Abstract
The disruption of the extracellular disc matrix is a major hallmark of disc degeneration. This has previously been shown to be associated with an up-regulation of major matrix metalloproteinase (MMP) expression and activity. However, until now hardly any data are available for MMP/TIMP regulation and thereby no concept exists as to which MMP/TIMP plays a major role in disc degeneration. The objective of this study was, therefore, to identify and quantify the putative up-regulation of MMPs/TIMPs on the mRNA and protein level and their activity in disc material in relation to clinical data and histological evidence for disc degeneration. A quantitative molecular analysis of the mRNA expression levels for the MMPs (MMPs-1, -2, -3, -7, -8, -9, -13) and the MMP inhibitors (TIMPs-1 and -2) was performed on 37 disc specimens obtained from symptomatic disc herniation or degeneration. In addition, disc specimens from patients without disc degeneration/herniation (=controls) were analyzed. Expression of MMPs-1, -2, -3, -7, -8, -9, -13 and TIMPs-1, -2 was analyzed using quantitative RT-PCR, normalized to the expression level of a house keeping gene (GAPDH). Gene expression patterns were correlated with MMP activity (in situ zymography), protein expression patterns (immunohistochemistry), degeneration score (routine histology) and clinical data. MMP-3 mRNA levels were consistently and substantially up-regulated in samples with histological evidence for disc degeneration. A similar but less pronounced up-regulation was observed for MMP-8. This up-regulation was paralleled by the expression of TIMP-1 and to a lesser extent TIMP-2. In general, these findings could be confirmed with regard to protein expression and enzyme activity. This study provides data on the gene and protein level, which highlights the key role of MMP-3 in the degenerative cascade leading to symptomatic disc degeneration and herniation. Control of the proteolytic activity of MMP-3 may, therefore, come into the focus when aiming to develop new treatment options for early disc degeneration.
Keywords: Disc degeneration, MMP-3, Proteolytic activity, Quantitative real-time RT-PCR
Introduction
The intervertebral disc is composed of a dense connective tissue which can be divided into the fibrocartilaginous annulus fibrosus and the more gelatinous nucleus pulposus. The motion segment thereby functions as a shock absorber and resists tensile and torsion forces. The matrix is mainly made up of collagen fibers that are responsible for the tissue tension while intermingled proteoglycans provide flexibility and hydrate the disc. Recent systematic histo-morphological analysis provided circumstantial evidence that a disruption of the disc matrix occurs in the young adult age and that this is closely and significantly associated with age-related disc degeneration [5]. The occurrence of major clefts and tears are hallmarks of the degenerative cascade, which finally lead to a loss of extracellular disc matrix. These features have been shown to be linked to the expression and activity of major matrix metalloproteinases (MMPs) [16, 31, 36].
MMPs are a family of proteolytic enzymes that participate in the degradation of all major matrix components, including glycoproteins, proteoglycans and especially collagen. Under normal physiological conditions, the efficiency of these enzymes is regulated by their own activation mechanism as well as by endogenous tissue inhibitors of metalloproteinases (TIMP-1, -2, -3). In various pathological processes, however, a dysbalance between MMPs and TIMPs plays a pivotal role in tissue degradation and decomposition [3]. According to their substrate specificity and gene structure, the vast number of MMPs (up to now ~24 members) can be classified into six groups: the collagenases (MMP-1, -8, -13, and -18) are the only proteases that cleave native interstitial collagen. The gelatinases (MMP-2, and -9) digest denatured collagens and non-helical collagen in basement membranes, while the stromelysins (MMP-3, and -10) and matrilysins (MMP-7 and -26) mainly degrade the protein core of proteoglycans and small non-collagenous proteins. The membrane-type MMPs (MT-MMPs or MMP-14, -15, -16 and -17) and the so-called “minor MMPs” (e.g. enamelysin MMP-20 and epilysin MMP-28) have specific roles either in the activation of various proteases at the cell membrane (MT-MMPs) or in the organ-specific developmental processes (e.g. enamelysin MMP-20 at the dental development) [19, 34].
Recent studies have shown that particularly MMP-1, -2 and -3 are increasingly expressed in degenerated disc tissue samples as evidenced by biochemistry, immunohistochemistry (protein), in situ hybridization (mRNA expression) and enhanced enzymatic, gelatinolytic activity (in situ zymography) [1, 8, 9, 15, 18, 22, 29, 31, 33, 36]. Despite these preliminary data, it remains unclear which member(s) of the MMP family are mainly responsible for the matrix disruption and decomposition. Furthermore, the role of the endogenous MMP inhibitors, the TIMPs, is also widely unknown and first reports ascribe them an important function in the pathological misregulation of the proteolytic activity [16]. Although immunohistochemical techniques provide excellent data on tissue localizations, they do not allow a quantification of these processes.
In the present study, various MMPs/TIMPs were analyzed on the gene/protein level as well as with regard to their activity and data was correlated with morphological and clinical data. The aim of this study was to identify enzymes and their inhibitors that are involved in disc destruction, using a broad methodological spectrum and an extensive set of specimens from both degenerated/herniated and normal discs.
Materials and methods
Study population
This study was performed on a series of well-defined fresh surgical specimens to prevent the well-known rapid degradation of mRNA. The study group comprised of 37 lumbar specimens obtained during surgical procedures, i.e. lumbar discectomies and interbody fusions. In addition, we were able to obtain fresh lumbar disc tissue samples from two young, previously healthy adults (22 and 28 years old) with polytrauma and fracture of the vertebral bodies where the discs were removed during surgical stabilization procedures (controls). Both samples neither showed gross morphological alterations nor enhanced histological degeneration signs. In the patient group, it was recorded whether the disc specimen was obtained from a disc exhibiting a protrusion (contained) or whether it was an extruded or sequestrated disc (non-contained). The average age of the 21 females and 16 males in the study group was 53.8 years (range 29–89 years). All patients underwent surgery for disc protrusion, herniation or degenerative disc disease. The distribution of the samples across the disc levels was as follows: L2/3 (n = 6), L3/4 (n = 5), L4/5 (n = 15), L5/S1 (n = 11). Clinical data on low back pain history, related disability and imaging findings (radiographs, MRI) were available for all cases (Table 1).
Table 1.
