Abstract
Tizanidine and aceclofenac individually have shown efficacy in the treatment of low back pain. The efficacy and tolerability of the combination have not yet been established. The objective of the study was to evaluate the efficacy and safety of aceclofenac-tizanidine fixed dose combination against aceclofenac alone in patients with acute low back pain. This double-blind, double-dummy, randomized, comparative, multicentric, parallel group study enrolled 197 patients of either sex in the age range of 18–70 years with acute low back pain. The patients were randomized to receive either aceclofenac (100 mg)–tizanidine (2 mg) b.i.d or aceclofenac (100 mg) alone b.i.d for 7 days. The primary efficacy outcomes were pain intensity (on movement, at rest and at night; on VAS scale) and pain relief (on a 5-point verbal rating scale). The secondary efficacy outcomes measures included functional impairment (modified Schober’s test and lateral body bending test) and patient’s and investigator’s global efficacy assessment. aceclofenac–tizanidine was significantly superior to aceclofenac for pain intensity (on movement, at rest and at night; P < 0.05) and pain relief (P = 0.00) on days 3 and 7. There was significant increase in spinal flexion in both the groups from baseline on days 3 and 7 with significant difference in favour of the combination group (P < 0.05). There were significantly more number of patients with excellent to good response for the aceclofenac–tizanidine treatment as compared to aceclofenac alone (P = 0.00). Both the treatments were well tolerated. In this study, aceclofenac–tizanidine combination was more effective than aceclofenac alone and had a favourable safety profile in the treatment of acute low back pain.
Keywords: Aceclofenac-tizanidine, Acute LBP, Aceclofenac
Background
Acute low back pain (LBP) is amongst the most common conditions requiring a consultation with physicians [11, 14, 17]. Despite the high prevalence of LBP, diagnosis of the precise cause is usually difficult, and specific causes can be determined in only in ~15–20% of LBP patients [12, 13]. Although cases of acute back pain may be self-limiting and resolve spontaneously (almost regardless of the treatment), many patients experience recurrent symptoms or persistent functional limitations [4]. Not surprisingly, the World Health Organization (WHO) focuses its LBP initiative on sub-acute back pain as an intermediate stage towards chronic pain [8].
Despite several guidelines for diagnostic and therapeutic approaches that have been developed and implemented in different countries during the past decade [3, 9, 22] mainly based on early mobilization, experts in the field increasingly appreciate that early and effective analgesia is pivotal and should combine various mechanisms to enhance efficacy and reduce toxicity [6, 19].
Fixed dose combinations of non-steroidal anti-inflammatory drugs (NSAIDs) such as aceclofenac and skeletal muscle relaxant like tizanidine may maximize pain relief by two different mechanisms. NSAIDs are widely used in the treatment of acute and chronic LBP, with most patients being treated in primary care [5, 20].
Aceclofenac, a phenylacetic acid derivative is an NSAID indicated for the symptomatic treatment of pain and inflammation in various conditions including acute and chronic pain [7]. In a previous double-blind study [1], 227 patients with acute LBP received either aceclofenac (2 × 100 mg daily) or diclofenac resinate (2 × 75 mg daily) for 10 days. There was significant difference in favour of aceclofenac for all subjective ratings of pain [21].
Tizanidine is a centrally active skeletal muscle relaxant. It is indicated for the symptomatic treatment of painful muscle spasms due to musculo-skeletal disorders and of spasticity of various origins at higher dose levels. In combination with NSAID, it is superior to NSAID alone [2]. Besides pain relief, addition of tizanidine reduces the consumption of NSAID and tranquillizer, thereby reducing the side effects potential. Lepisto [15] compared tizanidine (2 mg, three times daily for 7 days) and placebo as sole therapy in a double-blind study of 30 patients with acute skeletal muscle spasms. There was a statistically significant change in favour of tizanidine in scores for muscle tension, tenderness and straight leg raising. Another study on pain syndrome (reflex muscular-tonic, myofascial, acute compression radiculopathy) showed significant pain relief with tizanidine and patients were able to discontinue analgesics and tranquilizers. In few patients, the doses of NSAIDs were noticeably reduced.
Also, tizanidine as a single agent has similar effect as that of diazepam [10] and chlormezanone [2]. Studies comparing the efficacy of tizanidine with diazepam and chlormezanone found tizanidine to be as effective as other agents in reducing spasticity. Whilst there is much controversy about the role of muscle spasm in acute back injury [2], there is no doubt that when the condition is extremely painful, a muscle relaxing drug in combination with an analgesic may be a useful way of managing the situation.
