Abstract
Few studies have investigated the factors related to the disability and physical function in degenerative lumbar spondylolisthesis using axially loaded magnetic resonance imaging (MRI). Therefore, we aimed to investigate the effect of axial loading on the morphology of the spine and the spinal canal in patients with degenerative spondylolisthesis of L4–5 and to correlate morphologic changes to their disability and physical functions. From March 2003 to January 2004, 32 consecutive cases (26 females, 6 males) with degenerative L4–5 spondylolisthesis, grade 1–2, intermittent claudication, and low back pain without sciatica were included in this study. All patients underwent unloaded and axially loaded MRI of the lumbo-sacral spine in supine position to elucidate the morphological findings and to measure the parameters of MRI, including disc height (DH), sagittal translation (ST), segmental angulation (SA), dural sac cross-sectional area (DCSA) at L4–5, and lumbar lordotic angles (LLA) at L1–5 between the unloaded and axially loaded condition. Each patient’s disability was evaluated by the Oswestry Disability Index (ODI) questionnaire, and physical functioning (PF) was evaluated by the Physical Function scale proposed by Stucki et al. (Spine 21:796–803, 1996). Three patients were excluded due to the presence of neurologic symptoms found with the axially loaded MRI. Finally, a total of 29 (5 males, 24 females) consecutive patients were included in this study. Comparisons and correlations were done to determine which parameters were critical to the patient’s disability and PF. The morphologies of the lumbar spine changed after axially loaded MRI. In six of our patients, we observed adjacent segment degeneration (4 L3–L4 and 2 L5–S1) coexisting with degenerative spondylolisthesis of L4–L5 under axially loaded MRI. The mean values of the SA under pre-load and post-load were 7.14° and 5.90° at L4–L5 (listhetic level), respectively. The mean values of the LLA under pre-load and post-load were 37.03° and 39.28°, respectively. There were significant correlations only between the ODI, PF, and the difference of SA, and between PF and the post-loaded LLA. The changes in SA (L4–L5) during axial loading were well correlated to the ODI and PF scores. In addition, the LLA (L1–L5) under axial loading was well correlated to the PF of patients with degenerative L4–L5 spondylolisthesis. We suggest that the angular instability of the intervertebral disc may play a more important role than neurological compression in the pathogenesis of disability in degenerative lumbar spondylolisthesis.
Keywords: Degenerative lumbar spondylolisthesis, Axially loaded MRI, Disability, Physical functioning, Segmental angulation
Introduction
Patients with degenerative lumbar spondylolisthesis may suffer from low back pain and disability, which greatly impacts their daily lives. It is most often seen in the lumbar spine at the L4/L5 level; slippage of vertebrae occurs if the facet joint locking mechanism fails, and will progress to a static or unstable condition over time [13].
The etiologies of disability are multifactorial in degenerative lumbar spondylolisthesis; the leading factors responsible for disability are spinal instability and neurological compromise [8]. Other pathological factors associated with disabilities in patients with degenerative lumbar spondylolisthesis include chemical factors such as inflammation, discogenic pain, facet arthropathy, and myofascial pain from ligaments or paraspinal muscles [8]. Detection of the relevant factors associated with disability or physical functioning in degenerative spondylolisthesis is very important. However, there is only limited clinical data on the roles of spinal instability and neurological compromise in degenerative spondylolisthesis-related disability. Dynamic radiographs are currently used for detecting spinal instability. However, Soini et al. suggested that dynamic radiographs may only have limited value for diagnosis of instability of the lumbar spine in cases with disc degeneration [15].
Axially loaded magnetic resonance imaging (MRI) or computed tomography (CT) simulates the upright position under normal gravity and mimics the condition of axial compression in the lumbar spine, enabling detection of dynamic or occult changes, such as mechanical compression of the dural sac or nerve root [3, 11]. Changes of the dural sac cross-sectional area (DCSA) of the spinal canal can be detected after axially loaded MRI or CT. Under axial loading, minimal alterations in intersegmental spatial relationships and more morphological changes could be found, including increased protrusion of the disc, decreased disc height, decreased DCSA and induced subluxation of the facet joint [3, 11]. Some changes could be correlated with clinical symptoms, and some would probably result in revised treatment protocols.
