Abstract
Choosing the appropriate graft material to participate in the healing process in posterolateral spinal fusion continues to be a challenge. Combining synthetic graft materials with bone marrow aspirate (BMA) and autograft is a reasonable treatment option for surgeons to potentially reduce or replace the need for autograft. FormaGraft, a bone graft material comprising 12% bovine-derived collagen and 88% ceramic in the form of hydroxyapatite (HAp) and beta tricalcium phosphate (β-TCP) was evaluated in three possible treatment modalities for posterior spinal fusion in a standard rabbit model. These three treatment groups were FormaGraft alone, FormaGraft soaked in autogenous BMA, and FormaGraft with BMA and iliac crest autograft. No statistically demonstrable benefits or adverse effects of the addition of BMA were found in the current study based on macroscopic, radiology or mechanical data. This may reflect, in part, the good to excellent results of the collagen HA/TCP composite material alone in a well healing bony bed. Histology did, however, reveal a benefit with the use of BMA. Combining FormaGraft with autograft and BMA achieved results equivalent to autograft alone. The mineral and organic nature of the material provided a material that facilitated fusion between the transverse processes in a standard preclinical posterolateral fusion model.
Keywords: Spinal fusion, Bone graft, Collagen, Hydroxyapatite, Bone marrow aspirate
Introduction
Bone graft substitutes provide surgeons a potential alternative in bone grafting procedures where the availability of the local autograft is limited or in the case of multiple levels of revision spinal fusion procedures. These materials need to satisfy the intraoperative and handling requirements of the surgeon as well as provide a reliable and reproducible in vivo response to facilitate healing and fusion between adjacent bones. Clinical studies comparing different materials and their in vivo responses are lacking. Preclinical data, however, based on a standard and well-reported model can provide a basis for comparison as well as understanding. Posterolateral fusion in adult rabbits [2–4, 8, 10–12, 20] is one such model that provides insight into the in vivo performance of bone graft materials. Calcium phosphate materials based on hydroxyapatite (HAp) [2–4, 8, 10–12, 20] or beta tricalcium phosphate (β-TCP) alone [13, 16] have been reported with varied levels of osteoconductive response in the rabbit posterolateral fusion model. The addition of osteoinductive molecules (e.g. bone morphogenetic proteins) or tissue (autologous bone graft or demineralized bone matrix) to such materials represents a potential strategy to improve the performance of these osteoconductive scaffolds. The addition of a collagen matrix to these ceramic materials represents a simple strategy to couple the osteoconductive response of the two phases (mineral and organic) as well as the porosity and interconnectivity within the materials to support and encourage cell proliferation and differentiation. Bone marrow aspirate or autograft can also be added to potentially improve the healing of osteoconductive materials [1, 2, 5–7, 9, 14, 15, 17–22].
FormaGraft® (NuVasive® Inc, San Diego, CA) is a composite bone graft material comprising 12% bovine-derived collagen and 88% ceramic in the form of HAp and β-TCP. This material has the potential advantage of long-term osteoconductivity for new bone ongrowth of the HAp component and the early release of calcium ions from the β-TCP component, which is rapidly degraded and replaced by new woven bone. HAp and β-TCP have a long and successful clinical history and are radiopaque for excellent intraoperative and postoperative visibility. The ceramic particles, with interconnected porosity to support and promote neovascularization and bone ingrowth, are entrapped in a fibrillar type I bovine collagen network, which is highly biocompatible with low immunogenicity. The collagen network provides the FormaGraft with excellent handling properties, adsorption of proteins and scaffold for cells, as well as a convenient means for delivery of bone marrow aspirate (BMA) intraoperatively for the surgeon.
This study evaluated the performance of a calcium phosphate–collagen composite bone graft material (FormaGraft, NuVasive, San Diego) in the common New Zealand white rabbit model for posterolateral spinal fusion. In particular, the effect of adding cellular components from either BMA or iliac crest autograft with BMA was investigated and compared to using the synthetic bone graft material alone and compared to iliac crest autograft alone (positive control) and decorticated group with no graft (negative control).
Materials and methods
Single-level posterolateral lumbar fusion was performed at L5–L6 in 69 adult New Zealand white rabbits as reported and developed by Dr. Boden et al. [2] following institutional ethical approval. The study design is outlined in Table 1. A sample size of n = 6 was used in the FormaGraft groups at 6 weeks, with radiographic and histological end points performed, while a sample size of n = 12 was used at 12 weeks where mechanical testing was also performed. This sample size was chosen based on a priori power assessment with an alpha of 0.05 and beta of 0.7.
Table 1.