These considerations led us to conduct a randomized comparative study to evaluate the clinical benefits of adding tizanidine to aceclofenac in treatment of patients with acute LBP.
Study objective
The primary objective of this study was to evaluate the efficacy of aceclofenac–tizanidine combination in comparison with aceclofenac in patients with acute LBP. The secondary objective was to assess the safety of study medications.
Materials and methods
Study design
This was a double-blind, double-dummy, multicentric, parallel group, prospective, comparative, randomized study conducted at: J. J. Hospital, Mumbai; Indira Gandhi Government Medical College, Nagpur; Mahatma Gandhi Medical College and Hospital, Jaipur; and B. J. Medical College and Sassoon General Hospital, Pune. The study protocol was approved by the institutional review board at each centre, and all patients provided written informed consent prior to participation. The execution and monitoring of the study were conducted in accordance with the requirements of good clinical practice.
Subject selection criteria
Male and female patients in the age range of 18–70 years with localized, uncomplicated acute lumbosacral pain, associated with degenerative spinal disorders (confirmed radiologically), of recent onset [1–30 day(s)] and with pain intensity at rest of at least 6 on a 10-point visual analogue scale (VAS) were included in this study.
The following patients were excluded: those patients with suspicion of a serious underlying spinal condition such as sciatica and nonspecific back symptoms related to abdominal, pelvic or thoracic pathology; those with prior history of sensory and/or motor deficits in the lower extremities and lumbosacral facet syndrome; those with malignancy, osteoporosis or previous history of lumbar spine surgery; those with any possibly confounding severe pain relating to the back, fibromyalgia, symptomatic disc herniation, spondylolisthesis higher than grade 2, severe spinal stenosis, acute LBP due to prolapsed intervertebral disc (PID) and who had received aceclofenac or tizanidine therapy in the past 1 week before inclusion in the study. Patients with history of hypersensitivity to NSAIDS or tizanidine and those with prior history of asthma or hypersensitivity potentially requiring concomitant treatment, and had used steroids within 4 weeks of study entry, were excluded. Those patients requiring drugs that affect platelet functions and coagulation were excluded from the study. Patients with history of peptic ulcers, duodenal ulcers, gastrointestinal bleeding, or bleeding disorders were not recruited in this study. Patients with abnormal renal and liver function and with significant unstable medical disease were also excluded from the study. Pregnant and lactating women and females of childbearing potential, who did not use contraceptives, were not enrolled in this study. All patients underwent a detailed neurological examination involving the spine and lower extremities. Neurological examination was performed by the principal investigators at each centre who were blinded to the study medication.
Treatment procedure
Patients fulfilling the inclusion and exclusion criteria were randomized to a double-blind, double-dummy treatment for 7 days with aceclofenac (100 mg)–tizanidine (2 mg) fixed dose combination tablets b.i.d (with aceclofenac 100 mg placebo) or aceclofenac (100 mg) alone b.i.d (with aceclofenac 100 mg–tizanidine 2 mg fixed dose combination placebo).
Efficacy criteria
The primary efficacy outcome measures included pain intensity on movement, at rest, at night and pain relief scores. The pain intensity was captured on a VAS ranging from “0 to 10” where a score of “0” represents “no pain” and “10” represents “worst” possible pain. Pain relief (relative to the amount of pain before enrolment) was assessed on a 5-point verbal rating scale with categories 1 = complete relief, 2 = good, 3 = moderate, 4 = slight, 5 = no relief. Pain intensity was measured on days 1 (baseline) 2, 3, 4, 5, 6 and 7. Pain relief was assessed on days 3 and 7.
The secondary efficacy outcome measures included functional impairment and global efficacy assessment. The functional impairment was assessed at all three visits (baseline, and days 3 and 7) using modified Schober’s test [16] and lateral body-bending test [18]. While performing the body-bending test, the patient was asked to stand with feet 15 cm apart. The position of the tips of the index finger was marked on the skin of both the thighs, and the patient was asked to bend maximally to the left and right side. The maximum reach of the index finger was marked on the skin of both the thighs. The distance between the upper and lower marks was measured on both the sides.
The patients’ and investigators’ overall assessment of study medication was recorded at the final visit using a 5-point verbal rating scale (0 = no change, 1 = poor, 2 = moderate, 3 = good, 4 = excellent).