If spinal instability exists, patients with degenerative spondylolisthesis will exhibit mechanical back pain; their symptoms and signs are posture-related and may present as symptoms typical of spinal stenosis, such as intermittent claudication [14]. The Dynawell, a device for applying axial loading during computed tomography (CT) or MRI, was invented to simulate an upright lumbar spine [17]. Many studies related to the changes in the spinal canal in the normal and stenotic spine have been performed [4]. One study found that the DCSA and the morphology of the spinal canal differed in different states of loading. A significant decrease in the DCSA of asymptomatic subjects (56%) and spinal stenosis patients (76%) was seen [4]. Twenty-nine percent of symptomatic patients showed “additional valuable imaging information”, including DCSA changes > 15 mm2, synovial cysts, lateral recess stenosis and herniated intervertebral discs. About 80% of patients with lumbar stenosis had significant narrowing of the spinal canal (at least 15 mm2).
By using axially loaded MRI, we can detect the extent and changes of intervertebral disc height (DH), sagittal translation (ST), segmental angulation (SA), DCSA at L4–5, and lumbar lordotic angles (LLA) at L1–5 before and after loading, and the additional morphological changes under axial loading in degenerative lumbar spondylolisthesis.
However, the roles of these parameters in spinal disability and physical function have not yet been determined in patients with degenerative lumbar spondylolisthesis. We sought to understand the correlation between these parameters and the severity of physical disabilities and physical function; we therefore designed this prospective clinical study to determine which critical factors were responsible for the symptoms of disability and physical functioning in degenerative spondylolisthesis of L4–5, by means of axially loaded MRI.
Materials and methods
Thirty-two consecutive patients (6 males, 26 females), with diagnoses of degenerative L4–L5 spondylolisthesis, grade 1 or 2 slippage, were included in this study. Patients with degenerative scoliosis were excluded in this study. All patients had back pain without sciatica or leg pain, and had intermittent claudication.
Axial loading was done using the Dynawell instrument (Dynawell Int. AB, Billdal, Sweden). Patients were placed in the supine position without changing their position during examination and wore a harness with their feet positioned against a footplate on the compression device. By adjusting the side straps, we controlled the axial load on the lumbar spine. Axial compression of the lumbar spine was affected by loading the patient with 50% body weight on the foot-plate; this was maintained for 5 min before re-imaging to mimic the influence of gravity or loading on the lumbar spine in the upright position. We aimed to focus the geometrical factors on MRI, not on radiograph. All patients underwent preload MRI examinations (T1), and then immediately underwent axially loaded MRI (T1 and T2) for both sagittal and axial images of the lumbo-sacral spine. All patients underwent preload MRI examinations of the lumbo-sacral spine (1.5T Signa Cvi, GE medical systems, Milewaukee, Wis), and then immediately underwent axially loaded MRI by phase array spinal coil. The preload sequences included sagittal fast spin-echo T1-weighted (FSE T1 W) images (repetition time/echo time [TR/TE]: 350/10 ms; numbers of excitation [NEX]: 4; echo train: 3;4.0 thickness/0.4 space), sagittal FSE T2WI with fat saturation (TR/TE: 4000/97 ms; NEX: 4; echo train: 20; 4.0 thk/0.4 sp), axial FSE T1WI (TR/TE: 550/9 ms; NEX: 4; echo train: 2; 4.0thk/1.2 sp). The post-load sequences included sagittal FSET2WI with fat saturation (TR/TE 4000/97 ms; NEX: 4; echo train: 20; 4.0 thk/0.4 sp), axial FSE T1WI (TR/TE/excitations 550/9 ms; NEX: 4; echo train: 2; 4.0thk/1.2 sp), and axial FSE T2WI (TR/TE/excitations 8000/106 ms; NEX: 4; echo train: 20; 4.0 thk/1.2 sp). (FOV 28 × 28, matrix 256 × 512, etc.) Three patients were excluded from this study after axial loading, due to the occurrence of intolerable back pain in one patient, and numbness and sciatica in the other two; one of these two patients sustained persistent sciatica and numbness after axial loading. The electrophysiology study revealed lumbosacral radiculopathy. The sciatica improved 2 months later after conservative treatment. In the end, a total of 29 (5 males, 24 females) consecutive patients were included in this study.