Study design
| Group | 6 weeks (n) | 12 weeks (n) |
|---|---|---|
| FormaGraft alone | 6 | 12 |
| FormaGraft + BMA | 6 | 12 |
| FormaGraft + BMA + autograft | 6 | 12 |
| Autograft (positive control) | 3 | 6 |
| Empty (negative control) | 3 | 3 |
The animals were anesthetized for surgery using isoflurane/oxygen inhalation. A midline incision was made in the skin, and the intermuscular plane between the multifidus and longissimus muscles was bluntly incised to expose the L5 and L6 transverse processes. A high-speed burr (Midas Rex, Medtronic, Memphis, TN) was used to decorticate the transverse processes in a uniform and reproducible manner in all animals, 10 mm from the base of the transverse processes. The groups examined in this study are outlined in Table 1. In the FormaGraft and FormaGraft plus BMA groups, two 10 × 20 mm strips of the material were stacked on top of each other; for the FormaGraft plus autograft group, one 10 × 20 mm strip of material had 0.75 ml of autograft laid on top of the FormaGraft over the decorticated transverse processes. For animals in the FormaGraft plus BMA, a total of 3 ml of bone marrow was aspirated from the proximal tibia through a puncture in the anteromedial cortex and harvested with a bone marrow biopsy needle. The FormaGraft strips were soaked with BMA in a 1:1 mix, based on volume, and allowed to soak for 5 min prior to implantation between the decorticated transverse processes. A total volume of 3 ml of corticocancellous bone was harvested from the iliac crests and morcelized in the autograft group. A total of 1.5 ml of autograft was placed on each side over the decorticated transverse processes. The volume of autograft used was measured using a 3 ml sterile syringe prior to placement into the posterolateral space. In the empty group, the transverse processes were decorticated using a high-speed burr but no graft materials were placed between the transverse processes. The facial and skin incisions were closed with 3-0 and 4-0 absorbable suture (Dexon, US Surgical, Norwalk CT), respectively. The animals were monitored daily until being killed at the designated survival time of either 6 or 12 weeks via lethal cardiac injection of sodium pentobarbital (Lethobarb, Virbac, Australia).
Time zero surgery was performed on two cadaveric rabbits to provide a baseline for radiographic end points used in the study with no healing. The surgical procedure was identical to the above for the FormaGraft alone group for radiographic end points of the material alone in the posterolateral site. Two 10 × 20 mm strips were placed over the decorticated transverse processes or one 10 × 20 mm strip with 0.75 cm3 of autograft as described above. The skin was closed and the specimens were processed for time zero radiographs as described below.
End points
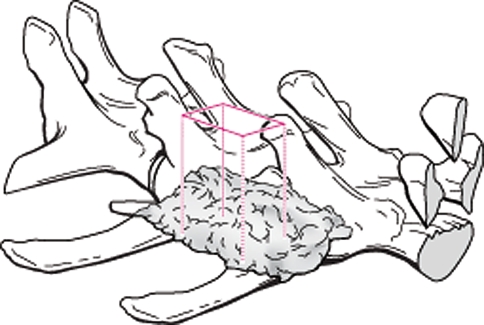
At the time of harvest, the general integrity of the incision was noted, along with any evidence of macroscopic reaction of the underlying subcutaneous tissues. Radiographs of the harvested spines were taken with a high-resolution device (Faxitron HP, Wheeling, IL) with an anteroposterior (AP) view and digital films (Agfa). DICOM data were viewed using DICOMWorks v1.3.5 in a blinded fashion. Grading fusion from these radiographs was difficult, considering that the inorganic mineral components of FormaGraft had a dense signal even at time zero. Bone mineral density (BMD, g/cm2) was measured using a Lunar DPXL pencil beam machine (Lunar, Madison, WI) in regions of interest of the graft alone between the transverse processes (Fig. 1). BMD data were analyzed using a two-way ANOVA with SPSS for Windows, followed by a Games Howell post hoc test (SPSS Version 15, SPSS Inc., Chicago, IL). Each spine was subsequently examined using a clinical computed tomography scanner (CT, Toshiba Asteion, Japan). Images were taken in the axial plane at a slice thickness of 0.5 mm using 100 kV and 60 mAs. DICOM data were imported and analyzed with MIMICS software (Version 11, Materialise, Leuven, Belgium). Three-dimensional models were created to visualize the fusion masses between the transverse processes using a standardized minimum threshold of 226. The fusions were also evaluated in the axial, coronal and sagittal planes in the raw CT images to assess the presence of residual material, new bone and continuity of fusions between the transverse processes.
Fig. 1.