Statistical plan
Efficacy analyses were performed for the clinically evaluable patient population and safety analysis for all the randomized patients who received at least one dose of study medication. Patients from both the study groups were evaluated at baseline for homogeneity with respect to demographic and disease characteristics. The basic descriptive statistics were calculated.
The primary efficacy outcome measures were compared at baseline on days 3 and 7 using two sample t test. Test of proportions in contingency tables were made using χ2 test or, if the expected cell frequencies were small, it was compared by Fisher’s exact test. The statistical significance level was set at the level 0.05.
Patients who had received at least one dose of study medication were evaluated for safety assessment. Comparisons of the two groups with respect to changes in laboratory parameters were evaluated by t test or Mann–Whitney U test as appropriate. Statistical analysis was performed using statistical software MINITAB 14.
Results
A total of 203 patients were screened for the study. Out of this, 197 patients reported for baseline visit. These 197 were randomized to either aceclofenac–tizanidine (n = 101) therapy or aceclofenac alone (n = 96) and were followed for up to 7 days.
Out of 197 patients, 185 (94 in the aceclofenac–tizanidine group and 91 in the aceclofenac group) patients completed the study. There were three patients (two from the aceclofenac–tizanidine group and one from the aceclofenac group), who refused further participation in the study. There was one protocol violation in the aceclofenac–tizanidine group and four patients from both the groups were lost to follow-up.
Both the treatment groups were comparable with respect to demography and baseline disease characteristics (Table 1). This study enrolled 120 males (60.90%) and 77 females (39.09%); with age ranging from 18 to 65 years with average age of 43.37 ± 11.55 years and average weight 63 ± 9.09 kg. Efficacy was evaluated in 185 patients who completed this study.
Table 1.
Demography and disease characteristics of patients at baseline
| Parameters | Aceclofenac–tizanidine (n = 101) | Aceclofenac (n = 96) | P value |
|---|---|---|---|
| Males (%) | 62 (61.39) | 58 (60.42) | 0.646 |
| Females (%) | 39 (38.61) | 38 (39.58) | |
| Mean age in years ± SD (range) | 43.30 ± 12.16 (18–65) | 43.45 ± 10.94 (19–62) | 0.927 |
| Mean weight (kg) ± SD | 63.29 ± 9.72 | 62.71 ± 8.47 | 0.656 |
| Mean height (cm) ± SD | 164.67 ± 8.31 | 164.65 ± 7.54 | 0.981 |
| Pulse rate (beats/min) ± SD | 76.79 ± 6.11 | 76.30 ± 4.93 | 0.536 |
| Body temperature (°F) (mean ± SD) | 97.78 ± 1.22 | 97.47 ± 1.38 | 0.096 |
| Respiration rate (breaths/min) (mean ± SD) | 16.21 ± 2.71 | 15.79 ± 2.66 | 0.278 |
| Systolic blood pressure (mm Hg) (mean ± SD) | 121.7 ± 9.56 | 121.97 ± 8.81 | 0.839 |
| Diastolic blood pressure (mm Hg) (mean ± SD) | 78.63 ± 4.89 | 78.94 ± 3.99 | 0.633 |
Primary efficacy outcome
Pain on movement, at rest and at night
All patients in both treatment groups showed significant improvement in pain on movement, at rest and at night from baseline on days 3 and 7 (P < 0.05). There was significantly better improvement in the combination group compared to the monotherapy group (P < 0.05; Table 2).
Table 2.
