Abstract
Generalized low bone mass and osteopenia in both axial and peripheral skeletons have been reported in adolescent idiopathic scoliosis (AIS). However, the mechanism and causes of bone loss in AIS have not been identified. Therefore, this study examined the relationship between the osteogenic and adipogenic differentiation abilities of mesenchymal stem cells (MSCs) and bone mass in 19 patients with AIS and compared these with those of 16 age- and gender-matched patients with lower leg fracture. Mean lumbar spinal bone mineral density (LSBMD) in AIS patients was found to be lower than in controls (P = 0.037) and the osteogenic differentiation abilities and alkaline phosphatase activities of MSCs from patients were also found to be lower than those of controls (P = 0.0073 and P = 0.001, respectively), but the abilities of the MSCs of patients and controls to undergo adipogenic differentiation were similar. The osteogenic differentiation ability was found to be positively correlated with alkaline phosphatase activity in the AIS group. However, the osteogenic and adipogenic abilities were not found to be correlated with LSBMD in either patients or controls. These findings suggest that the decreased osteogenic differentiation ability of MSCs might be one of the possible mechanisms leading to low bone mass in AIS. However, we did not determine definite mechanisms of low bone mass in AIS. Therefore, further study with large scale will be needed to identify the mechanism involved.
Keywords: Adolescent idiopathic scoliosis, Bone mineral density, Mesenchymal stem cells, Osteogenic differentiation ability
Introduction
Adolescent idiopathic scoliosis (AIS) is a complex three-dimensional deformity of the spine that usually occurs mostly in girls between the ages of 10 and 14. The etiology and pathogenesis of idiopathic scoliosis remain unclear despite the number of studies performed. Furthermore, the cause of scoliosis is believed to be multifactorial because of the known associations between the development of scoliosis and growth, hormonal secretion, gravity, and other factors [1, 10, 11, 13, 16, 17, 19, 27, 29, 31]. However, none of these factors has been conclusively shown to have a causative role in the development of AIS.
The association between osteopenia and idiopathic scoliosis was first reported by Burner et al. [3] in 1982 using the Singh index. Generalized reduced bone mass and osteopenia in both axial and peripheral skeletons have been reported in AIS [4, 6, 8, 28, 30] and abnormal histomorphometric bone cell activity has been reported in bone biopsies in AIS [7]. Low bone mass in AIS patients is likely to persist through to adulthood [5] and there is a growing concern that adolescents with idiopathic scoliosis have a lower peak bone mass, which would increase the risk of osteoporosis development and its related complications in later life [5, 6]. However, the mechanisms and causes of bone loss in AIS have not been identified.
Osteoporosis is characterized by a reduction in skeletal mass caused by an imbalance between bone resorption and bone formation. Furthermore, it is generally recognized that osteoporosis is characterized by a reduced bone-forming ability that is mediated by functional defects and reduced numbers of osteoblasts and their precursors [18], and it has been suggested that the functional characteristics of mesenchymal stem cells (MSCs) and changes in the regulation of their differentiation pathway may have important implications in some osteogenic disorders [12, 21]. It has also been postulated that age-related defects in osteoblast numbers and functions may be due to quantitative and qualitative stem cell defects [2]. Rodriguez et al. [24] postulated that the osteogenic alterations, associated with osteoporosis, may be explained in part by changes in the functional dynamic response of progenitor bone cells. Nuttall et al. [21] reported that the reduced bone volume associated with osteoporosis is accompanied by increased adipose tissue formation in marrow. In addition, Rodriguez et al. [24] found that increased bone marrow adipocyte production in osteoporosis is counterbalanced by diminished osteogenic cell production and several studies have suggested a relationship between senile osteoporosis and activities of MSCs. However, few studies have addressed the associations between the osteogenic and adipogenic differentiation abilities of MSCs and bone mass in AIS. Accordingly, we undertook to study the relationships between the osteogenic and adipogenic differentiation abilities of MSCs and bone mass in AIS girls and compared these with corresponding relations in healthy controls.