The bone mineral density was assessed using a Lunar DPXL DEXA machine. A region of interest within the center of the implant encompassing the entire fusion mass (excluding the transverse processes) on the right and left sides was used. DEXA density (g/cm2) increased with time for all FormaGraft groups, while the autograft decreased with time
Assessment of the stiffness at the fused level was performed prior to dissection qualitatively using manual palpation for samples at 12 weeks in a blinded fashion. Spines were gently loaded manually in cantilever bending with the distal vertebral body held fixed to evaluate flexion at the site. The deformation of the transverse processes at the surgical site was monitored visually. Samples were graded as either stable or unstable. The mechanical properties of the spines from the 12-week groups were examined in a quantitative fashion using destructive mechanical testing in tension. Samples were carefully dissected so that only the fusion mass participated to resist the tensile load. The facet joints, intertransverse membrane, and posterior and supratransverse ligaments were sharply dissected using a #15 scalpel blade prior to loading on the testing machine. Dissection of the supporting structures of the spine allowed the biomechanical performance to be a function of the fusion between fused levels without contribution of the other structures. Uniaxial tension was applied at a constant rate of 5 mm/min using a testing jig that allowed a degree of freedom to minimize off-axis loading. Spines were fixed in the testing rig using a 3.2 mm stainless steel pin drilled through each vertebra in the AP direction and attached to an MTS 858 Bionix testing machine (MTS, Eden Prairie, MN) and a 2kN load cell. Samples were fixed in the machine while in load control to avoid any preloading prior to testing. Load/displacement data were continually recorded at a rate of 100 Hz and the peak load, stiffness and energy to peak load were calculated for each specimen using an in-house MatLab program (MatLab, Natick, MA). Mechanical data were analyzed using a one-way ANOVA followed by a Games Howell post hoc test (SPSS Version 15).
All specimens were fixed in cold phosphate-buffered formalin solution for a minimum of 48 h and decalcified in 10% of formic acid–phosphate buffered formalin solution for routine paraffin histology. The left-hand side of the fusion was sectioned in the coronal plane, and the right-hand side sectioned in the sagittal plane after decalcification. Two coronal blocks (anterior and posterior) and four sagittal blocks (midline to lateral) were obtained for each animal. From each block, 5 μm sections cut using a Leica microtome (Leica Microsystems GnbH, Wetzlar, Germany) were stained with haemotoxylin and eosin (H&E), tetrachrome and pentachrome for blinded examination of new bone formation and host-graft material interactions with the adjacent bony beds of the transverse processes and vertebral body, as well as midway between the transverse processes with the muscle on the dorsal aspect and the intertransverse membranes on the ventral aspect.
The FormaGraft at time zero prior to implantation was examined with scanning electron microscopy (Hitatchi 3400, Tokyo, Japan), X-ray fluorescence (XRF, Phillips PW 2400) and Fourier transform infrared spectroscopy (FTIR) (Avatar Model 370 FTIR) to characterise the graft material.
Results
No significant inflammatory reaction to the implanted material was noted on macroscopic inspection at harvest. Manual palpation of the fused level revealed stable constructs bilaterally in nearly all animals with 2 sides out of 24 (8.3%) in each of the FormaGraft plus BMA group and FormaGraft plus autograft and BMA group found to be unstable at 12 weeks on manual assessment. Autograft at 12 weeks was found to be unstable in 2 of 12 sites (16.7%).
Radiography
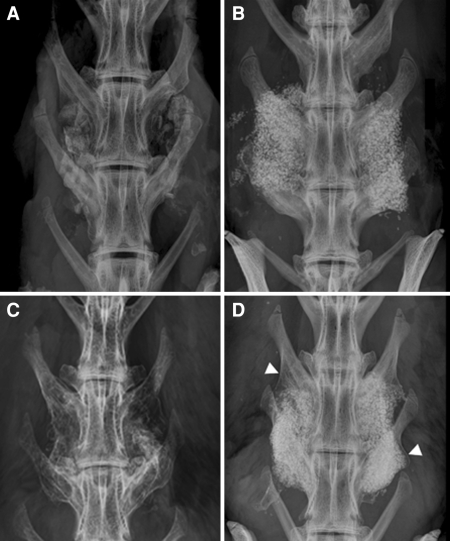
AP Faxitron radiographs of FormaGraft at time zero showed a structure predominated by dense particles of the ceramic phases. Distinct and clearly defined edges were present at time zero, which was not seen in any of the implanted grafts at 6 or 12 weeks. The mineral particles were coarsely arranged and overall the implant had a strong radiopacity, which would ultimately make it difficult to critically assess new bone within the graft materials based on radiographs alone. Typical radiographs at 12 weeks following surgery are presented in Fig. 2. Radiographs confirmed the presence of the FormaGraft material at both 6 and 12 weeks following surgery. Evidence of new bone formation at the margins of the material was present at 6 and 12 weeks in all animals with FormaGraft as well as autograft alone with no differences between groups being observed. Softening of the edges of the FormaGraft was observed from 0 to 6 weeks and progressed with time at 12 weeks. This is consistent with resorption of the composite graft coupled with new bone formation; however, a precise breakdown of these two processes could not be determined based on radiographs alone. Assessment of the evidence of new bone formation or resorption of the inorganic mineral component within the material itself was difficult due to the radiopacity. Radiographic fusion rate of the autograft control was 50% (3/6 sites) at 6 weeks and increased to 83% (10/12 sites) at 12 weeks. The negative control (decorticated empty) group did not show signs of radiographic fusion in any case.
Fig. 2.