Mean changes in baseline pain intensity score on days 3 and 7 using 10-point VAS scale
| Parameters | Aceclofenac–tizanidine (n = 94) | Aceclofenac (n = 91) | P value |
|---|---|---|---|
| Day 3 | |||
| Pain on movement (±SD) | −2.94 ± 1.59 | −1.81 ± 1.04 | 0.00 |
| Pain at rest (±SD) | −3.01 ± 1.51 | −1.90 ± 1.13 | 0.00 |
| Pain at night (±SD) | −3.02 ± 1.51 | −1.92 ± 1.26 | 0.00 |
| Day 7 | |||
| Pain on movement (±SD) | −6.09 ± 2.34 | −3.98 ± 1.86 | 0.00 |
| Pain at rest (±SD) | −5.88 ± 2.14 | −4.35 ± 2.06 | 0.00 |
| Pain at night (±SD) | −5.76 ± 2.12 | −4.40 ± 2.15 | 0.00 |
Pain relief
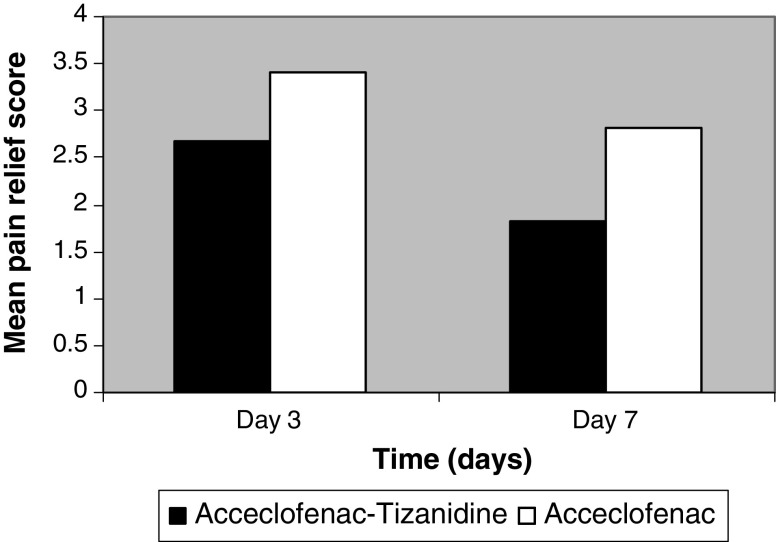
The average pain relief scores showed statistically significant improvement for the aceclofenac–tizanidine group compared to the aceclofenac alone on days 3 (P = 0.00) and 7 (P = 0.00; Fig. 1).
Fig. 1.
Average pain relief score on days 3 and 7 for both the study treatment
Secondary efficacy outcome
Modified Schober’s test and lateral body-bending test
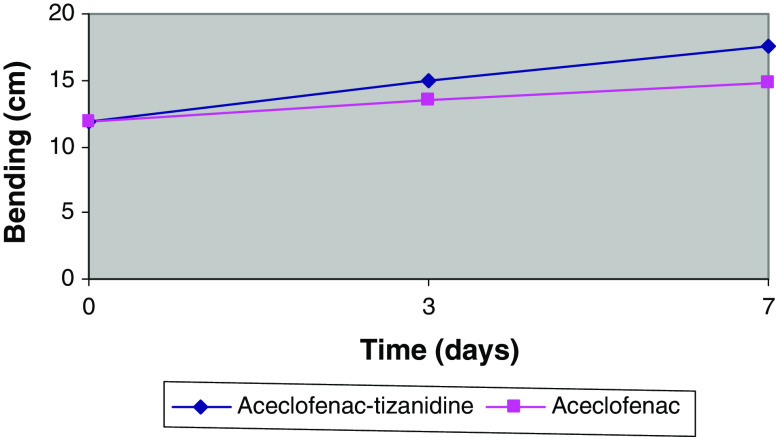
The modified Schober’s test showed progressive improvement in spinal flexibility on days 3 and 7 in both treatment groups; however, the improvement was higher in the combination group compared to the aceclofenac group (Table 3; P < 0.05). Likewise, lateral bending test measurement on day 7 increased to a greater extent, thus indicating significantly increased spinal flexion in aceclofenac–tizanidine treated patients compared with the aceclofenac group (P = 0.005; Fig. 2).
Table 3.
Mean changes in modified Schober’s test
| Modified Schober’s test (degrees) | Aceclofenac–tizanidine (n = 94) | Aceclofenac (n = 91) | P value |
|---|---|---|---|
| Baseline (±SD) | 6.13 ± 8.46 | 6.83 ± 8.46 | 0.570 |
| Day 3 (±SD) | 17.63 ± 8.35 | 12.08 ± 8.16 | 0.00 |
| Day 7 (±SD) | 27.9 ±11.7 | 19.6 ± 10.6 | 0.00 |
Fig. 2.