Clinical and histological characterization of the study population
| # | Age | Sex | Level | Disc pathology | Imaging | Pain duration (weeks) | HDSa |
|---|---|---|---|---|---|---|---|
| 1 | 56 | Female | L4/5 | Contained | Disc degeneration | 9 | 7 |
| 2 | 54 | Female | L2/3 | Contained | Disc degeneration | 20 | 11 |
| 3 | 33 | Male | L4/5 | Non-contained | Sequestration | 6 | 12 |
| 4 | 30 | Female | L5/S1 | Non-contained | Extrusion | 1 | 8 |
| 5 | 70 | Female | L4/5 | Non-contained | Extrusion | 10 | 10 |
| 6 | 54 | Male | L4/5 | Contained | Disc degeneration | 8 | 9 |
| 7 | 37 | Female | L4/5 | Non-contained | Extrusion | 20 | 10 |
| 8 | 39 | Male | L4/5 | Non-contained | Extrusion | 14 | 12 |
| 9 | 29 | Female | L3/4 | Non-contained | Sequestration | 14 | 10 |
| 10 | 47 | Female | L5/S1 | Contained | Disc degeneration | 30 | 11 |
| 11 | 36 | Male | L5/S1 | Contained | Disc degeneration | 22 | 11 |
| 12 | 45 | Male | L5/S1 | Non-contained | Extrusion | 5 | 8 |
| 13 | 45 | Female | L5/S1 | Non-contained | Extrusion | 15 | 10 |
| 14 | 35 | Male | L3/4 | Contained | Protrusion | 9 | 11 |
| 15 | 40 | Female | L4/5 | Contained | Disc degeneration | 2 | 7 |
| 16 | 49 | Female | L5/S1 | Contained | Disc degeneration | 16 | 10 |
| 17 | 65 | Male | L4/5 | Contained | Disc degeneration | 20 | 11 |
| 18 | 48 | Female | L4/5 | Non-contained | Extrusion | 5 | 9 |
| 19 | 89 | Female | L5/S1 | Non-contained | Extrusion | 6 | 9 |
| 20 | 70 | Male | L3/4 | Non-contained | Extrusion | 20 | 11 |
| 21 | 49 | Female | L5/S1 | Contained | Disc degeneration | 3 | 7 |
| 22 | 58 | Female | L5/S1 | Non-contained | Extrusion | 2 | 6 |
| 23 | 67 | Female | L2/3 | Non-contained | Sequestration | 5 | 8 |
| 24 | 39 | Female | L4/5 | Non-contained | Extrusion | 4 | 9 |
| 25 | 65 | Female | L5/S1 | Non-contained | Sequestration | 4 | 7 |
| 26 | 69 | Male | L4/5 | Contained | Disc degeneration | 8 | 11 |
| 27 | 50 | Male | L3/4 | Contained | Disc degeneration | 4 | 9 |
| 28 | 77 | Male | L2/3 | Non-contained | Sequestration | 23 | 12 |
| 29 | 63 | Male | L4/5 | Non-contained | Extrusion | 26 | 13 |
| 30 | 62 | Male | L3/4 | Contained | Disc degeneration | 4 | 8 |
| 31 | 58 | Female | L5/S1 | Non-contained | Extrusion | 3 | 13 |
| 32 | 73 | Female | L5/S1 | Non-contained | Sequestration | 7 | 9 |
| 33 | 66 | Male | L2/3 | Non-contained | Sequestration | 15 | 11 |
| 34 | 48 | Female | L4/5 | Contained | Disc degeneration | 21 | 10 |
| 35 | 65 | Female | L4/5 | Non-contained | Sequestration | 18 | 10 |
| 36 | 71 | Male | L2/3 | Contained | Disc degeneration | 8 | 8 |
| 37 | 59 | Male | L3/4 | Contained | Protrusion | 5 | 6 |
aHDS modified according to Boos et al. [5]
Tissue preparation
After removal of the specimen, the material was immediately dissected into three parts:
The first portion of the specimen was immediately immersed in RNA-later solution (Qiagen, Hilden, Germany) in order to ensure its conservation within less than 15 min after removal.
The second portion was immediately fixed in 3.7% buffered formaldehyde (pH 7.4). Following routine fixation, this material was embedded in paraffin for the preparation of thin sections (2–4 μm), which were placed on silanized glass slides for routine and histochemical stainings (H&E, Elastica-van Gieson’s connective tissue stain, Alcian blue-PAS, Masson-Goldner) as well as immunohistochemical analysis as previously described [5].
The third portion of the material was immediately deep frozen at –80°C for the preparation of in situ zymography on freshly frozen sections.
RNA extraction and cDNA synthesis
From the tissue samples that had been fixed in RNA-later solution (1st portion of the material), total RNA was extracted using the RNeasy Protect Midi Kit (Qiagen, Hilden, Germany) according to the recommendations of the manufacturer. The tissue was homogenized in a mechanical homogenizer (Ultraturrax, IKA, Staufen, Germany). The resulting tissue homogenate was centrifuged and the remaining pellet was digested with Proteinase K (10 μg/ml, 15 min, room temp.; Qiagen, Hilden, Germany) before being applied to a RNeasy Midi column together with the supernatant from the centrifugation step. Following extraction, the RNA was quantified photometrically and cDNA was synthesized from equal concentrations of each RNA preparation using oligo dT primers provided in the cDNA Synthesis Kit (First Strand cDNA Synthesis Kit, Pharmacia Biotech, Freiburg, Germany) following the manufacturer’s instructions.
Real-time quantitative RT-PCR
Quantitative RT-PCR was performed on a Light-Cycler (Roche, Mannheim, Germany) using specific primers in combination with the Light-Cycler FastStart Master Sybr®Green I (Roche). The Light Cycler-Primer Sets for MMP-1, -2, -3, -7, -8, -13 and that of tissue inhibitors (TIMPs) TIMP-1 and -2 target genes as well as for a series of housekeeping genes were purchased from Search LC (Heidelberg, Germany). PCR conditions were set according to the manufacturer’s instructions provided with the primer sets.
Prior to the quantification of the target genes, an appropriate house keeping gene had to be determined. Since previous studies had shown differences between various parameters [2], we performed a pre-test by comparative analysis of various usually applied genes, such as β-actin, HPRT, cyclophillin B, PBGD, glucose-6-phosphate (G6P) and GAPDH. Out of those six genes that are usually used as house keeping genes, β-actin, HPRT, cyclophillin B, PBGD and G6P revealed differences between various samples which are consistent with previous observations on cell cultures of tumor cells [2]. Due to the extensive testing on disc tissue, GAPDH proved to be the most constant and replicable parameter so that this housekeeping gene was selected for all subsequent experiments. Technical details have previously been reported [2].
For calculation of the MMP/TIMP target gene expressions, the number of transcripts obtained by quantitative RT-PCR was divided by the number of GAPDH housekeeping gene transcripts. In this manner, target gene expressions for all IVD samples were normalized giving transcription level results that could be compared with each other. All amplification reactions were performed in triplicate. Mean and standard deviation were calculated.