Typical radiographs are presented for autograft at 6 and 12 weeks (a and c) and FormaGraft alone at 6 and 12 weeks (b and d). The increase in fusion maturity with autograft as well as FormaGraft is present with the development of neocortex laterally. A progression of new bone enveloping the graft emanating from the transverse processes (arrows) was found between 6 and 12 weeks, which was also accompanied by resorption of the implant material in that area
Computed tomography
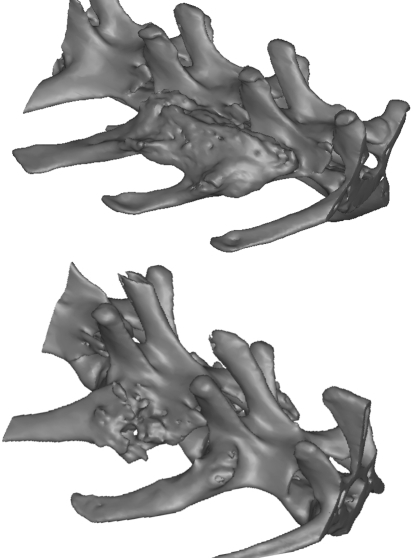
Fusion between the transverse processes was also investigated from a review of three-dimensional models and evaluation in three planes (coronal, sagittal and axial views) using models created using MIMICS (Materialise, Belguim). CTs at 6 weeks revealed the clear presence of FormaGraft with the rectangular shape and loss of detail at the margins, which progressed at 12 weeks where the rectangular shape was less evident, suggesting continued resorption and/or remodeling coupled with new bone formation. In the FormaGraft plus autograft group, the pieces of autograft appeared integrated with the FormaGraft as early as 6 weeks and were still evident on top of the FormaGraft at 12 weeks. The fusion rate from CT for the autograft only group was 50% (3 of 6 sites) at 6 weeks and 75% (8 of 12 sites) at 12 weeks. The CT analysis was an effective technique for assessing the fusions by examination of the axial, coronal and sagittal views; however, the presence of FormaGraft at 6 and 12 weeks made it difficult to discriminate new bone from remaining inorganic material of the FormaGraft. CT was however helpful in assessing non-fusions with autograft alone especially at 6 weeks (Fig. 3).
Fig. 3.
Reconstructions of 3D were created based on DICOM data using MIMICS. The lack of a solid fusion mass is clearly highlighted in an animal treated with autograft alone at 6 weeks. The presence of residual mineral phase with the FormaGraft resulted in a solid mass of material between the transverse processes at 6 weeks when reconstructed into 3D models
Bone mineral density
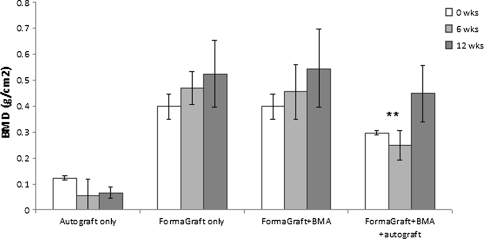
Dual energy X-ray absorptiometry (DEXA) was conducted on all specimens to characterize the progression of potential new bone growth and graft resorption. The right- and left-hand sides were pooled for each group for analysis. DEXA values were greater when the transverse processes were included; however, the overall results were the same when bone mineral density (BMD) was assessed between the transverse processes. The DEXA data between the transverse processes are presented in Fig. 4. BMD for autograft decreased with time from 0 to 12 weeks (*P = 0.054) as the autograft was resorbed and remodeled. BMD when two strips of FormaGraft were used was greater compared to a single strip plus BMA and autograft (**P < 0.05) at 6 weeks. No differences were detected between two strips of FormaGraft at time zero and one strip of FormaGraft plus BMA and autograft at 12 weeks (P = 0.874). BMD increased between 6 and 12 weeks for all FormaGraft groups, but no differences were detected.
Fig. 4.
BMD for autograft decreased with time from 0 to 12 weeks (*P = 0.054) as the autograft was resorbed and remodeled. BMD when two strips of FormaGraft were used was greater compared to a single strip plus BMA and autograft (**P < 0.05) at 6 weeks. No difference, however, was detected between two strips of FormaGraft at time zero and one strip of FormaGraft plus BMA and autograft at 12 weeks (P = 0.874). BMD increased between 6 and 12 weeks for all FormaGraft groups, but no differences were detected
Mechanical testing
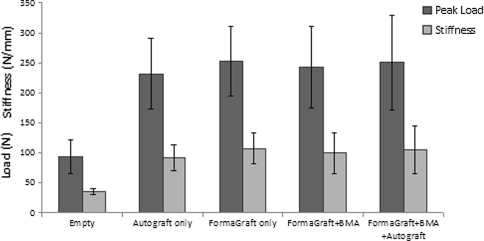
Manual palpation of the fusion mass was not a reliable end point in this study, although instability at individual levels was detected in 2 sites out of 24 (8.3%) in the FormaGraft + BMA and 2 sites out of 24 (8.3%) in the FormaGraft + BMA + autograft groups at 12 weeks. Destructive uniaxial tensile testing of the spines at 12 weeks revealed similar biomechanical performance in the FormaGraft groups compared to the positive control (iliac crest autograft) with a strong correlation between peak load and stiffness (R2 = 0.845, P < 0.001) (Fig. 5), while the correlation with energy was weaker (R2 = 0.504, P < 0.001, data not shown). The negative control (decorticated empty) was significantly inferior to the test groups (*P < 0.05). Failure occurred in a transverse fashion between the transverse processes bilaterally within the fusion mass during tensile testing. No failure at the pin sites was observed during mechanical testing.