Body bending (mean degrees of bending to the left and right) against time during treatment with aceclofenac–tizanidine and aceclofenac
Patient’s overall efficacy assessment for treatment
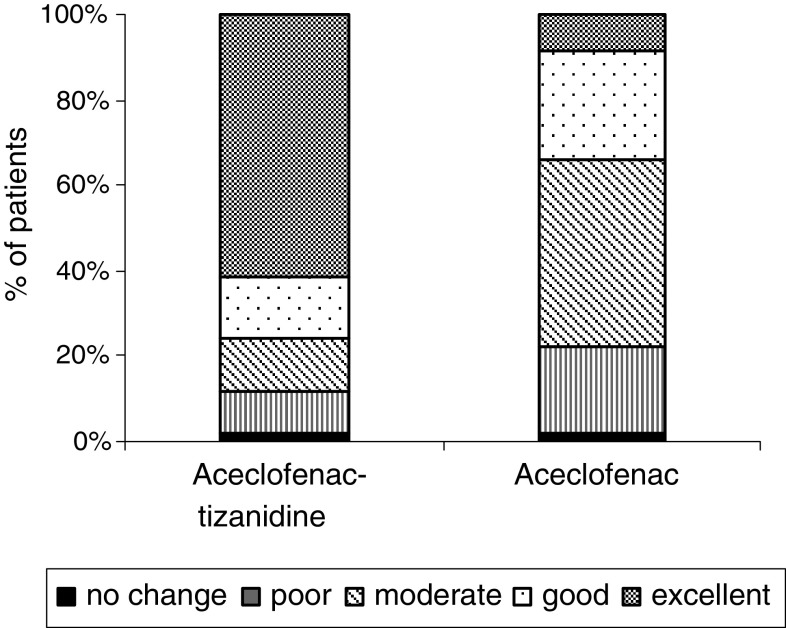
There were significantly more number of patients who reported excellent to good response in the combination therapy compared to the monotherapy group (P < 0.05). The aceclofenac–tizanidine was rated as excellent to good in 75.53% of patients compared to 34.06% of patients on the aceclofenac group. (Fig. 3).
Fig. 3.
Patient’s overall efficiency assessment at the end of the study
Investigator’s overall efficacy assessment for treatment
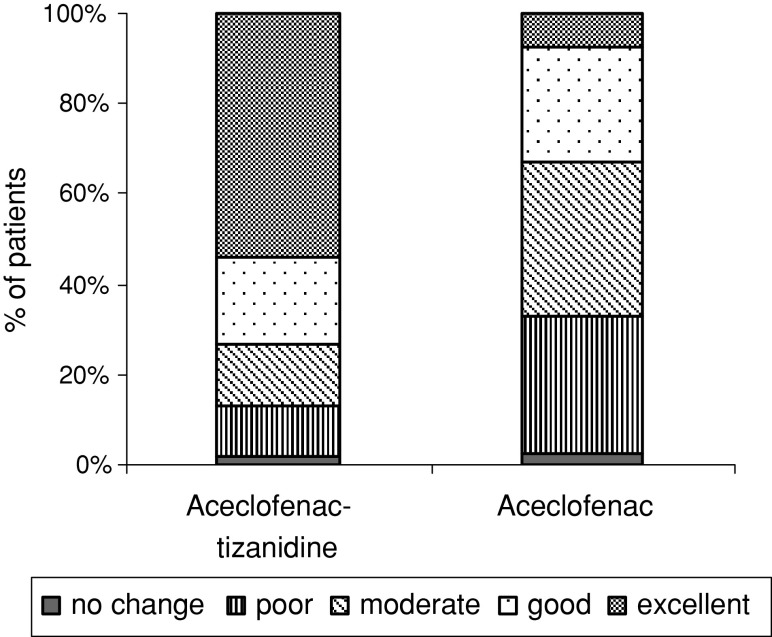
The investigator’s overall efficacy assessment was significantly better in the aceclofenac–tizanidine group than in the aceclofenac group (P < 0.05). The aceclofenac–tizanidine was rated as excellent to good in 73.40% of patients compared to 32.96% of patients on the aceclofenac group. (Fig. 4).
Fig. 4.
Investigator’s overall efficacy assessment at the end of study
Safety
Those patients who received at least one dose of study medication were included in the safety analysis. A total of 197 patients were analysed and 24 patients (12%) reported four adverse events during this study. The adverse events reported in this study are tabulated in Table 4. The commonly reported adverse events were vomiting and dizziness. The less common adverse events were dyspepsia and drowsiness. The incidence of adverse event with aceclofenac–tizanidine combination was similar to that with aceclofenac. In the investigator’s judgement, the reported adverse events were of mild to moderate intensity and possibly related to the study drugs. All the adverse events subsided on their own.
Table 4.