By using axially loaded MRI, we planned to depict the possible morphological changes in detail, including the changes in DCSA, buckling of the ligamentum flavum, bulging of a herniated disc, narrowing of a lateral recess, asymmetric distortion of the dural sac, facet joint subluxation, facet joint synovial cyst, etc., in patients with degenerative spondylolisthesis.
DH is a good indicator for disc flexibility. ST and SA are two factors associated with spinal stability. DCSA, a good neural compression indicator, is the factor associated with the severity of spinal canal stenosis. Therefore, we measured MRI parameters, including the intervertebral DH, ST, SA, DCSA at the L4–5 level, and LLA at the L1–5 level before and after loading in degenerative spondylolisthesis.
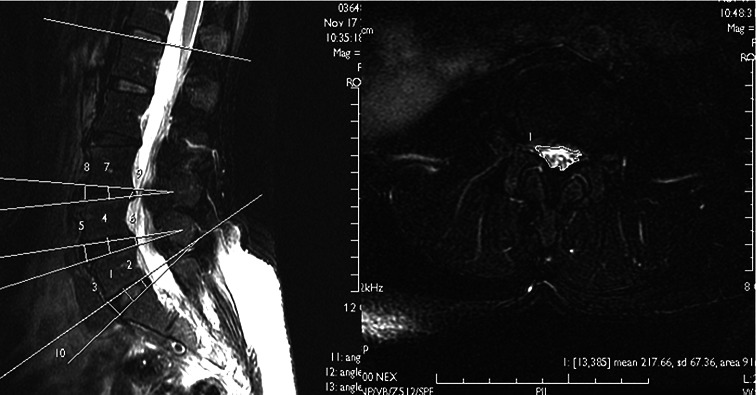
The DH, ST, SA at L4–5, and LLA at L1–5 were measured from sagittal lumbar MRI, and the DCSA at L4–L5 was measured from axial lumbar MRI using a measurement program on a digital image view station (Magic View 1000; Siemens) (Fig. 1). DH was measured from the means of anterior, middle, and posterior disc height. We measured the net sagittal displacement between two vertebrae as ST; we also deemed the disc angle difference between the upper and lower endplates to be disc SA before and after loading. DCSA was measured by depicting the area surrounding the dural sac on Axial T1WI.
Fig. 1.
By using axially loaded MRI [Sagittal FSE T2WI (TR/TE: 4000/97 ms; NEX: 4) with fat saturation and Axial FSE T2WI (TR/TE: 8000/106 ms; NEX: 4) with fat saturation], we measured MRI parameters including the intervertebral disc height (DH), sagittal translation (ST), segmental angulation (SA), and dural sac cross section area (DCSA) before and after loading in degenerative spondylolisthesis
Each patient’s disability was evaluated by the Oswestry Disability Index (ODI) questionnaire proposed by Fairbank et al. in 1980 [7], and physical functioning (PF) was also evaluated by the Physical Function scale proposed by Stucki et al. in 1996 [16]. Statistical comparisons and correlations between the differences of DH, ST, SA, DSCA, LLA between loaded and unloaded MRI, and the ODI and PF were performed to determine which parameters were critical to the patient’s disability and PF. Informed consent was obtained from all patients for inclusion of all of their medical data in this document.
Statistical analysis
We used the JMP software package for statistical analysis. Means and standard errors of ODI and PF scores, and stratification by age and sex, are shown in Table 2. To adjust for potential confounders, we performed multiple linear regression analysis to determine, which relevant factors contributed to the disability (ODI) and PF of degenerative L4–L5 spondylolisthesis. The effect estimates were expressed for a change of each factor by an inter-quartile range. P values below 0.05 indicated statistical significance.
Table 2.