Materials and methods
We studied 19 adolescent girl patients who had been admitted to our department for the surgical correction of scoliosis. The Cobb angles of the curves were measured on plain radiographs. All patients aged between 11 and 14 years presenting to the adolescent idiopathic scoliosis were asked to voluntary provide bone marrow (BM) for measurement of MSCs activities and to examine a bone mineral density of lumbar spine and proximal femur. Sixteen age- and gender-matched patients with lower leg fracture were used as the control group. All the control subjects had a straight spine and a normal forward bending test on the physical examination with no history of spinal disease. Subjects with a history of congenital deformities, neuromuscular disease, endocrine disease, skeletal dysplasia, connective tissue abnormalities or mental retardation were excluded from the study. All subjects and their parents provided informed consent before the examination and measurements. The study was approved by the Clinical Research Ethics Committee of the university and the hospital.
Anthropometric measurement
Anthropometric measurements included body height and body weight. A standard standing whole spine antero-posterior radiograph was taken for each AIS patient. A standard technique was used for the measurement of the Cobb’s angle. For AIS patients, corrected height was derived from Bjure’s formula (log y = 0.011x − 0.177, where y is the loss of trunk height (cm) due to the deformed spine and x is the greatest Cobb angle of the primary curve) [5]. Body mass index (BMI) was determined by dividing weight (kg) by the square of the uncorrected height (m2).
Dual energy X-ray absorptiometry
Lumbar spinal bone mineral density (LSBMD) and femoral neck BMD (FNBMD) of the non-dominant proximal femur were measured by dual-energy X-ray absorptiometry (DEXA) (XR-36; Norland Corp., Fort Atkinson, WI). LSBMD was measured in L1 through L4 with anterior-posterior view. The scoliotic curvature of the spine in AIS patients may present difficulties in measuring the spinal BMD reliably. To minimize this problem, the amount of rotation in patients with AIS was determined by pre-scanning of the spine and then the LSBMD was measured in the neutral position [28].
Measurement of osteogenic and adipogenic differentiation abilities
Mononuclear cells were separated from BM by centrifugation in a Ficoll–Hypaque gradient (density = 1.077 g/cm3; Sigma, St. Louis, MO), suspended in alpha-modified essential medium (α-MEM) containing 20% fetal bovine serum (FBS), and were seeded at a concentration of 1 × 106 cells/cm2. The cultures were maintained at 37° in a humidified atmosphere containing 5% CO2. Colonies of fibroblastic cells began to appear in culture flasks at 5–7 days after plating BM-nucleated cells from the iliac crest. The patient-derived MSCs formed colonies in primary culture that had characteristic, spindle-shaped morphologic features. When monolayers of adherent cells had reached 80% confluence, cells were trypsinized (0.25% trypsin; Sigma), and resuspended in α-MEM containing 20% FBS and subcultured at a concentration of 1 × 103 cells/cm2. The cells were expanded in vitro by successive subculturing and then used in the described experiments.
To examine expressions of cell surface markers, cells were fixed with 4% paraformaldehyde for 10 min and then permeabilized with 0.1% triton X-100 for 10 min. The blocking and dilution solution used, consisting of phosphate-buffered saline (PBS), 1% bovine serum albumin (BSA), and 1% serum (Sigma) from similar species in which the secondary antibodies had been raised. The slides were blocked for 30 min, incubated sequentially for 30 min each with the antibodies against CD29-PE (1:100), CD44-FITC (1:100), CD133 (1:100), CD34 (1:100), or CD14(1:100), and fluorescein- or phycoerythrin-coupled anti-mouse or anti-mouse immunoglobulin G (IgG) antibodies. Between each step, slides were washed with PBS plus 0.3% BSA.
The MSCs from initial third passages were harvested to 90% confluence and counted. The period for doubling the cell number was determined after plating the constant number of cells (1 × 103 cells/cm2) to a 12-well plate.