Adverse events reported in both the treatment groups
| Adverse events | Aceclofenac–tizanidine (n = 101) | Aceclofenac (n = 96) |
|---|---|---|
| Vomiting | 5 | 7 |
| Dizziness | 5 | 4 |
| Dyspepsia | 0 | 1 |
| Drowsiness | 2 | 0 |
The laboratory evaluations were done at baseline (screening visit) and at the end of therapy (day 7) and were performed in the hospital’s central laboratories. Mean changes from baseline for various laboratory parameters were evaluated on day 7 (end of therapy) for all the patients. There were no clinically significant trends evident across the treatment groups. No serious laboratory abnormality was reported in any patient in this study.
Discussion
The results of this double-blind, double-dummy, multicentre, parallel group, randomized study show that aceclofenac–tizanidine is effective in lowering the pain intensity in patients with acute, uncomplicated LBP. Moreover, the combination shows a trend towards superiority in its efficacy and tolerability compared with monotherapy.
The symptomatic treatment of acute LBP is generally carried out using anti-inflammatory drugs in order to reduce inflammation and pain. Frequently, however, the patient presents with muscle spasm causing the condition to be more painful and produce functional impairment. In such cases, the use of muscle relaxant drugs may be useful [2].
In this study, the addition of tizanidine to aceclofenac in the treatment of acute LBP appears to have been beneficial, particularly with regard to patients with moderate to severe pain at night and at rest, when the predominant pain present may have been due to muscle spasm. The results show that the combination gives a significantly more rapid improvement in pain at night (P = 0.00) and at rest (P = 0.00). Pain on movement was significantly reduced in patients treated with aceclofenac–tizanidine (P = 0.00), which further supports a quicker recovery due to the addition of tizanidine. Analyses of the secondary efficacy parameters consistently supported a higher efficacy of combination, which showed a greater improvement in spinal flexibility and a greater reduction in functional disability than monotherapy.
Furthermore, the global efficacy assessments showed that patients and physicians had better perceptions of the efficacy for aceclofenac–tizanidine compared to aceclofenac alone. In addition, the superior efficacy of combination compared to monotherapy, leading to less pain and improved functioning, could result in a better “global appreciation” of the drug and consequently better tolerability.
No serious adverse events were reported. No significant drug-related changes to blood pressure, pulse rate, blood biochemistry or haematology were observed with both the study treatments. Although two patients treated with aceclofenac–tizanidine reported drowsiness as an adverse event, this may be a desirable effect since patients with severe acute LBP are often treated with sedation, analgesia and bed rest [2].The combination of aceclofenac–tizanidine is well tolerated and the tolerability profile was similar as reported in literature where both these drug were administered separately [2, 21].
Conclusion
The results of this study suggest that tizanidine is a useful adjunct to the aceclofenac in the treatment of acute LBP in general practice. The combination was found to be superior to aceclofenac monotherapy with respect to efficacy. Both the study treatments were found to be safe and well tolerated in the studied population.
Acknowledgments
This study has been sponsored by Ipca Laboratories Limited. The authors would like to thank Ms. Smita Talekar, for her technical assistance right from the inception of the study, and Mr. Mitesh Sharma, biostatistician for statistical analysis and data management for this study. Dr. Anil Pareek and Nitin Chandurkar, who are employees of Ipca Laboratories Limited, were involved in the conceptualization, coordination and execution of the study at all the centres. The experiment complies with the current laws of the country in which they were performed inclusive of the ethics approval.