Fig. 5.
Destructive uniaxial tensile testing of the spines at 12 weeks revealed similar biomechanical performance in the FormaGraft groups compared to the positive control (iliac crest autograft) with a strong correlation between peak load and stiffness (R2 = 0.845, P < 0.001). The negative control (decorticated empty) was significantly inferior to the test groups (P < 0.05)
Histology
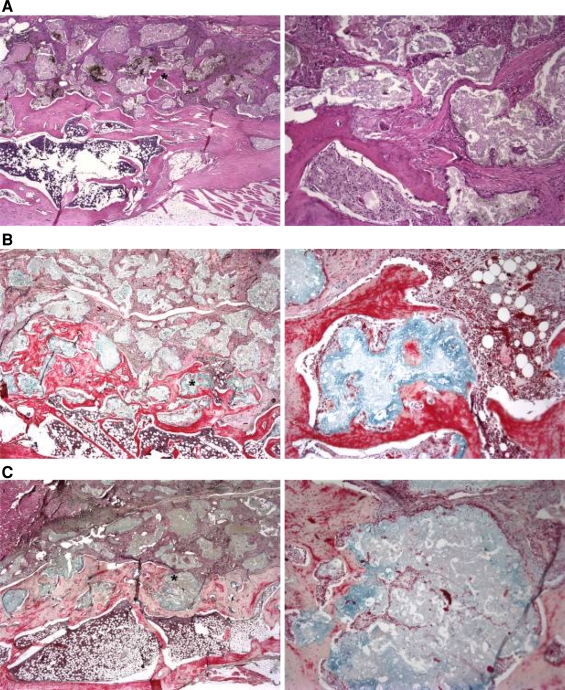
The coronal and sagittal paraffin histology sections demonstrated the osteoconductive nature of FormaGraft when in intimate contact with a bony bed at 6 and 12 weeks. New bone formation directly on and within the material was observed at 6 weeks, which progressed at 12 weeks. Histology revealed the acellular collagenous component of the FormaGraft at 6 weeks. A mild inflammatory/foreign body response was present adjacent to the mineral and collagenous phases at 6 weeks, although this subsided by 12 weeks, supporting the biocompatibility of FormaGraft in this model. New bone formation was noted around the ceramic particles, especially at the interface with the transverse processes at 6 weeks, which progressed and remodeled at 12 weeks. At 12 weeks, new bone was noted around and within the porous domains of the ceramic particles adjacent to the decorticated transverse processes (Fig. 6). The addition of BMA or autograft provided stimulus for local bone formation in the center of the fusion mass, away from the transverse processes, which was otherwise not seen with FormaGraft alone. At 12 weeks, evidence of a bony cortex was discovered on the periphery of the implanted FormaGraft strips. The autograft treatment alone revealed the presence of residual autograft at 6 and 12 weeks with the establishment of a new cortex by 12 weeks, consistent with the radiographs as well as other reports of this model.
Fig. 6.
a FormaGraft alone at 12 weeks (H&E); b FormaGraft plus BMA (Pentachrome); c FormaGraft + BMA + Autograft Pentachrome. New bone formation was noted around the ceramic particles especially at the interface with the transverse processes at 6 weeks, which progressed and remodeled at 12 weeks. At 12 weeks, there was new bone, intimate with the ceramic phases of FormaGraft as well as within the porous domains of the ceramic particles (×2 and ×20 objectives)
In vitro characterization of ceramic
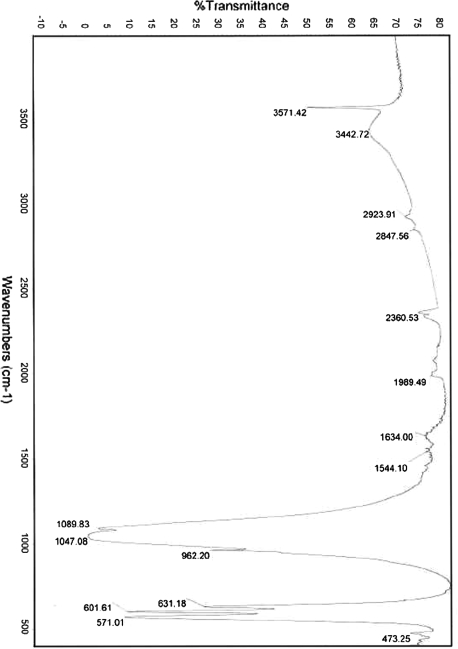
FTIR of the ceramic particles revealed a pattern consistent with biphasic calcium phosphate, with peaks for hydroxyapatite as well as the tricalcium phosphate components of FormaGraft (Fig. 7). XRF showed the volume of CaO to be 56%, that of P2O5 41% and SiO2 1.5%. Scanning electron microscopy of the FormaGraft strip showed porous ceramic granules with a rough, microstructured surface entrapped within a loose collagen network (Fig. 8).
