc degeneration: potentials and shortcomings. Eur Spine J. 2007;16:447–468. doi: 10.1007/s00586-006-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips FM, An H, Kang JD, Boden SD, Weinstein J. Biologic treatment for intervertebral disc degeneration: summary statement. Spine. 2003;28:S99. doi: 10.1097/00007632-200308011-00017. [DOI] [PubMed] [Google Scholar]
- 48.Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308:401–407. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- 49.Richardson SM, Hughes N, Hunt JA, Freemont AJ, Hoyland JA. Human mesenchymal stem cell differentiation to NP-like cells in chitosan-glycerophosphate hydrogels. Biomaterials. 2008;29:85–93. doi: 10.1016/j.biomaterials.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Risbud MV, Guttapalli A, Stokes DG, Hawkins D, Danielson KG, Schaer TP, Albert TJ, Shapiro IM. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 51.Roughley P, Hoemann C, DesRosiers E, Mwale F, Antoniou J, Alini M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials. 2006;27:388–396. doi: 10.1016/j.biomaterials.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 52.Slade SC, Keating JL. Unloaded movement facilitation exercise compared to no exercise or alternative therapy on outcomes for people with nonspecific chronic low back pain: a systematic review. J Manip Physiol Ther. 2007;30:301–311. doi: 10.1016/j.jmpt.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Sive JI, Baird P, Jeziorsk M, Watkins A, Hoyland JA, Freemont AJ. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91–97. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8:888–896. doi: 10.1016/j.spinee.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 55.Specchia N, Pagnotta A, Toesca A, Greco F. Cytokines and growth factors in the protruded intervertebral disc of the lumbar spine. Eur Spine J. 2002;11:145–151. doi: 10.1007/s00586-001-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–411. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 57.Takada T, Nishida K, Doita M, Kurosaka M. Fas ligand exists on intervertebral disc cells: a potential molecular mechanism for immune privilege of the disc. Spine. 2002;27:1526–1530. doi: 10.1097/00007632-200207150-00009. [DOI] [PubMed] [Google Scholar]
- 58.Tapp H, Deepe R, Ingram JA, Kuremsky M, Hanley EN, Jr, Gruber HE. Adipose-derived mesenchymal stem cells from the sand rat: transforming growth factor beta and 3D co-culture with human disc cells stimulate proteoglycan and collagen type I rich extracellular matrix. Arthr Res Ther. 2008;10:R89. doi: 10.1186/ar2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tapp H, Hanley EN, Jr, Patt JC, Gruber HE. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med (Maywood) 2009;234:1–9. doi: 10.3181/0805-MR-170. [DOI] [PubMed] [Google Scholar]
- 60.Tsai TT, Guttapalli A, Oguz E, Chen LH, Vaccaro AR, Albert TJ, Shapiro IM, Risbud MV. Fibroblast growth factor-2 maintains the differentiation potential of nucleus pulposus cells in vitro: implications for cell-based transplantation therapy. Spine. 2007;32:495–502. doi: 10.1097/01.brs.0000257341.88880.f1. [DOI] [PubMed] [Google Scholar]
- 61.Vadalà G, Sobajima S, Lee JY, Huard J, Denaro V, Kang JD, Gilbertson LG. In vitro interaction between muscle-derived stem cells and nucleus pulposus cells. Spine J. 2008;8:804–809. doi: 10.1016/j.spinee.2007.07.394. [DOI] [PubMed] [Google Scholar]
- 62.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 63.Wei A, Brisby H, Chung SA, Diwan AD. Bone morphogenetic protein-7 protects human intervertebral disc cells in vitro from apoptosis. Spine J. 2008;8:466–474. doi: 10.1016/j.spinee.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto Y, Mochida J, Sakai D, Nakai T, Nishimura K, Kawada H, Hotta T. Upregulation of the viability of nucleus pulposus cells by bone marrow-derived stromal cells: significance of direct cell-to-cell contact in coculture system. Spine. 2004;29(14):1508–1514. doi: 10.1097/01.BRS.0000131416.90906.20. [DOI] [PubMed] [Google Scholar]