Immunohistochemistry
The immunostainings were essentially performed as described previously in detail [12, 15–17]. For the localization of MMPs-1, -2, -3 and -9, we used specific polyclonal antibodies (MMP-1, Alexis, Switzerland; MMP-2, -3, -9, BioVision, CA, USA). Briefly, pretreated deparaffinized slides were incubated with the specific primary antibody. Following washing steps, a secondary antibody system coupled with the avidin–biotin complex (Vector, Burlingame, USA) was used in order to localize the antigen. As a chromogenic substrate, we used diaminobenzidine (DAKO, Hamburg, Germany).
Control sections using either non-specific immunoglobulins of comparable concentration (“isotype control”) or after omission of the specific primary antibody (“blank control”) were run in parallel.
In situ zymography
In situ zymography was performed as an internal control for the biological activity of the enzymes under investigation. We localized the gelatinolytic activity in tissue sections of fresh disc samples. This technique was applied as previously described [36] which is a slight modification of the in situ zymography method used widely in oncology [20, 21, 26]. Briefly, standardized, 5-μm-thick frozen sections of the fresh material were placed on 1.5% gelatine films (which contain 12% polyacrylamide for the polymerization of the gel) and incubated in a wet chamber for 16 h. Subsequently, the slides were stained with Poinceau red (Merck, Darmstadt, Germany) as previously described [36] thereby avoiding over-staining effects. The loss of staining was scored by two independent observers with respect to the histological tissue alterations in a semi-quantitative manner. Since the gelatine films differed slightly in thickness, the bleaching of the sample area was normalized to the gelatine staining surrounding the sample-layered area. Similarly, any slight differences in the thickness of the sections were also normalized with respect to the surrounding staining intensity. Thereby, mainly the gelatinolytic activity of MMPs is detected, i.e. the activity of MMP-2 and -9 is monitored. However, recently [2] it has been shown that high activity levels of stromelysins (MMP-3, -10, -11) can also induce gelatine degradation.
Evaluation and statistical analysis
All slides of the routine stainings were first subjected to a qualitative examination to determine the histological degeneration score (HDS) as previously described [5]. The observations were classified as following: cell proliferation (clustering of chondrocytes), tear and cleft formation, granular matrix changes and mucoid degeneration of the ground substance. Cell death was omitted from the scoring because it was often difficult to reliably assess this parameter in the surgical specimens. A distinction was made between annular and nuclear disc tissue on the basis of histo-morphological criteria, especially density and arrangement of the collagen network, which was evaluated under polarized light, as previously described [5].
For analysis of gene expression results, we first compared the absolute values, but also evaluated the level of amplification using five levels: none (no amplification or minimal amplification, maximum 4-fold base line level); minor (5–50-fold base line level), moderate (50–500-fold base line level), strong (500–5,000 100-fold amplification levels) and very strong (>5,000-fold base line level) each compared to the housekeeping gene level. The immunohistochemical stainings were evaluated morphometrically on a semi-quantitative approach. The mean number of stained cells per 100 cells was evaluated in nuclear and annular regions as done in previous studies [24, 25]. The in situ zymographic slides were evaluated semi-quantitatively with respect to the extent of the gelatinolytic activity. Therefore, the reduction of gelatine staining in comparison to the surrounding background staining of the gelatine film was semi-quantitatively scored as 0 = none, 1 = minor, 2 = intermediate, 3 = major.
The correlation of mRNA levels and clinical/morphological data was explored with a Spearman’s rank correlation test. Similarly, the inter-correlation of the MMPs and TIMPs was investigated with a Spearman test. The level of statistical significance was set at 0.05, two-tailed.
Results
Histological observations and determination of the HDS
The histo-morphological appearance of the material was almost identical to that described in previous studies on surgical material [5, 36]. Accordingly, both the qualitative extent of changes (chondrocyte proliferation, cleft formation, granular changes, mucoid matrix degeneration) and the ranking of the extent of those alterations provided very similar results as seen in previous comparable cases (Table 1) [5]. The present study material was assumed to be representative with respect to the histopathological changes for age-related disc degeneration. Furthermore, no significant difference was noted between disc tissue samples with and without herniation in terms of degeneration grade.
Gene expression
In this analysis, mRNA levels were determined by normalizing the transcripts of the MMP and TIMP target genes by the housekeeping gene GAPDH which had proven to provide a constant expression level in experimental settings as previously described [2].
The assessments on the two samples from previously asymptomatic trauma patients (controls) showed in both cases either no detectable or very low levels of MMP gene expression ranging for all MMPs tested between 0- and 4-fold increase when compared to GAPDH, suggesting an either absent or minimal expression of all MMPs. Based on this data, our threshold for non-increased mRNA levels was set at 5-fold.
In symptomatic patients, large variations in gene expression were observed related to the individuals and MMPs. None of the investigated MMPs and TIMPs exhibited a correlation to the type of pathology, i.e. disc degeneration without herniation, disc protrusion, disc extrusion or sequestration. Similarly, no statistical significant difference was observed when comparing contained and non-contained disc herniations (Table 2).
Table 2.