Fig. 7.
FTIR of the ceramic particles revealed a pattern consistent with biphasic calcium phosphate, with peaks for hydroxyapatite as well as the tricalcium phosphate components of FormaGraft
Fig. 8.
SEM revealed significant surface microstructure on the ceramic granule (left) and a loose collagen network (right)
Discussion
Corticocancellous autograft harvested from the iliac crest provides surgeons with a reliable bone graft to participate in the biology of fusion, but with well-known morbidities. Synthetic materials to potentially replace, augment or extend autograft have extensively investigated. Fundamental principles of bone healing need to be satisfied in order to achieve fusion. These include a stable, mechanical environment, a well-prepared bone bed, some type of matrix to support or encourage cellular attachment and proliferation, and the appropriate local milieu of biological factors required in the complex bone-healing cascade. Considering these requirements, it is no surprise that autograft, when present in sufficient quantity and quality and in the correct patient, achieves excellent results for fusion in animals as well as humans. Along the same lines, the use of a synthetic calcium phosphate phase alone, even under ideal conditions, presents a significant biological challenge to achieve bony fusion even in a preclinical rabbit model. The bone graft substitute, FormaGraft, examined in the current study combines a type I collagen matrix coupled with a faster resorbing TCP inorganic and a more stable HAp osteoconductive component. The FTIR spectrum (Fig. 7) illustrates the organic and inorganic signature of this material to contain both HA as well as TCP. While this material and other similar ceramics do not have the cellular population or the growth factors at the time of surgery, the current study supports that the composite material makeup of FormaGraft acts as a matrix for the attachment and proliferation of cells to achieve spinal fusion in a standard preclinical animal model in the rabbit alone.
FormaGraft alone and in combination with autograft and bone marrow aspirate performed in an equivalent manner in this rabbit model compared to autograft alone, and outperformed decortication alone with no graft material. FormaGraft provides a collagen matrix for cellular infiltration coupled with the inorganic hydroxyaptatite and tricalcium phosphate to provide local calcium and phosphate ions as well as the osteoconductive nature of these materials. The strips of FormaGraft were placed directly over the decorticated transverse processes spanning the entire level. The use of two strips of FormaGraft did however make radiographic evaluation of new bone and implant resorption difficult even with the use of computed tomography. The addition of BMA softened the FormaGraft material at the time of surgery and facilitated placement in the posterolateral gutter. The corticocancellous autograft harvest from the iliac crest increased operative time even in this preclinical model, as expected, similar to humans. A total of 3 ml of autograft in particulate form was harvested from the iliac crests and 1.5 ml was used for each side in our model. This amount was chosen considering the decorticated region of the transverse processes was 10 mm from the midline. Furthermore, we did not decorticate to the tips of the transverse processes, and thus additional autograft was not required to be placed in the posterolateral gutters. The results of the current study support the use of 1.5 ml of autograft in the rabbit posterolateral model to achieve fusion rates equivalent to a more aggressive harvest of autograft from the iliac crests and a larger area of decortication.
No statistically demonstrable benefits or adverse effects of the addition of BMA were found in the current study based on macroscopic, radiology or mechanical data. This may reflect, in part, the good to excellent results of the collagen HA/TCP composite material alone in a well-healing bony bed in the 6-month-old rabbit model. However, qualitative differences present at the histological level were detected with the addition of BMA with respect to new bone formation, although these differences did not manifest themselves in the macroscopic, radiographic or mechanical endpoints. This highlights the sensitivity of different end points where differences need to be large for radiographs to demonstrate the effects or sample size increased to large numbers to detect smaller differences between treatment groups.
The well-reported model developed by Boden was used in this study [2]. This model provides an excellent preclinical evaluation of materials for spinal fusion and its usefulness is reflected in its wide acceptance. The nature of this posterolateral model provides insight into the osteoconductive response of a material as well as a more generalized osteogenic response, when you consider the material–host interactions adjacent to a bony bed (osteoconductive response) and in the middle of the transverse process (osteogenic response). This rabbit model also does not incorporate any mechanical fixation (e.g., pedicle screws) often used in humans and is not destabilized, such as to require hardware for mechanical stability.
The end points reported in the current study were based on those of Boden et al., with some differences. Radiographic assessment of the fusion mass and implant resorption of materials with inorganic mineral phases are difficult due to the presence of remaining graft materials. In this study, the difficulty in assessing fusion by plain film radiograph was offset by the additional end points of DEXA, histology and mechanical testing, each of which added further information on the quality of the fusion. These end points are not relevant in the clinical scenario, although in that case the clinician has the option of monitoring the fusion status from displacements on flexion/extension films. The DEXA and CT data in the current study demonstrated the initial stages of implant resorption difficult to critically assess on plain films. Changes compared to time zero data were evident in the computed tomography data with softening of the edges and changes in the appearance of the mineral phase. The use of CTs in the clinical setting may not be considered ideal due to the additional radiation and associated risks, but is a useful tool in the preclinical setting. Note that the CTs were taken after euthanasia in this animal model.