Mean values of the DCSA, DH, SA, ST at the L4–5 level and LLA at L1–5 under pre-load and post-load
| Mean value | Pre-load | Post-load |
|---|---|---|
| L45–DCSA (mm2) | 108.28 | 89.03 |
| L45–DH (mm) | 9.04 | 8.36 |
| L45–SA | 7.14 | 5.90 |
| L45–ST (mm) | 1.80 | 1.46 |
| L1–5 LLA (degrees) | 37.03 | 39.28 |
Results
The morphologies of the lumbar spine changed after axially loaded MRI. Additional valuable imaging information for axially loaded MRI is listed in Table 1 (expressed by the case numbers and the corresponding percentages in our series), including the significant changes of DCSA (more than 15 mm2) in 13 patients, spinal stenosis with DCSA less than 100 mm2 in preload (11 cases) and post-load status (16 cases), the presence of buckling of the ligamentum flavum (5 cases), the presence of a herniated disc (6 cases), narrowing of the lateral recess (7 cases), asymmetric distortion of the dural sac (5 cases), facet joint subluxation (1 case), and facet joint synovial cyst (1 case) after axial loading. In the more symptomatic patients, we found some morphological changes after axially loaded MRI. Surprisingly, we observed that six patients had adjacent segment degeneration (4 L3–L4 and 2 L5–S1) coexisting with degenerative spondylolisthesis of L4–L5 under axially loaded MRI (Fig. 2).
Table 1.
Additional imaging information of L4–5 from axially loaded MRI
| ∆ DCSA > 15 mm2 | Pre-load < 100 mm2 | Post-load < 100 mm2 | Buckling of lig flavum | Herniated disc | Narrowing of the lateral recess | Asymmetric distortion of the dural sac | Facet joint subluxation | Facet joint Synovial cyst |
|---|---|---|---|---|---|---|---|---|
| 13/29 (44.8%) | 11/29 (37.9%) | 16/29 (55.2%) | 5/29 (17.2%) | 6/29 (20.7%) | 7/29 (24.1%) | 5/29 (17.2%) | 1/29 (3.4%) | 1/29 (3.4%) |
Fig. 2.
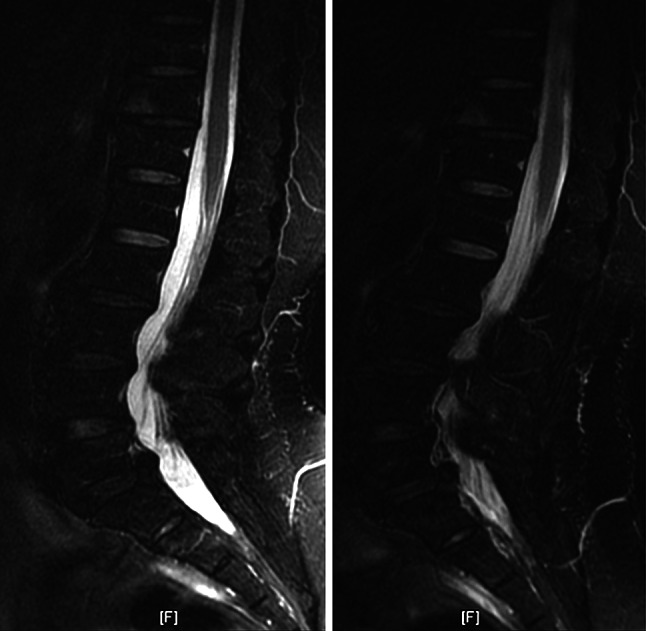
A 60-year-old female had adjacent segment degeneration (L3–L4 and L5–S1) coexisting with degenerative spondylolisthesis of L4–L5 under axially loaded MRI [preload Sagittal FSE T2WI (TR/TE: 4000/97 ms; NEX: 4) with fat saturation and post-load Sagittal FSE T2WI (TR/TE: 4000/97 ms; NEX: 4) with fat saturation]
The mean values of the DCSA, DH, SA, ST at the L4–5 level and LLA at the L1–5 under pre-load and post-load were recorded in Table 2. The mean values of the SA under pre-load and post-load were 7.14° and 5.90° at L4–L5 (listhetic level), respectively. The mean values of the LLA under pre-load and post-load were 37.03° and 39.28°, respectively.