Correlation matrix of clinical and morphological findings with mRNA expression levels of MMPs and TIMPs
| Variable | MMP-1 | MMP-2 | MMP-3 | MMP-7 | MMP-8 | MMP-9 | MMP-13 | TIMP-1 | TIMP-2 |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| rho | –0.291 | –0.176 | 0.363* | –0.148 | –0.222 | 0.054 | –0.224 | –0.460** | –0.357* |
| sig. | 0.081 | 0.297 | 0.027 | 0.382 | 0.186 | 0.752 | 0.183 | 0.004 | 0.030 |
| Age | |||||||||
| rho | 0.118 | 0.078 | –0.147 | –0.043 | –0.077 | 0.207 | 0.059 | 0.071 | –0.160 |
| sig. | 0.488 | 0.647 | 0.386 | 0.800 | 0.650 | 0.219 | 0.731 | 0.676 | 0.345 |
| Pain duration | |||||||||
| rho | –0.124 | –0.112 | 0.444** | 0.251 | 0.325* | –0.051 | –0.010 | –0.127 | –0.036 |
| sig. | 0.465 | 0.510 | 0.006 | 0.134 | 0.050 | 0.764 | 0.953 | 0.455 | 0.832 |
| Disc pathology | |||||||||
| rho | –0.049 | –0.074 | –0.050 | 0.031 | –0.037 | 0.101 | –0.052 | 0.003 | –0.166 |
| sig. | 0.772 | 0.663 | 0.770 | 0.857 | 0.829 | 0.552 | 0.761 | 0.986 | 0.326 |
| Disc level | |||||||||
| rho | 0.097 | –0.045 | –0.232 | 0.244 | 0.018 | –0.054 | –0.050 | 0.089 | 0.029 |
| sig. | 0.568 | 0.791 | 0.167 | 0.146 | 0.918 | 0.753 | 0.770 | 0.601 | 0.864 |
| HDS score | |||||||||
| rho | –0.187 | –0.100 | 0.600** | 0.123 | 0.342* | 0.040 | –0.199 | –0.036 | 0.014 |
| sig. | 0.267 | 0.554 | 0.0001 | 0.469 | 0.039 | 0.815 | 0.238 | 0.832 | 0.936 |
| Cell proliferation | |||||||||
| rho | –0.006 | 0.092 | 0.464** | 0.059 | 0.141 | –0.041 | –0.156 | –0.045 | –0.136 |
| sig. | 0.970 | 0.589 | 0.004 | 0.729 | 0.405 | 0.811 | 0.356 | 0.789 | 0.422 |
| Tears/clefts | |||||||||
| rho | –0.041 | –0.071 | 0.365* | 0.130 | 0.356* | –0.029 | –0.108 | 0.096 | –0.001 |
| sig. | 0.810 | 0.675 | 0.026 | 0.442 | 0.031 | 0.867 | 0.524 | 0.570 | 0.996 |
| Granular changes | |||||||||
| rho | –0.249 | –0.090 | 0.292 | –0.045 | 0.147 | –0.011 | –0.188 | 0.001 | 0.138 |
| sig. | 0.137 | 0.597 | 0.080 | 0.790 | 0.385 | 0.947 | 0.265 | 0.996 | 0.414 |
| Mucoid degeneration | |||||||||
| rho | –0.041 | –0.164 | 0.454** | 0.281 | 0.318 | 0.071 | 0.052 | –0.086 | –0.063 |
| sig. | 0.809 | 0.332 | 0.005 | 0.092 | 0.055 | 0.678 | 0.761 | 0.612 | 0.709 |
| MMP-1 | |||||||||
| rho | 1.000 | 0.716** | –0.178 | 0.265 | –0.319 | 0.184 | 0.686** | 0.393* | 0.439** |
| sig. | 0.0001 | 0.291 | 0.113 | 0.054 | 0.274 | 0.0001 | 0.016 | 0.006 | |
| MMP-2 | |||||||||
| rho | 0.716** | 1.000 | 0.047 | 0.144 | –0.336* | 0.178 | 0.562** | 0.359* | 0.321 |
| sig. | 0.0001 | 0.781 | 0.396 | 0.042 | 0.291 | 0.0001 | 0.029 | 0.052 | |
| MMP-3 | |||||||||
| rho | –0.178 | 0.047 | 1.000 | 0.074 | 0.242 | 0.184 | 0.146 | –0.281 | –0.237 |
| sig. | 0.291 | 0.781 | 0.662 | 0.149 | 0.276 | 0.389 | 0.092 | 0.158 | |
| MMP-7 | |||||||||
| rho | 0.265 | 0.144 | 0.074 | 1.000 | 0.106 | –0.038 | 0.279 | 0.193 | 0.162 |
| sig. | 0.113 | 0.396 | 0.662 | 0.534 | 0.821 | 0.095 | 0.253 | 0.338 | |
| MMP-8 | |||||||||
| rho | –0.319 | –0.336* | 0.242 | 0.106 | 1.000 | 0.141 | –0.328* | –0.018 | 0.033 |
| sig. | 0.054 | 0.042 | 0.149 | 0.534 | 0.405 | 0.047 | 0.916 | 0.846 | |
| MMP-9 | |||||||||
| rho | 0.184 | 0.178 | 0.184 | –0.038 | 0.141 | 1.000 | 0.336* | –0.100 | –0.114 |
| sig. | 0.274 | 0.291 | 0.276 | 0.821 | 0.405 | 0.042 | 0.556 | 0.502 | |
| MMP-13 | |||||||||
| rho | 0.686** | 0.562** | 0.146 | 0.279 | –0.328* | 0.336* | 1.000 | 0.205 | 0.263 |
| sig. | 0.0001 | 0.0001 | 0.389 | 0.095 | 0.047 | 0.042 | 0.223 | 0.116 | |
| TIMP-1 | |||||||||
| rho | 0.393* | 0.359* | –0.281 | 0.193 | –0.018 | –0.100 | 0.205 | 1.000 | 0.482** |
| sig. | 0.016 | 0.029 | 0.092 | 0.253 | 0.916 | 0.556 | 0.223 | 0.003 | |
| TIMP-2 | |||||||||
| rho | 0.439** | 0.321 | –0.237 | 0.162 | 0.033 | –0.114 | 0.263 | 0.482** | 1.000 |
| sig. | 0.006 | 0.052 | 0.158 | 0.338 | 0.846 | 0.502 | 0.116 | 0.003 | |
* Correlation is significant at the 0.05 level (two-tailed)
** Correlation is significant at the 0.01 level (two-tailed)
Stromelysins (MMP-3)
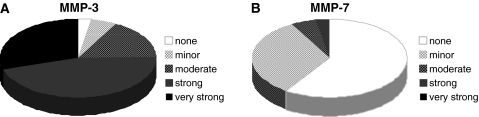
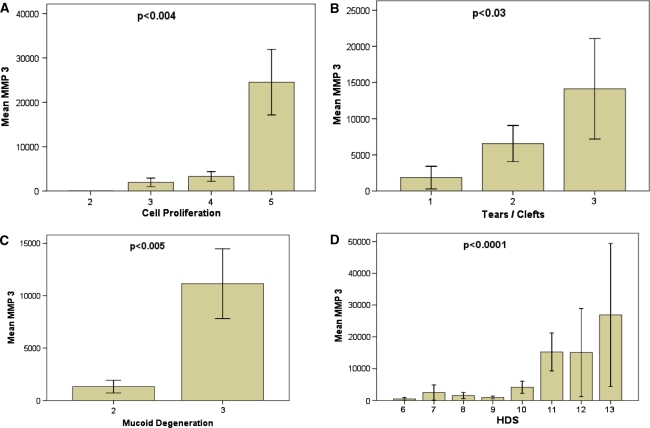
All but one case showed increased MMP-3 (previously termed stromelysin-1) levels, with the strongest increase in mRNA expression (Fig. 1) compared to all other MMPs analyzed (up to approx. 50,000-fold increase, mean 7429). The expression of MMP-3 was more elevated in males than females (rho = 0.363, p = 0.027) and in patients with a longer duration of the current pain episode (rho = 0.444, p = 0.006). In addition, MMP-3 showed the strongest correlation (rho = 0.6, p < 0.001) to histo-morphological matrix alterations (Fig. 2; Table 2).
Fig. 1.
Expression profile of MMP-3 and MMP-7. Pie charts representing the amount of cases with an increase of RNA levels for MMP-3 (a) and MMP-7 (b) compared to the housekeeping gene. A 50-fold increase was termed minor, up to 500-fold moderate, up to 5,000-fold strong and more than 5,000-fold very strong
Fig. 2.