Manual assessment of the fusion mass has been reported by other investigators (reference here). This is a qualitative end point and to control between studies. While manual palpation can distinguish a non-fused segment, it is a subjective assessment of the stiffness (N/mm) rather than the strength (N) of the fusion. Destructive mechanical testing using a simple uniaxial tensile test was performed to evaluate the ultimate properties of the fusions at 12 weeks. Samples were dissected and tested in such a fashion as to isolate the fusion mass as well as minimize potential off-axis loading in the mechanical testing. The testing jigs used in this study were a modification of the jigs recommended for use in the ISO and ASTM testing of pedicle screws (F1717). The degree of freedom in rotation combined with fixing the samples in the testing machine in load control helped keep the loads at 0 N prior to distracting the samples to failure. The use of pins through the vertebral body removed the need for embedding media such as metal alloys or methacrylate-based cements.
Histology in the current study was examined in the sagittal as well as coronal planes on all samples. The material–host reaction adjacent to the bony bed aids in the examination of the osteoconductive potential. In contrast, the local environment midway between the transverse processes could be considered as more challenging based on the distance from the native bone bed. The sagittal sections also have the additional benefit of examining how the material–host reaction occurs with local muscle present on the dorsal aspect of the fusion mass.
This study was limited in that longer time points beyond 12 weeks were not examined. The short-term follow-up and minimal resorption of the graft material do not provide insight into the in vivo performance of this material as resorption continues and further bone remodeling occurs.
Conclusions
This study has shown that FormaGraft has equivalent performance to particulate iliac crest autograft in the NZ white rabbit model of posterolateral spinal fusion. Fusion was difficult to assess by traditional radiography; however, DEXA showed a progression of new bone with time, as did histology. Histology showed new bone growing in and around the ceramic particles and at 12 weeks a new cortical shell was evident around the implant. Analysis of the histology also revealed a clear benefit from the addition of either BMA or autograft, although this benefit was not as clear from the other end points. Destructive mechanical testing, a direct measurement of properties of fusion, showed that each of the FormaGraft groups had a trend toward superior biomechanical performance when compared to autograft, and was statistically superior when compared to the empty controls. Thus, FormaGraft is an efficacious device when used alone, or preferentially with BMA or autograft, in the New Zealand white rabbit model.
Acknowledgments
Support for this study was provided by NuVasive, Inc. San Diego, CA. The authors would like to acknowledge the support of John Rawlinson, Greg Mitchell and Molly Barnhart for the animal-related aspects of this study.
References
- 1.Becker S, Maissen O, Ponomarev I, Igor P, Stoll T, Thierry S, Rahn B, Wilke I, Ingo W. Osteopromotion by a beta-tricalcium phosphate/bone marrow hybrid implant for use in spine surgery. Spine. 2006;31:11–17. doi: 10.1097/01.brs.0000192762.40274.57. [DOI] [PubMed] [Google Scholar]
- 2.Boden SD, Martin GJ, Morone M, Ugbo JL, Titus L, Hutton WC. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine. 1999;24:320–327. doi: 10.1097/00007632-199902150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Boden SD, Martin GJ, Morone MA, Ugbo JL, Moskovitz PA. Posterolateral lumbar intertransverse process spine arthrodesis with recombinant human bone morphogenetic protein 2/hydroxyapatite-tricalcium phosphate after laminectomy in the nonhuman primate. Spine. 1999;24:1179–1185. doi: 10.1097/00007632-199906150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Bozic KJ, Glazer PA, Zurakowski D, Simon BJ, Lipson SJ, Hayes WC. In vivo evaluation of coralline hydroxyapatite and direct current electrical stimulation in lumbar spinal fusion. Spine. 1999;24:2127–2133. doi: 10.1097/00007632-199910150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Cinotti G, Patti AM, Vulcano A, Della Rocca C, Polveroni G, Giannicola G, Postacchini F. Experimental posterolateral spinal fusion with porous ceramics and mesenchymal stem cells. J Bone Joint Surg Br. 2004;86:135–142. [PubMed] [Google Scholar]