References
- 1.Batlle-Gualda E, Román Ivorra J, et al. Aceclofenac vs paracetamol in the management of symptomatic osteoarthritis of the knee: a double-blind 6-week randomized controlled trial. Osteoarthr Cartil. 2007;15(8):900–908. doi: 10.1016/j.joca.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Berry H, Hutchinson DR. Tizanidine and ibuprofen in acute low-back pain: results of a double-blind multicentre study in general practice. J Int Med Res. 1988;16:83–91. doi: 10.1177/030006058801600202. [DOI] [PubMed] [Google Scholar]
- 3.Burton AK, Waddell G. Clinical guidelines in the management of low back pain. Baillieres Clin Rheumatol. 1998;12:17–35. doi: 10.1016/S0950-3579(98)80004-6. [DOI] [PubMed] [Google Scholar]
- 4.Carey TS, Garret J, Jackman A, et al. The outcomes and costs of care for acute low back pain among patients seen by primary care practitioners, chiropractors, and orthopedic surgeons: The North Carolina Back Pain Project. N Engl J Med. 1995;333:913–917. doi: 10.1056/NEJM199510053331406. [DOI] [PubMed] [Google Scholar]
- 5.Cherkin DC, Wheeler KJ, Barlow W, Deyo RA. Medication use for low back pain in primary care. Spine. 1998;23:607–614. doi: 10.1097/00007632-199803010-00015. [DOI] [PubMed] [Google Scholar]
- 6.Desmeules J, Rollanson V, Piguet V, Dayer P. Clinical pharmacology and rationale of analgesic combination. Eur J Anaesthesiol Suppl. 2003;28:7–11. [PubMed] [Google Scholar]
- 7.Dooley M, Spencer CM, Dunn CJ. Aceclofenac: a reappraisal of its use in the management of pain and rheumatic disease. Drugs. 2001;61(9):1351–1378. doi: 10.2165/00003495-200161090-00012. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich GE. Back pain. J Rheumatol Suppl. 2003;67:26–31. [PubMed] [Google Scholar]
- 9.European Commission COST B13 Management Committee European guidelines for the management of low back pain. Acta Orthop Scand Suppl. 2002;73:20–25. doi: 10.1080/000164702760379503. [DOI] [PubMed] [Google Scholar]
- 10.Fryda-Kaurimsky Z, Muller-Fassbender H. Tizanidine (DS 103–282) in the treatment of acute paravertebral muscle spasm: a controlled trial comparing tizanidine with diazepam. J Int Med Res. 1981;9:501–505. doi: 10.1177/030006058100900613. [DOI] [PubMed] [Google Scholar]
- 11.Hagen KB, Hilde G, Jamtvedt G, Winnem M (2004) Bed rest for acute low back pain and sciatica. Cochrane Database Syst Rev (4):CD001254 [DOI] [PubMed]
- 12.Hildebrandt J. Does unspecific low back pain really exist? Z Orthop Ihre Grenzgeb. 2004;142:139–145. doi: 10.1055/s-2004-822623. [DOI] [PubMed] [Google Scholar]
- 13.Jackson KC., II Pharmacotherapy in lower back pain. Drugs Today (Barc) 2004;40:765–772. doi: 10.1358/dot.2004.40.9.850496. [DOI] [PubMed] [Google Scholar]
- 14.Jensen S. Back pain: clinical assessment. Aust Fam Physician. 2004;33(393–395):397–401. [PubMed] [Google Scholar]
- 15.Lepisto P. Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the Cochrane Collaboration. J Int Med Res. 1981;9(6):501–505. [Google Scholar]
- 16.MacRae IF, Wright V. Measurement of back movements. Ann Rheum Dis. 1969;28:584–589. doi: 10.1136/ard.28.6.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manek NJ, MacGregor AJ. Epidemiology of back disorders: prevalence, risk factors, and prognosis. Curr Opin Rheumatol. 2005;17:134–140. doi: 10.1097/01.bor.0000154215.08986.06. [DOI] [PubMed] [Google Scholar]
- 18.Million R, Hall W, Nilsen KH, Baker RD, Jayson MIV. Assessment of the progress of the back pain patient. Spine. 1982;7:204. doi: 10.1097/00007632-198205000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Raffa RB, Clark-Vetri R, Tallarida RJ, Wertheimer Al. Combination strategies for pain management. Expert Opin Pharmacother. 2003;4:1697–1708. doi: 10.1517/14656566.4.10.1697. [DOI] [PubMed] [Google Scholar]
- 20.Rossignol M, Abenhaim L, Bonvalot Y, Gobeille D, Shrier I. Should the gap be filled between guidelines and actual practice for management of low back pain in primary care? Spine. 1996;21:2893–2898. doi: 10.1097/00007632-199612150-00021. [DOI] [PubMed] [Google Scholar]
- 21.Schattenkirchner M, Milachowski KA. A double-blind, multicentre, randomised clinical trial comparing the efficacy and tolerability of aceclofenac with diclofenac resinate in patients with acute low back pain. Clin Rheumatol. 2003;22(2):127–135. doi: 10.1007/s10067-003-0710-9. [DOI] [PubMed] [Google Scholar]
- 22.Van Tulder MW, Tunt M, Pennick V, et al. Quality of primary care guidelines for acute low back pain. Spine. 2004;29:E357–E362. doi: 10.1097/01.brs.0000137056.64166.51. [DOI] [PubMed] [Google Scholar]