Correlation of MMP-3 with histo-morphologic alterations. Histo-morphologic age-related alterations of the disc indicated by cell proliferation (a), tears and clefts formation (b), and mucoid degeneration (c) exhibited a significant correlation to the increase in mRNA expression for MMP-3. This was also reflected in the overall histologic degeneration score (d). Error bars represent standard error of the mean
Matrilysins (MMP-7)
Gene expression levels of MMP-7 were elevated in 15 out of 37 samples (mean 58). Even though the substrate specificity of MMP-7 and MMP-3 is very similar, we could not detect a significant correlation between their expression levels. In contrast to MMP-3, MMP-7 did not exhibit any correlation to histo-morphological matrix alterations or clinical findings (Fig. 1; Table 2).
Collagenases (MMP-1, -8, -13)
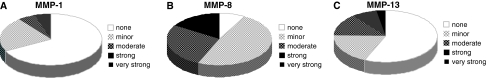
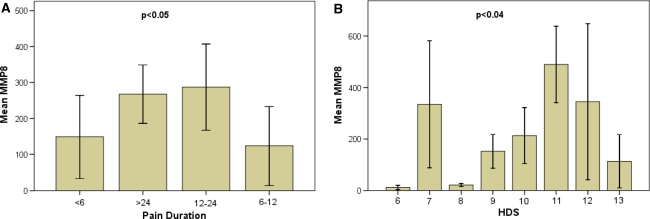
Although MMP-1, -8 and-13 all belong to the same family of MMPs, i.e. collagenases, they did not show a comparable pattern and correlations could only be observed between some of the family members. However, no significant correlation was observed with regard to the disc pathology, i.e. disc sequester, contained/non-contained disc. Gene expression of MMP-1 (previously termed “interstitial collagenase”) was minorly or moderately enhanced in 12 cases and strongly enhanced in 2 cases (mean 56) (Fig. 3). Expression levels of MMP-1 strongly correlated with MMP-13 (rho = 0.686, p < 0.0001), but not with MMP-8 (Table 2). In contrast, levels for MMP-8 increased minorly in 18 and moderately in 10 cases and strongly in 6 cases (overall mean 261) (Fig. 3) and showed a positive correlation to the pain duration (rho = 0.325, p < 0.05) and histo-morphological degeneration (rho = 0.342, p = 0.039) (Fig. 4). MMP-8 was not correlated with MMP-1, but we found an inverse correlation with MMP-13 levels (rho = –0.328, p = 0.047).
Fig. 3.
Expression profile of collagenases MMP-1, MMP-8 and MMP-13. Pie charts representing the amount of cases with an increase of RNA level for MMP-1 (a), MMP-1 (b) and MMP-13 (c) compared to the housekeeping gene. A 50-fold increase was termed minor, up to 500-fold moderate, up to 5,000-fold strong and more than 5,000-fold very strong
Fig. 4.
Correlation of MMP 8 with pain duration and histo-morphologic alterations. Pain duration (a) and histo-morphologic age-related alterations (HDS) (b) exhibited a significant correlation to the increase in mRNA expression for MMP-8. Error bars represent standard error of the mean
Gelatinases (MMP-2, -9)
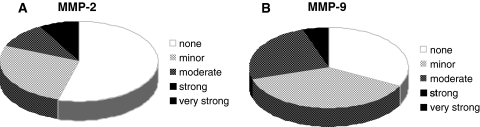
In all investigated IVD tissue samples, gelatinase mRNA levels were generally lower compared to collagenase mRNA levels. There was neither a significant correlation between MMP-2 and MMP-9 nor with clinical parameters or histo-morphological parameters. MMP-2 expression exerted a minor or moderate increase in 14 cases and a strong increase in 3 cases (mean 80) (Fig. 5). MMP-2 correlated with all collagenases, especially with MMP-1 (rho = 0.716, p < 0.0001) and MMP-13 (rho = 0.562, p < 0.0001) and to a lesser extent and inversely with MMP-8 (rho = –0.336, p = 0.042) (Table 2).
Fig. 5.
Expression profile of gelatinases MMP-2 and MMP-9. Pie charts representing the amount of case with an increase of RNA level for MMP-2 (a) and MMP-9 (b) compared to the housekeeping gene. A 50-fold increase was termed minor, up to 500-fold moderate, up to 5,000-fold strong and more than 5,000-fold very strong
Tissue inhibitors of MMPs (TIMP-1, -2)
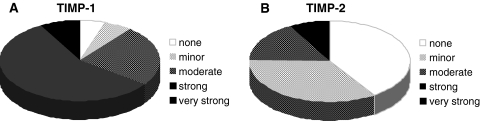
Besides the analysis of major MMPs, we analyzed the expression levels for the two most important endogenous MMP inhibitors, TIMP-1 and -2. We observed a heterogeneous expression pattern of the two inhibitors with lower levels of TIMP-2 in the majority of the IVD specimen. TIMP-1 mRNA levels were enhanced in all but two specimens, with a moderate increase in 9 cases, a strong increase in 21 cases and a very strong increase in 3 cases (mean 2726) (Fig. 6). TIMP-1 positively correlated with MMP-1 expression levels (rho = 393, p = 0.016). For TIMP-2, a moderate increase was noted in 16 cases and a strong increase in 6 cases (120- and 2,400-fold increase, mean 135). TIMP-2 also showed a positive correlation to MMP-1, similar to TIMP-1 (rho = 0.439, p = 0.006). For both inhibitors, no correlation with type and level of disc pathology or histo-morphological parameters could be detected.
Fig. 6.
Expression profile of TIMP-1 and TIMP-2. Pie representing the amount of cases with an increase of RNA level for TIMP-1 (a) and TIMP-2 (b) compared to the housekeeping gene. A 50-fold increase was termed minor, up to 500-fold moderate, up to 5,000-fold strong and more than 5,000-fold very strong
Immunohistochemistry
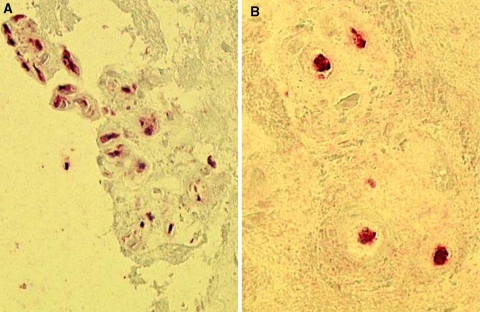
In order to investigate protein levels, we additionally performed an immunohistochemical analysis of the formalin-fixed and paraffin-embedded material. Due to limited amount of material, we focused on MMP-1, -2, -3 and -9. All enzymes could be detected, with highest values for MMP-3 (mean 65%, range 15–85%, positive cells) (Fig. 7a), supporting the data obtained by gene expression. MMP-1 was also detectable (mean 55%, range 10–65%, positive cells) (Fig. 7b), while nuclear staining of MMP-2 and -9 was less pronounced (MMP-2: mean 20%, range 0–40%; MMP-9: mean 10%, range 0–30%, positive cells). There was mainly but not exclusively a positive staining for the MMPs in those areas with tissue disruption (clefting). There was no statistical significant association between the number of labeled cells, mRNAs expression levels (MMPs) and in situ zymography results.