We found that age and sex might interfere with ODI and PF scores in our population (Table 3). Distribution of L4–5 predictors under axially loaded MRI in our population was expressed in Table 4. After adjustment for sex and age, significant associations were found between the ODI, PF and the difference of SA (Table 5), and the PF and the post-loaded LLA (P = 0.02) (Table 6), only. However, there were no significant relationships between the ODI, PF and other parameters. We also checked for the associations of L2–3, L3–4, and L5–S1 levels and no levels showed significant results in our population (data not shown). We also did not find significant associations between the parameter of DCSA that could be evaluated by MRI only, and ODI or PF. We found that the disability was more severe and symptomatic if the patients had additional morphological changes, such as herniated disc, facet joint synovial cyst, facet joint subluxation, or lateral recess narrowing.
Table 3.
Mean and standard errors of ODI and PF scores in our study population
| N | ODI | PF | |
|---|---|---|---|
| Age | |||
| <55 | 9 | 10.0 ± 1.3 | 18.1 ± 0.4 |
| 55–65 | 11 | 11.6 ± 0.8 | 17.2 ± 0.6 |
| ≥65 | 9 | 18.8 ± 2.9 | 14.2 ± 1.2 |
| Sex | |||
| Male | 5 | 19.6 ± 4.9 | 14.4 ± 1.9 |
| Female | 24 | 12.0 ± 1.0 | 17.0 ± 0.5 |
| Total | 29 | 13.3 ± 1.2 | 16.6 ± 0.5 |
Table 4.
Distribution of predictors of MRI parameters under axially loaded MRI in our population
| Mean | SE | Min | Max | IQR | |
|---|---|---|---|---|---|
| L4–5 DCSA difference | 28.8 | 5.7 | 0.0 | 122.0 | 9.0–36.5 |
| L4–5 DH difference | 1.1 | 0.1 | 0.2 | 3.4 | 0.7–1.6 |
| L4–5 SA difference | 2.8 | 0.6 | 0.0 | 15.0 | 1.0–4.0 |
| L4–5 ST difference | 1.0 | 0.2 | 0.0 | 4.1 | 0.0–1.5 |
| L1–5 LLA (pre-load) | 37.0 | 2.4 | 9.0 | 58.0 | 28.0–49.0 |
| L1–5 LLA (post-load) | 39.3 | 2.3 | 4.0 | 65.0 | 32.0–45.5 |
SE standard error, IQR inter-quartile range
Table 5.
Association of ODI and PF scores with L4–5 MRI parameter differences under axially loaded MRI through multiple linear regression models
| ODI | PF | |||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P value | Estimate | 95% CI | P value | |
| DCSA difference | 0.99 | −0.96 to 2.94 | 0.31 | 0.03 | −0.80 to 0.91 | 0.90 |
| DH difference | 0.09 | −2.69 to 2.87 | 0.95 | −0.12 | −1.31 to 1.08 | 0.84 |
| SA difference | 0.89 | 0.25 to 1.54 | 0.04 | −0.95 | −1.68 to −0.23 | 0.01 |
| ST difference | −0.65 | −3.36 to 2.07 | 0.63 | 0.81 | −0.32 to 1.94 | 0.15 |
Models were adjusted by age and sex
Effect estimates were expressed for a change of each factor by IQR
Table 6.
Association of ODI and PF scores with L1–5 LLA through multiple linear regression models
| ODI | PF | |||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P value | Estimate | 95% CI | P value | |
| L1–5 LLA (pre-load) | −0.13 | −3.70 to 3.4 | 0.94 | 0.88 | −0.59 to 2.3 | 0.23 |
| L1–5 LLA (post-load) | −1.03 | −3.38 to 1.34 | 0.37 | 1.08 | 0.16 to 2.01 | 0.02 |
Models were adjusted by age and sex
Effect estimates were expressed for a change of each factor by IQR
Discussion
Degenerative spondylolisthesis is a common cause of low back pain, instability and disability, and can affect a patient’s quality of life. The etiologies of disability in patients with degenerative lumbar spondylolisthesis are multifactorial, and include mechanical factors such as spinal instability, neurological compromise, chemical factors, and other factors such as discogenic pain, facet arthropathy, and myofascial pain from ligaments or paraspinal muscles, etc. [8]. Therefore, it is very important for us to detect the critical factors affecting disability or physical functioning in degenerative spondylolisthesis.