After plating a constant number of cells in 12-well plates, osteogenic differentiation was induced by culturing MSCs of confluent state for 2 weeks in an osteogenic medium (10% FBS, 0.1 μmol/l dexamethasone, 10 μmol/l β-glycerophosphate, and 50 μg/ml ascorbic acid in α-MEM). Degrees of extra-cellular matrix (ECM) calcification were quantified by alizarin red S staining. Osteogenic differentiation was quantified by measuring areas stained with alizarin red S using an image analysis program (Meta Morph, Universal Imaging, Downing-town, PA). Measurements were performed in duplicate, and experiments were repeated three to four times. To measure alkaline phosphatase activities, cells were washed twice with Tris-buffered saline (50 nmol/l Tris and 150 mmol/l NaCl; pH 7.4), scraped off plates, and lysed by sonication. Cell lysates were reacted at 37°C for 1 h in a buffer containing 0.1 mol/l 2-amino-2-methyl-1,3-propanediol, 5 mmol/l MgCl2 (pH 10.0), and 10 μmol/l p-nitrophenylphosphate. Alkaline phosphatase activities were expressed as mol p-nitrophenol/min/mg protein. Protein contents were determined using a protein assay kit (Bio-Rad, Hercules, CA) with BSA as the standard.
After plating a constant number of cells in 12-well plate, MSCs were grown until confluent. No differences in cell sizes were evident between patients and control (data not shown). Adipogenic differentiation was induced by culturing the MSCs for 2 weeks in an adipogenic medium (10% FBS, 1 μmol/l dexamethasone, 100 μg/ml 3-isobutyl-1-methylxanthine, 5 μg/ml insulin, and 60 μmol/l indomethacin in α-MEM), and was assessed by an Oil Red O (Sigma) staining as an indicator of intracellular lipid accumulation. Cells were fixed in 4% neutral buffered formalin for 10 min followed, washed with 3% isopropanol, incubated with newly filtered Oil Red O staining solution for 1 h at room temperature, and rinsed with double-distilled H2O. To obtain quantitative data, 1 ml of isopropyl alcohol was added to stained culture dishes. Optical densities of Oil Red O staining were measured at 405 nm.
Statistical analysis
Statistical analysis was performed using SPSS 11.5 software for Windows (SPSS, Chicago, IL). Data were expressed by mean ± standard deviation. Groups were compared using the Mann–Whitney test and the correlations were determined using Pearson’s or Spearman’s correlation, as appropriate. A P < 0.05 was regarded as statistically significant.
Results
Table 1 presents a summary of the results of the AIS patients and controls. Mean weight, BMI and corrected BMI for patients were 41.6 ± 4.0 kg, 17.4 ± 1.5 kg/m2 and 17.1 ± 1.6 kg/m2, respectively, and mean weight and BMI for controls were 43.3 ± 6.7 kg and 18.3 ± 2.7 kg/m2. There was no significant difference of the mean weight, BMI and corrected BMI between two groups. Mean LSBMD and FNBMD for patients were 0.714 ± 0.057 g/cm2 and 0.728 ± 0.043 g/cm2, respectively, and for controls 0.765 ± 0.072 g/cm2 and 0.739 ± 0.074 g/cm2, respectively. The mean LSBMD in patients with AIS was decreased compared with that in controls (P = 0.037). There was no significant difference in FNBMD between the AIS and the control subjects.
Table 1.
Results of the AIS patients and control subjects
| AIS | Control | P-value | |