Fig. 7.
Immunohistochemical detection of MMP-1 and MMP-3. a Immunostaining for MMP-3 at a cleft margin with numerous positively labeled cells (red staining, case #28). b Several nuclear cells are also positive for MMP-1 (red staining, case #5)
In situ zymography
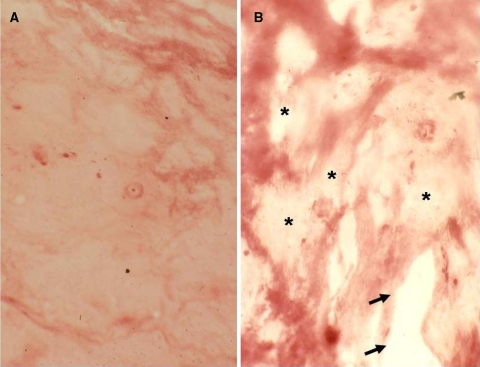
Out of the 37 samples with molecular analysis, 24 samples were available with sufficient and representative material for in situ zymography. In eight cases, either no material was available for this analysis or the material was too small/restricted. In five cases, the material was not representative for the material for molecular analysis. In 18 of the 24 evaluated samples, an intermediate to major gelatinolytic activity was seen, while the other 6 cases did only show minor gelatinolysis (Fig. 8a). No direct correlation was seen with regard to the expression levels of the gelatinases. Enzyme activity was mainly seen in areas of the nucleus pulposus, but less frequently annular regions were affected. All disc samples with major histological signs of disc degeneration, in particular with tear and cleft formation, revealed enhanced proteolytic activity as determined by in situ zymography (Fig. 8b). There was an obvious association of gelatinolytic activity and cleft formation.
Fig. 8.
In situ zymography of intervertebral disc samples. a Section through a disc sample with minimal gelatinolytic activity with mostly preserved gelatin matrix (red staining, Score 1, case #22). b Extensive gelatinolytic activity (HDS 10, case #5) with loss of the red-stained gelatin matrix substrate (asterisk). Note focal clefts along the lightly stained activity areas (arrows)
Discussion
Low back pain is one of the most frequent causes for work failure in Western industrial countries. Despite this enormous socio-economic burden, the causes for disc degeneration are still poorly understood, especially with regard to the molecular events that are involved. In particular, the processes that lead to the destruction and resorption of extracellular disc matrix are unclear. Recently, extensive analyses have been undertaken to fill the above-mentioned knowledge gap. An increasing puzzle of data on distinct molecular changes has been described during disc degeneration. This puzzle comprises of enhanced apoptosis of annular disc cells [30], occurrence of phagocytic phenotypic disc cell changes [25], up-regulation of various cytokines, such as TNF-α [35] and TGF-β [23], significant changes of extracellular matrix composition [24, 32] and significantly altered expression and activity of MMPs [16, 31, 36]. The latter have recently been postulated to play a pivotal role in destruction and resorption of the degenerating disc [36].
The MMPs are a group of at least 24 different enzymes that are unique in their capability to cleave various extracellular matrix constituents. MMP enzyme activity is controlled by endogenous inhibitors, the “tissue inhibitors of metalloproteinases” (TIMPs), which comprise four members. TIMP-1 and -2 are the two most important and widespread proteins involved in human MMP regulation. TIMP-3 and -4 are found in various tissues, but seem to be of minor clinical significance. Although recent research activities started to put an emphasis on elucidating the role as well as the interplay of MMPs and TIMPs, studies up to now used single detection methods or small sample sizes; in addition, samples were mostly taken from patients with degenerative disc disease, but no comparison to healthy tissue was sought [16, 31, 36]. Therefore, the aim was to perform a comprehensive study on the role and the interplay of MMPs and TIMPs with regard to mRNA levels, protein levels and enzyme activity, using not only discs from patients with disc disease, but also putative healthy discs.
Recent studies used immunolocalization, in situ hybridization and in situ zymography for the study of MMP/TIMP localization, synthesis and activation in disc tissue samples [16, 31, 36]. These studies provided considerable insight into the role of the MMP/TIMPs in discs:
Major MMPs are expressed in enhanced amounts in disc tissue with signs of disc degeneration.
MMPs are synthesized by local cells.
Synthesis seems to be up-regulated in discs with degenerative lesions.
Enzymatic activity as evidenced by gelatinolytic activity is mostly seen in areas of tissue destruction (such as cleft and tear formation).
TIMPs are synthesized in parallel with major MMPs.
The amount of TIMPs synthesized seems to be up-regulated in correlation to disc tissue degeneration.
Despite some recent investigations by Le Maitre et al. [16], it still remains largely unclear whether TIMP expression really parallels expression of major MMPs or whether it is lower or higher than the MMP levels. Furthermore, the role of the various individual MMPs remains unclear, since morphological techniques provide no—or at best only partially—quantitative data on the MMP changes. In contrast, biochemical analytical techniques, such as gel zymography, provide semi-quantitative data on distinct MMPs; however, they do not allow insight into the regional distribution of the enzymes [6]. In order to overcome these drawbacks, we designed this study that uses a combination of morphology and quantitative analytical procedures. Since tissue samples for both analyses should be small to avoid tissue bias due to substantial tissue heterogeneity [1], we chose the quantitative RT-PCR technique.
Up to now, quantitative PCR has mainly been applied to experimental settings, such as animal models of disc degeneration [1, 10, 17, 28, 33, 37]. In summary, these models provide clear evidence that either acute or more protracted lesion of annular and/or nuclear disc tissue leads to an initial up-regulation of major MMPs, such as MMP-3 [18], MMP-13 [17, 28] and MMPs-1 and -9 [1]. In contrast, several other studies showed unchanged or even slightly decreased expression levels for MMPs, such as MMP-1 [37] in particular experimental settings, such as under high pressure (30 atm cyclic pressure). In general, the changes in the experimental studies could be related to pre-operative baseline expression levels and the changes differed between 2- and 8-fold.