- 6.Goshima J, Goldberg VM, Caplan AI. The origin of bone formed in composite grafts of porous calcium phosphate ceramic loaded with marrow cells. Clin Orthop Relat Res. 1991;27:4–283. [PubMed] [Google Scholar]
- 7.Johnson KD, Frierson KE, Keller TS, Cook C, Scheinberg R, Zerwekh J, Meyers L, Sciadini MF. Porous ceramics as bone graft substitutes in long bone defects: a biomechanical, histological, and radiographic analysis. J Orthop Res. 1996;14:351–369. doi: 10.1002/jor.1100140304. [DOI] [PubMed] [Google Scholar]
- 8.Karaismailoglu TN, Tomak Y, Andac A, Ergun E. Comparison of autograft, coralline graft, and xenograft in promoting posterior spinal fusion. Acta Orthop Traumatol Turc. 2002;36:147–154. [PubMed] [Google Scholar]
- 9.Khoueir P, Oh BC, Di Risio DJ, Wang MY. Multilevel anterior cervical fusion using a collagen-hydroxyapatite matrix with iliac crest bone marrow aspirate: an 18-month follow-up study. Neurosurgery. 2007;61:963–970. doi: 10.1227/01.neu.0000303192.64802.c6. [DOI] [PubMed] [Google Scholar]
- 10.Konishi S, Nakamura H, Seki M, Nagayama R, Yamano Y. Hydroxyapatite granule graft combined with recombinant human bone morphogenic protein-2 for solid lumbar fusion. J Spinal Disord Tech. 2002;15:237–244. doi: 10.1097/00024720-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Kraiwattanapong C, Boden SD, Louis-Ugbo J, Attallah E, Barnes B, Hutton WC. Comparison of Healos/bone marrow to INFUSE(rhBMP-2/ACS) with a collagen–ceramic sponge bulking agent as graft substitutes for lumbar spine fusion. Spine. 2005;30:1001–1007. doi: 10.1097/01.brs.0000160997.91502.3b. [DOI] [PubMed] [Google Scholar]
- 12.Minamide A, Kawakami M, Hashizume H, Sakata R, Tamaki T. Evaluation of carriers of bone morphogenetic protein for spinal fusion. Spine. 2001;26:933–939. doi: 10.1097/00007632-200104150-00017. [DOI] [PubMed] [Google Scholar]
- 13.Minamide A, Kawakami M, Hashizume H, Sakata R, Yoshida M, Tamaki T. Experimental study of carriers of bone morphogenetic protein used for spinal fusion. J Orthop Sci. 2004;9:142–151. doi: 10.1007/s00776-003-0749-0. [DOI] [PubMed] [Google Scholar]
- 14.Muschler GF, Matsukura Y, Nitto H, Boehm CA, Valdevit AD, Kambic HE, Davros WJ, Easley KA, Powell KA (2005) Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Relat Res 242–251. doi:10.1097/01.blo.0000149812.32857.8b [DOI] [PMC free article] [PubMed]
- 15.Muschler GF, Nitto H, Matsukura Y, Boehm C, Valdevit A, Kambic H, Davros W, Powell K, Easley K (2003) Spine fusion using cell matrix composites enriched in bone marrow-derived cells. Clin Orthop Relat Res 102–118. doi:10.1097/00003086-200302000-00018 [DOI] [PMC free article] [PubMed]
- 16.Namikawa T, Terai H, Suzuki E, Hoshino M, Toyoda H, Nakamura H, Miyamoto S, Takahashi N, Ninomiya T, Takaoka K. Experimental spinal fusion with recombinant human bone morphogenetic protein-2 delivered by a synthetic polymer and beta-tricalcium phosphate in a rabbit model. Spine. 2005;30:1717–1722. doi: 10.1097/01.brs.0000172155.17239.fa. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa M, Myoui A, Ohgushi H, Ikeuchi M, Tamai N, Yoshikawa H. Bone tissue engineering using novel interconnected porous hydroxyapatite ceramics combined with marrow mesenchymal cells: quantitative and three-dimensional image analysis. Cell Transpl. 2004;13:367–376. doi: 10.3727/000000004783983819. [DOI] [PubMed] [Google Scholar]
- 18.Ohgushi H, Goldberg VM, Caplan AI. Heterotopic osteogenesis in porous ceramics induced by marrow cells. J Orthop Res. 1989;7:568–578. doi: 10.1002/jor.1100070415. [DOI] [PubMed] [Google Scholar]
- 19.Ohgushi H, Goldberg VM, Caplan AI. Repair of bone defects with marrow cells and porous ceramic. Experiments in rats. Acta Orthop Scand. 1989;60:334–339. doi: 10.3109/17453678909149289. [DOI] [PubMed] [Google Scholar]
- 20.Tay BK, Le AX, Heilman M, Lotz J, Bradford DS. Use of a collagen–hydroxyapatite matrix in spinal fusion. A rabbit model. Spine. 1998;23:2276–2281. doi: 10.1097/00007632-199811010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Walsh WR, Harrison J, Loefler A, Martin T, Van Sickle D, Brown MK, Sonnabend DH (2000) Mechanical and histologic evaluation of Collagraft in an ovine lumbar fusion model. Clin Orthop Relat Res 258–266. doi:10.1097/00003086-200006000-00031 [DOI] [PubMed]
- 22.Zerwekh JE, Kourosh S, Scheinberg R, Kitano T, Edwards ML, Shin D, Selby DK. Fibrillar collagen–biphasic calcium phosphate composite as a bone graft substitute for spinal fusion. J Orthop Res. 1992;10:562–572. doi: 10.1002/jor.1100100411. [DOI] [PubMed] [Google Scholar]