|---|---|---|---|
| Age (years) | 13.5 ± 0.88 | 13.1 ± 0.8 | 0.2202 |
| BMI (kg/m2) | 17.4 ± 1.5 | 18.3 ± 2.7 | 0.5184 |
| cBMI (kg/m2) | 17.1 ± 1.6 | 18.3 ± 2.7 | 0.2142 |
| LSBMD (g/cm2) | 0.714 ± 0.057 | 0.765 ± 0.072 | 0.0370 |
| FNBMD (g/cm2) | 0.728 ± 0.043 | 0.739 ± 0.074 | 0.6549 |
| Doubling time (h) | 30.4 ± 5.5 | 29.6 ± 4.9 | 0.4963 |
| Alkaline phosphatase activity (mol p-nitrophenol/min/mg) | 19.2 ± 2.1 | 22.8 ± 3.0 | 0.001 |
| Osteogenic differentiation (%) | 58.0 ± 5.3 | 64.5 ± 7.5 | 0.0073 |
| Adipogenic differentiation | 0.44 ± 0.19 | 0.42 ± 0.17 | 0.8296 |
cBMI corrected BMI
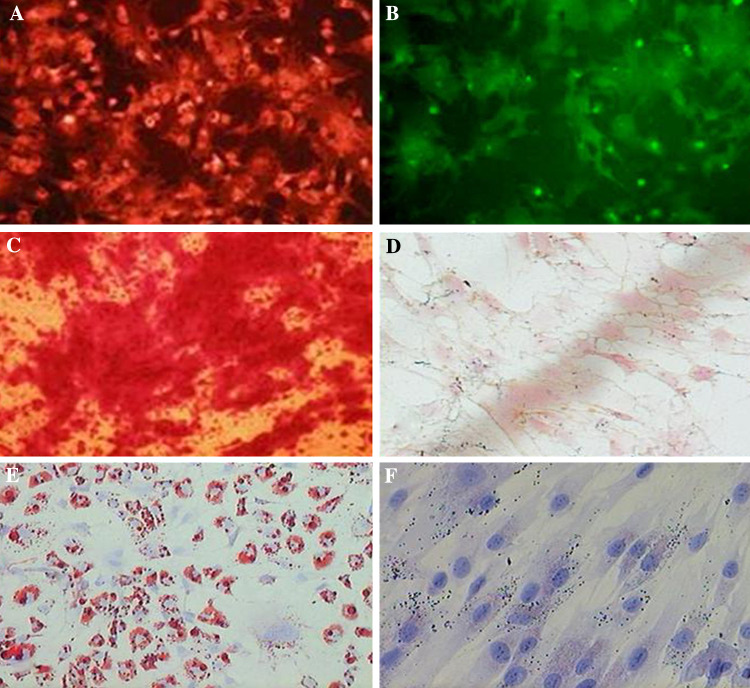
Surface marker expressions on MSCs were determined by immunocytochemistry using CD29-PE, CD44-FITC, CD34, CD14, and CD133 monoclonal antibodies in the undifferentiated state. MSCs were found to express CD29-PE and CD44-FITC, MSC markers (Fig. 1a, b), but not express the CD34, CD14, or CD133, hematopoietic cell markers.
Fig. 1.
MSC surface marker expression was determined by immunocytochemically using the monoclonal antibodies (a) CD29-PE and (b) CD44-FITC as markers. Localized perinuclear pattern was observed (magnification, ×200). c MSCs were cultured for 2 weeks in osteogenic medium and stained with alizarin red S stain to identify differentiated cells. Several red stained regions, indicative of the presence of a calcified extracellular matrix, were observed (magnification, ×200). d No calcification was observed in undifferentiated MSCs maintained in control osteogenic medium (magnification, ×200). e The MSCs were cultured for 2 weeks in the adipogenic medium and stained with Oil Red O. A significant fraction of the cells contained many intracellular lipid-filled droplets that had accumulated Oil Red O (magnification, ×200). f No lipid droplets were observed in undifferentiated MSCs maintained in control adipogenic medium (magnification, ×200)
MSCs were differentiated toward the osteogenic and adipogenic lineages using lineage-specific induction factors to examine their multilineage capacities. ECM calcification was assessed in MSCs by alizarin red S staining; calcified regions in cell monolayer were stained red. Consistent with osteogenesis, several red regions were observed in MSCs treated for 2 weeks in osteogenic medium (Fig. 1c). No calcification was observed in undifferentiated MSCs (Fig. 1d).
To determine if MSCs underwent adipogenesis, the cells were cultured in adipogenic medium and stained with Oil Red O. Significant fractions (approximately 80%) of the cells were found to contain many intracellular lipid-filled droplets that accumulated Oil Red O (Fig. 1e), but no lipid droplets were observed in undifferentiated MSCs (Fig. 1f).
The doubling time of the MSCs obtained from AIS patients was similar to that of the controls. A comparison of the osteogenic differentiation abilities of MSCs from the patients and controls revealed a decrease (P = 0.0073) in the osteogenic differentiation abilities of MSCs from patients. Furthermore, the alkaline phosphatase activities of MSCs from patients were significantly lower than those of control (P = 0.001). However, MSCs from patients and controls were similarly able to undergo adipogenic differentiation.