In contrast, only very few studies cover the determination of mRNA levels for different MMPs in human tissue samples. Very recently, Bertram et al. [4] studied scoliotic disc samples for the expression of several MMPs (MMP-3, -13 and -17) along with various anabolic tissue markers. No differences were seen in the expression levels between scoliotic tissue samples and non-scoliotic control discs from trauma operation specimens. However, no analysis was made with regard to age-related degeneration of different extent and no absolute values were given for the expression levels of MMPs in relation to a house keeping gene. In a further study on in vivo disc degeneration analysis, Cs-Szabo et al. [7] used quantitative RT-PCR to analyze proteoglycan mRNA changes in disc tissue samples. However, no morphological correlation was performed so that only very limited information was available on that issue.
With the present study, we were able to show that the individual values for the various MMPs differed significantly from sample to sample. However, there was a clear tendency showing that expression of distinct MMPs was significantly higher than others. This holds particularly true for MMP-3 which was dramatically up-regulated in all samples analyzed. On the contrary, MMP-7 and -9 were only moderately elevated in few samples. The remaining MMPs tested were intermediate with significant differences between samples and various MMPs from case to case. Furthermore, the two TIMPs tested similarly revealed an up-regulation which was more pronounced for TIMP-1 than for TIMP-2. These observations clearly demonstrate (and partly confirm) that
MMPs and TIMPs are up-regulated in disc tissue samples with signs of disc degeneration;
there is a correlation between histological disc degeneration and the expression of isolated MMPs, particularly MMP-3;
there is evidence for the biologic activity of MMP-2 and -9 as shown by the enhanced gelatinolytic activity during in situ zymography.
In order to test whether findings on the mRNA level can be confirmed on the protein level, we used an immunohistochemical approach for the most relevant MMPs. As the size of the tissue samples is very limited, staining with specific antibodies was chosen, rather than analysis of protein extracts via immunoblotting. Even though data could only be evaluated in a semi-quantitative manner, results on the protein level confirmed the extensive expression of various MMPs seen on the gene expression level. Again, MMP-3 was most extensively expressed, while the other MMPs revealed less extensive staining. A direct comparison on an individual basis was not performed since the (dynamic) transcriptional expression levels cannot directly be compared to the (static) immunohistochemical protein data. Furthermore, mRNA levels could be quantified numerically, while immunohistochemical stainings were evaluated by a morphometric approach. Nevertheless, our data clearly support the particular role of MMP-3 on various expression levels.
Our study identified major MMPs using disc material from symptomatic individuals with back pain. This is corroborated by the findings on two samples from acute trauma victims without histological evidence for relevant age-related disc alterations. In these samples, the expression levels for all MMPs were either not detectable or very low. This confirms that in symptomatic individuals a “veritable” increase in enzyme synthesis is present. However, since it is extremely difficult to obtain “normal” control material, the comparison between “normal” and “pathological” or “symptomatic” must be performed very cautiously. Despite this fact, we provide clear evidence that MMP-3 plays a central role during disc tissue degradation. In addition, MMP-8 is similarly up-regulated, showing a more consistent increase in the values than other MMPs. These data clearly identify few MMPs to be more involved in disc degradation than others. In addition, the immunohistochemical and in situ zymographic investigations clearly confirm these notions although there are some differences in the extent and level of expression between the transcriptional and the protein (and activity) levels. These differences, however, may be due to the fact that additional modulations, such as protein secretion and enzyme degradation, may interfere with the protein and activity data.
It is well known that MMPs possess different pH optima, even though exact values do not exist for all families and their members. However, it is known that MMP-3, compared to most other MMPs, shows a rather narrow pH window, with its optimum being around pH 5.5–6.5 [11], indicating that MMP-3 is particularly active under microenvironmental conditions that can be found during disc degeneration. Taking into consideration that many other MMPs possess significantly higher pH optima, e.g. pH 8.5 for MMP-2 [27], it becomes evident why these enzymes may not play a pivotal role during disc degeneration where pH levels are known to drop as low as pH 5.7.
Our data fits very well with that obtained in an in vitro model of disc cell alteration that mimics in vitro disc degeneration [12]. In those experiments, high levels of MMP-3 and -7 had been observed to be expressed in herniated disc material with MMP-3 leading to resorption of the disc material. It was furthermore shown that disc cells were stimulated by macrophages to produce significantly more MMP-3 than in the unstimulated situation. In turn, it was assumed that MMP-3 may act as a macrophage chemo-attractant. The application of recombinant MMP-3 leads to enhanced resorption processes [14], which is similar to that observed in vivo. Haro et al. [14] described TNF-α as a major inducer of MMP-3. Similarly, the generation of soluble TNF-α was essential for the induction of MMP-3 in disc co-cultures [13] which in turn was necessary to attract macrophage infiltration into herniated disc material. We have recently shown that TNF-α is also considerably expressed in degenerated non-herniated disc tissue [35]. We hypothesize that intradiscal activation of the TNF-α-cytokine network may be involved not only in mediating discogenic pain but also in stimulating a MMP-mediated cascade, leading to progressive tissue destruction.
We also provide quantitative data for the up-regulation of major endogenous MMP inhibitors, i.e. TIMPs-1 and -2, which are differently expressed between various samples. In general, the expression levels of TIMP-1 were higher than that of TIMP-2. Although we have as yet no data on the biological role of this up-regulation, we suggest that a dysbalance between MMPs and TIMPs may have an impact on the maintenance of the extracellular matrix. This is well in line with other previous observations which had included TIMP-3 as well [16].
Clinical relevance
In the present paper, we first provide (semi-)quantitative data on the gene and protein level, indicating an up-regulation of MMP-3 and -8 in clinical disc samples with a significant correlation to histo-morphologic matrix destruction. The central role of MMP-3 activation during disc degeneration deserves further intense study. With increasing knowledge, these MMPs may come into focus of a therapeutic approach that treats or at least slows down the degenerative cascade leading to low back pain.
Acknowledgments
This study was supported by a grant from the SynOs Stiftung für Orthopädische Chirurgie, Münsingen, Switzerland (Grant no. 1028-01) and the AOSPINE Switzerland SRN 02/103 and AOSBRC-07-03.