Axially loaded MRI is a useful tool for study of the anatomical changes of the spinal canal of the lumbar spine. It can also aid diagnosis of instability, or occult spinal disorders, such as equivocal herniated discs or stenosis, by simulating the upright position under normal gravity. These changes can influence treatment decisions for 25% of symptomatic patients with spinal stenosis [11]. However, there are only a few clinical studies on the roles of axially loaded MRI in degenerative spondylolisthesis-related disability.
The spine is a flexible structure; discs are viscoelastic and facet joints provide scant motion. The dynamic changes of the disc in patients with degenerative lumbar spondylolisthesis seem important, so we studied them by means of the Dynawell axial loading instrument to investigate morphological changes in the lumbar spine under axial loading, and to correlate the severity of disability and various geometrical factors, such as segmental instability, and dynamic changes of neural compression using a multiple regression model on axially loaded MRI. The status of segmental instability can be exhibited in SA and ST, while the dynamic changes of neural compression under loading, such as DCSA, influence the neurogenic claudication and might be another parameter of segmental instability.
In patients with degenerative spondylolisthesis of L4–5, the intervertebral disc of L4–5 degenerated and the locking mechanism of the facet joint of L4–5 failed, and will progress to a static or unstable condition over time [13]. The segmental instability of L4–5 may originate from the changes of morphology under axial loading, including the changes in DCSA, buckling of the ligamentum flavum, the presence of a herniated disc, facet joint synovial cyst, and facet joint subluxation, asymmetric distortion of the dural sac, and narrowing of the lateral recess after axial loading. Therefore, the SA, ST, DH, and DCSA are all possible pathological factors responsible for the segmental instability of vertebral structures in patients with degenerative spondylolisthesis. The severity of instability may be associated with the changes of such dynamic parameters. In our study, we could not find a significant correlation between DCSA and ODI; however, we found that the disability was more severe and symptomatic if patients had additional morphological changes, such as herniated disc, facet joint synovial cyst, facet joint subluxation, or lateral recess narrowing. Therefore, we believe that the morphological changes revealed by axial loading could be the signs of segmental instability, and the dynamic instability might be the pathogenesis of disability for degenerative spondylolisthesis.
Lumbar intersegmental instability has been defined as the ST of more than 4 mm and/or SA of more than 10° on dynamic flexion–extension of lumbo-sacral radiographs [1, 6, 10, 14]. We can accurately evaluate the severity of spinal canal compromise by calculating the DCSA. DCSA under 100 mm2 is defined as relative spinal stenosis, and DCSA less than 75 mm2 is defined as absolute spinal stenosis [14]. Indicators of potential disability for degenerative spondylolisthesis (L4–L5) include changes in disc height, ST, SA and changes in DCSA at L4–L5.
Danielson et al. [4] and Willen et al. [5] have reported mean DCSA decreases of 30 mm2 at L4–L5 in patients with suspected spinal stenosis. However, mean DCSA decreases were only 19.25 mm2 at L4–L5 in our series; the decreased change in DCSA may be attributed to the more severe degeneration and less dynamic flexibility in the lumbar degenerative spondylolisthesis of our series.