Correlations between variables are summarized in Tables 2 and 3. Chronological age was positively correlated with FNBMD in the patients and with LSBMD and FNBMD in controls. LSBMD was positively correlated with FNBMD in both groups. However, the BMI was not correlated with any parameter in either group. Osteogenic differentiation ability was positively correlated with alkaline phosphatase activity in patients, but osteogenic and adipogenic abilities were not correlated with LSBMD and FNBMD in either group.
Table 2.
Correlations of the parameters in patients with AIS
| Age | BMI | cBMI | LSBMD | FNBMD | Doubling time | Alkaline phosphatase activity | Osteogenic differentiation | Adipogenic differentiation | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | r | −0.2887 | −0.2381 | 0.4330 | 0.4934 | 0.1832 | −0.2554 | −0.1580 | 0.1549 | |
| P | 0.2307 | 0.3264 | 0.0641 | 0.0318 | 0.4529 | 0.2913 | 0.5184 | 0.5266 | ||
| BMI | r | −0.2887 | 0.3734 | −0.0482 | 0.0530 | −0.0020 | 0.3737 | −0.0207 | 0.2153 | |
| P | 0.2307 | 0.1153 | 0.8447 | 0.8293 | 0.9935 | 0.1151 | 0.9329 | 0.3761 | ||
| cBMI | r | −0.2381 | 0.3734 | −0.0161 | 0.0958 | −0.0187 | 0.3734 | −0.0098 | 0.2384 | |
| P | 0.3264 | 0.1153 | 0.9478 | 0.6965 | 0.9395 | 0.1153 | 0.9683 | 0.3256 | ||
| LSBMD | r | 0.4330 | −0.0482 | −0.0161 | 0.6152 | 0.3207 | 0.0444 | 0.2793 | 0.1110 | |
| P | 0.0641 | 0.8447 | 0.9478 | 0.0051 | 0.1806 | 0.8567 | 0.2468 | 0.6511 | ||
| FNBMD | r | 0.4934 | 0.0530 | 0.0958 | 0.6152 | 0.0785 | −0.0197 | 0.0255 | 0.3124 | |
| P | 0.0318 | 0.8293 | 0.6965 | 0.0051 | 0.7493 | 0.9362 | 0.9173 | 0.1928 | ||
| Doubling time | r | 0.1832 | −0.0020 | −0.0187 | 0.3207 | 0.0785 | −0.2638 | −0.1851 | −0.4898 | |
| P | 0.4529 | 0.9935 | 0.9395 | 0.1806 | 0.7493 | 0.2752 | 0.4481 | 0.0333 | ||
| Alkaline phosphatase activity | r | −0.2554 | 0.3737 | 0.3734 | 0.0444 | −0.0197 | −0.2638 | 0.5475 | 0.3688 | |
| P | 0.2913 | 0.1151 | 0.1153 | 0.8567 | 0.9362 | 0.2752 | 0.0153 | 0.1202 | ||
| Osteogenic differentiation | r | −0.1580 | −0.0207 | −0.0098 | 0.2793 | 0.0255 | −0.1851 | 0.5475 | −0.0816 | |
| P | 0.5184 | 0.9329 | 0.9683 | 0.2468 | 0.9173 | 0.4481 | 0.0153 | 0.7399 | ||
| Adipogenic differentiation | r | 0.1549 | 0.2153 | 0.2384 | 0.1110 | 0.3124 | −0.4898 | 0.3688 | −0.0816 | |
| P | 0.5266 | 0.3761 | 0.3256 | 0.6511 | 0.1928 | 0.0333 | 0.1202 | 0.7399 |
cBMI corrected BMI
Table 3.