References
- 1.Anderson DG, Izzo MW, Hall DJ, Vaccaro AR, Hilibrand A, Arnold W, Tuan RS, Albert TJ. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine. 2002;27:1291–1296. doi: 10.1097/00007632-200206150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Bachmeier BE, Albini A, Vene R, Benelli R, Noonan D, Weigert C, Weiler C, Lichtinghagen R, Jochum M, Nerlich AG. Cell density-dependent regulation of matrix metalloproteinase and TIMP expression in differently tumorigenic breast cancer cell lines. Exp Cell Res. 2005;305:83–98. doi: 10.1016/j.yexcr.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Bachmeier BE, Iancu CM, Jochum M, Nerlich AG. Matrix metalloproteinases in cancer: comparison of known and novel aspects of their inhibition as a therapeutic approach. Expert Rev Anticancer Ther. 2005;5:149–163. doi: 10.1586/14737140.5.1.149. [DOI] [PubMed] [Google Scholar]
- 4.Bertram H, Steck E, Zimmerman G, Chen B, Carstens C, Nerlich A, Richter W. Accelerated intervertebral disc degeneration in scoliosis versus physiological ageing develops against a background of enhanced anabolic gene expression. Biochem Biophys Res Commun. 2006;342:963–972. doi: 10.1016/j.bbrc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 5.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Crean JK, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22:2877–2884. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 7.Cs-Szabo G, Ragasa-San Juan D, Turumella V, Masuda K, Thonar EJ, An HS. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine. 2002;27:2212–2219. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 8.Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine. 1998;23:1612–1626. doi: 10.1097/00007632-199807150-00021. [DOI] [PubMed] [Google Scholar]
- 9.Gruber HE, Ingram JA, Hanley EN., Jr Immunolocalization of MMP-19 in the human intervertebral disc: implications for disc aging and degeneration. Biotech Histochem. 2005;80:157–162. doi: 10.1080/10520290500387607. [DOI] [PubMed] [Google Scholar]
- 10.Guehring T, Omlor GW, Lorenz H, Bertram H, Steck E, Richter W, Carstens C, Kroeber M. Stimulation of gene expression and loss of anular architecture caused by experimental disc degeneration—an in vivo animal study. Spine. 2005;30:2510–2515. doi: 10.1097/01.brs.0000186591.17114.e9. [DOI] [PubMed] [Google Scholar]
- 11.Gunja-Smith Z, Woessner JFJ. Activation of cartilage stromelysin-1 at acid pH and its relation to enzyme Ph optimum and osteoarthritis. Agents Actions. 1993;40:228–231. doi: 10.1007/BF01984067. [DOI] [PubMed] [Google Scholar]
- 12.Haro H, Crawford HC, Fingleton B, MacDougall JR, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-3-dependent generation of a macrophage chemoattractant in a model of herniated disc resorption. J Clin Invest. 2000;105:133–141. doi: 10.1172/JCI7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000;105:143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haro H, Komori H, Kato T, Hara Y, Tagawa M, Shinomiya K, Spengler DM. Experimental studies on the effects of recombinant human matrix metalloproteinases on herniated disc tissues—how to facilitate the natural resorption process of herniated discs. J Orthop Res. 2005;23:412–419. doi: 10.1016/j.orthres.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 17.MacLean JJ, Lee CR, Alini M, Iatridis JC. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120–1127. doi: 10.1016/j.orthres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Matsui Y, Maeda M, Nakagami W, Iwata H. The involvement of matrix metalloproteinases and inflammation in lumbar disc herniation. Spine. 1998;23:863–868. doi: 10.1097/00007632-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Nakada M, Nakamura H, Ikeda E, Fujimoto N, Yamashita J, Sato H, Seiki M, Okada Y. Expression and tissue localization of membrane-type 1, 2, and 3 matrix metalloproteinases in human astrocytic tumors. Am J Pathol. 1999;154:417–428. doi: 10.1016/S0002-9440(10)65288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura H, Ueno H, Yamashita K, Shimada T, Yamamoto E, Noguchi M, Fujimoto N, Sato H, Seiki M, Okada Y. Enhanced production and activation of progelatinase A mediated by membrane-type 1 matrix metalloproteinase in human papillary thyroid carcinomas. Cancer Res. 1999;59:467–473. [PubMed] [Google Scholar]
- 22.Nemoto O, Yamagishi M, Yamada H, Kikuchi T, Takaishi H. Matrix metalloproteinase-3 production by human degenerated intervertebral disc. J Spinal Disord. 1997;10:493–498. doi: 10.1097/00002517-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Nerlich AG, Bachmeier BE, Boos N. Expression of fibronectin and TGF-β1 mRNA and protein suggest altered regulation of extracellular matrix in degenerated disc tissue. Eur Spine J. 2004;14:17–26. doi: 10.1007/s00586-004-0745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nerlich AG, Schleicher E, Boos N. 1997 Volvo Award in basic science. Immunohistochemical markers of age-related changes in the human lumbar intervertebral disc. Spine. 1997;22:2781–2795. doi: 10.1097/00007632-199712150-00001. [DOI] [PubMed] [Google Scholar]
- 25.Nerlich AG, Weiler C, Zipperer J, Narozny M, Boos N. Immunolocalization of phagocytic cells in normal and degenerated intervertebral discs. Spine. 2002;27:2484–2490. doi: 10.1097/00007632-200211150-00012. [DOI] [PubMed] [Google Scholar]
- 26.Ohashi K, Nemoto T, Nakamura K, Nemori R. Increased expression of matrix metalloproteinase 7 and 9 and membrane type 1-matrix metalloproteinase in esophageal squamous cell carcinomas. Cancer. 2000;88:2201–2209. doi: 10.1002/(SICI)1097-0142(20000515)88:10<2201::AID-CNCR2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Okada Y, Morodomi T, Enghild JJ, Suzuki K, Yasui A, Nakanishi I, Salvesen G, Nagase H. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur J Biochem. 1990;194:721–730. doi: 10.1111/j.1432-1033.1990.tb19462.x. [DOI] [PubMed] [Google Scholar]
- 28.Omlor GW, Lorenz H, Engelleiter K, Richter W, Carstens C, Kroeber MW, Guehring T. Changes in gene expression and protein distribution at different stages of mechanically induced disc degeneration—an in vivo study on the New Zealand white rabbit. J Orthop Res. 2006;24:385–392. doi: 10.1002/jor.20055. [DOI] [PubMed] [Google Scholar]
- 29.Pockert AJ, Richardson SM, Le Maitre CL, Lyon M, Deakin JA, Buttle DJ, Freemont AJ, Hoyland JA. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60:482–491. doi: 10.1002/art.24291. [DOI] [PubMed] [Google Scholar]
- 30.Rannou F, Lee TS, Zhou RH, Chin J, Lotz JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A, Shyy JY. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164:915–924. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 32.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 33.Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, Kang JD. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5:14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 34.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 35.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine. 2005;30:44–53. doi: 10.1097/01.brs.0000174529.07959.c0. [DOI] [PubMed] [Google Scholar]
- 36.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. Expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenger KH, Woods JA, Holecek A, Eckstein EC, Robertson JT, Hasty KA. Matrix remodeling expression in anulus cells subjected to increased compressive load. Spine. 2005;30:1122–1126. doi: 10.1097/01.brs.0000162395.56424.53. [DOI] [PubMed] [Google Scholar]