Our data showed that the SA was the only critical factor associated with the disability and PF in degenerative spondylolisthesis of L4–5. We assume that the degenerated disc of spondylolisthesis with abnormal angular movement may be more susceptible to instability and posterior herniation under an axial force, and may cause neural compression, which is a possible cause of the disability and poor PF in patients with degenerative spondylolisthesis. Iguchi et al. [12] used dynamic flexion extension radiographs to evaluate the relationship of instability to lumbar symptoms, and suggested that ST contributes more than SA to lumbar symptoms; these results differed from ours evaluated with axially loaded MRI. Axially loaded MRI can provide more accurate parameters, detect dynamic or occult changes of neurological compromise or nerve root indentation, as well as some additional morphological changes. The direction of ST was perpendicular to the force of axial loading; therefore, the severity of translational instability is difficult to completely exhibit on axially loaded MRI. However, the ST of L4–L5 seemed not to be such an important factor in the disability or PF under axially loaded MRI in patients with degenerative spondylolisthesis. Fujiwara et al. [9] reported that axial rotational motion was most affected by disc degeneration. We assume that the disc degenerates in degenerative spondylolisthesis; therefore, SA might be a more important parameter for physical disability in lumbar degenerative spondylolisthesis, in view of our results.
Among these parameters, only changes in SA (at the L4–5 listhetic level) can be regarded as disability indicators. In this study, we were able to predict the severity of disability by measuring the difference of SA on loaded and unloaded MRI. The Dynawell instrumental application simulates the upright position, which aids the determination of spinal morphological changes under axial loading and helps us understand the severity of neurological compromise by measuring DCSA; it also provides additional information at the junctional level, such as herniated disc potential and the presence or absence of instability or stenosis at L3–4 or L5–S1. Determining the meaning of these changes (L3–4 or L5–S1) will require more study. The difference in angulatory motion of L4–5 can be considered an important factor of segmental instability, and it also correlates well with the severity of physical disability and PF. We believe that the angular instability of the intervertebral disc may play a more important role than neurological compression in the pathogenesis of disability in degenerative lumbar spondylolisthesis.
A previous study suggested that segmental vertebral fusion might accelerate the degenerative process at the adjacent levels [2]. In six of our patients, we observed a phenomenon in which some radiographically occult adjacent or junctional problems (potential L3–L4 and L5–S1 herniated disc, or ligmentum flavum) coexisted with degenerative spondylolisthesis of L4–L5. However, this problem was not evident during unloaded MRI, only through axially loaded MRI. Therefore, junctional degeneration might have occurred before segmental fusion in some patients with degenerative lumbar spondylolisthesis of L4–5. We suspect that pre-operative degeneration may play an important role in the determination of postoperative junctional problems in such patients. Using axially loaded MRI, we found some premature degeneration of L3–4 or L5–S1 coexisting with degenerative spondylolisthesis of L4–5, which may be associated with the compensatory stress accumulation at the junction segment (L3–4 or L5–S1) adjacent to the degenerated listhetic segment (L4–5). Disc degeneration at the junction level might be associated with future junctional problems, such as instability or stenosis of L3–4 or L5–S1 after fusion of the L4–5 vertebrae; this possibility requires further study.
Patients with degenerative lumbar spondylolisthesis differ in their symptoms, such as low back pain, intermittent claudication, sciatica, disability, etc. However, most people with low-grade spondylolithesis have no obvious symptoms and no need to seek medical care because they have no compelling symptoms. Furthermore, low-grade spondylolithesis is an incidental finding in many patients. Therefore, we think that neurological compression is not the only factor responsible for the severity of symptomatic patients with degenerative spondylolisthesis. Some occult instability expressed in the changes in SA under axial loading causes their symptoms to deteriorate. We suggest that the angular instability of the intervertebral disc may play a more important role than neurological compromise in the pathogenesis of disability in degenerative lumbar spondylolisthesis.
Conclusions
Axially loaded MRI using the Dynawell device, allows to demonstrate morphological changes in patients with symptomatic degenerative spondylolisthesis. Only SA at L4–5 is well correlated with physical disability (ODI) and PF; the post-load LLA was well correlated to the PF of patients with degenerative L4–L5 spondylolisthesis. Under axially loaded MRI, SA of L4–5 can be a good indicator of disability in patients with degenerative spondylolisthesis of L4–5.
Acknowledgments
The authors did not receive any outside funding or grants in support of their research for or preparation of this work. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
References
- 1.Boden SD, Wiesel SW. Lumbosacral segmental motion in normal individuals: have we been measuring instability properly? Spine. 1990;15:571–576. doi: 10.1097/00007632-199006000-00026. [DOI] [PubMed] [Google Scholar]
- 2.Caputy AJ, Luessenhop AJ. Long-term evaluation of decompressive surgery for degenerative lumbar stenosis. J Neurosurg. 1992;77:669–676. doi: 10.3171/jns.1992.77.5.0669. [DOI] [PubMed] [Google Scholar]
- 3.Cartolari R, Argento G, Cardello P, et al. Axial loaded computed tomography (AL-CT), and cine AL-CT. Riv Neuroradiol. 1998;11:275–286. [Google Scholar]
- 4.Danielson B, Willen J. Axially loaded magnetic resonance image of the lumbar spine in asymptomatic individuals. Spine. 2001;26:2601–2606. doi: 10.1097/00007632-200112010-00015. [DOI] [PubMed] [Google Scholar]
- 5.Danielson BI, Willen J, Gaulitz A, et al. Axial loading of the spine during CT and MR in patients with suspected lumbar spinal stenosis. Acta Radiol. 1998;39:604–611. doi: 10.3109/02841859809175484. [DOI] [PubMed] [Google Scholar]
- 6.Dupuis PR, Yong-Hing K, Cassidy JD, et al. Radiological diagnosis of degenerative lumbar spinal instability. Spine. 1985;10:262–266. doi: 10.1097/00007632-198504000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 8.Fritz JM, Delitto A, Welch WC, et al. Lumbar spinal stenosis: a review of current concepts in evaluation, management, and outcome measurements. Arch Phys Med Rehabil. 1998;79:700–708. doi: 10.1016/S0003-9993(98)90048-X. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara A, Lim TH, An HS, et al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine. 2000;25:3036–3044. doi: 10.1097/00007632-200012010-00011. [DOI] [PubMed] [Google Scholar]
- 10.Hanley EN Jr (1995) The indications for lumbar spinal fusion with and without instrumentation. Spine 20(suppl):143–153. doi:10.1097/00007632-199512151-00012 [PubMed]
- 11.Hiwatashi A, Danielson B, Moritani T, et al. Axial loading during MR imaging can influence treatment decision for symptomatic spinal stenosis. AJNR Am J Neuroradiol. 2004;25:170–174. [PMC free article] [PubMed] [Google Scholar]
- 12.Iguchi T, Kanemura A, Kasahara K, et al. Lumbar instability and clinical symptoms: which is the more critical factor for symptoms: sagittal translation or segment angulation? J Spinal Disord Tech. 2004;4:284–290. doi: 10.1097/01.bsd.0000102473.95064.9d. [DOI] [PubMed] [Google Scholar]
- 13.Jayakumar P, Nnadi C, Saifuddin A, et al. Dynamic degenerative lumbar spondylolisthesis: diagnosis with axial loaded magnetic resonance imaging. Spine. 2006;31:E298–E301. doi: 10.1097/01.brs.0000216602.98524.07. [DOI] [PubMed] [Google Scholar]
- 14.Morgan FP, King T. Primary instability of lumbar vertebrae as a common cause of low back pain. J Bone Joint Surg Br. 1957;39:6–22. doi: 10.1302/0301-620X.39B1.6. [DOI] [PubMed] [Google Scholar]
- 15.Soini J, Antti-Poika I, Tallroth K, et al. Disc degeneration and angular movement of the lumbar spine comparative study using plain and flexion–extension radiography and discography. J Spinal Disord. 1991;4:183–187. doi: 10.1097/00002517-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Stucki G, Daltroy L, Liang MH, et al. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine. 1996;21:796–803. doi: 10.1097/00007632-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Willen J, Danielson B, Gaulitz A, et al. Dynamic effects on the lumbar spinal canal: axially loaded CT-myelography and MRI in patients with sciatica and/or neurogenic claudication. Spine. 1997;22:2968–2976. doi: 10.1097/00007632-199712150-00021. [DOI] [PubMed] [Google Scholar]