Correlations of the parameters in control subjects
| Age | BMI | LSBMD | FNBMD | Doubling time | Alkaline phosphatase activity | Osteogenic differentiation | Adipogenic differentiation | ||
|---|---|---|---|---|---|---|---|---|---|
| Age | r | 0.0260 | 0.6666 | 0.5953 | −0.0634 | −0.1737 | −0.4102 | 0.0449 | |
| P | 0.9238 | 0.0048 | 0.0150 | 0.8155 | 0.5199 | 0.1146 | 0.8688 | ||
| BMI | r | 0.0260 | 0.2061 | 0.0530 | 0.0949 | −0.3248 | −0.1734 | −0.2254 | |
| P | 0.9238 | 0.4438 | 0.8454 | 0.7267 | 0.2196 | 0.5208 | 0.4014 | ||
| LSBMD | r | 0.6666 | 0.2061 | 0.8495 | −0.2480 | −0.1737 | −0.2101 | −0.2694 | |
| P | 0.0048 | 0.4438 | 0.0001 | 0.3545 | 0.5201 | 0.4348 | 0.3130 | ||
| FNBMD | r | 0.5953 | 0.0530 | 0.8495 | −0.4382 | −0.0471 | −0.2061 | −0.3527 | |
| P | 0.0150 | 0.8454 | 0.0001 | 0.0896 | 0.8624 | 0.4439 | 0.1803 | ||
| Doubling time | r | −0.0634 | 0.0949 | −0.2480 | −0.4382 | −0.1872 | 0.4088 | −0.3081 | |
| P | 0.8155 | 0.7267 | 0.3545 | 0.0896 | 0.4875 | 0.1159 | 0.2457 | ||
| Alkaline phosphatase activity | r | −0.1737 | −0.3248 | −0.1737 | −0.0471 | −0.1872 | 0.4480 | −0.3081 | |
| P | 0.5199 | 0.2196 | 0.5201 | 0.8624 | 0.4875 | 0.0818 | 0.2457 | ||
| Osteogenic differentiation | r | −0.4102 | −0.1734 | −0.2101 | −0.2061 | 0.4088 | 0.4480 | −0.0073 | |
| P | 0.1146 | 0.5208 | 0.4348 | 0.4439 | 0.1159 | 0.0818 | 0.9785 | ||
| Adipogenic differentiation | r | 0.0449 | −0.2254 | −0.2694 | −0.3527 | 0.2408 | −0.3081 | −0.0073 | |
| P | 0.8688 | 0.4014 | 0.3130 | 0.1803 | 0.3690 | 0.2457 | 0.9785 |
Discussion
A generalized low bone mass and osteopenia in both the axial and peripheral skeletons in AIS have been reported [4, 6, 8, 28, 30]. However, the exact mechanisms of bone loss in AIS patients are not fully identified. Studies have shown that BMD in children can be affected by factors like body weight, height, physical activity, or nutrition status [14, 15, 20, 25]. Regular exercise has also been reported to significantly increase peak bone mass [14, 20]. The relationship between AIS and low body-weight may indicate disordered eating and is thus a cause for concern, particularly in the light of the well-established relationship between eating psychopathology and osteoporosis [26]. However, no significant difference was found in physical activity between the AIS and the controls [6]. The present study showed that BMI was not correlated to any variable in either study group. It is generally accepted that BMD is influenced by body weight, height, and BMI. The current study did not show any correlations of BMD with other factors such as BMI, doubling time, alkaline phosphatase activity, osteogenic and adipogenic differentiation abilities. Nevertheless, the chronological age had significant correlations with BMD in both groups. However, the number of patients included was relatively small in our study; therefore, our results should be confirmed by a larger population-based study.
Many studies have addressed the relationship between MSCs activity and osteoporosis, but few have examined the association between MSC activity and bone mass in AIS. Therefore, we studied the relationship between MSC activity and bone mass in AIS girls and compared these findings with those of healthy controls.
MSCs isolated from osteoporotic donors demonstrate functional differences. Osteoporotic cells have lower proliferation rates than control cells, and respond differently to insulin-like growth factor-1 (IGF-1) [24]. For example, control MSC numbers were found to increase two- to three-fold in response to different concentrations of IGF-1, whereas MSCs derived from osteoporotic donors did not proliferate across a wide IGF-1 concentration range. In this study we did not observe differences in response to IGF-1 expressed by AIS and control cells. Results of our study showed that the proliferation rate of the MSCs obtained from the patients with AIS was similar to that of the control subjects.
Another important functional difference observed between the MSCs of osteoporotic patients and controls concerns their abilities to respond to stimuli that induce differentiation to the osteogenic lineage [9, 22, 24]. Thus, only MSCs derived from control donors cultured under osteogenic condition differentiate into osteogenic lineage as evidence the increased alkaline phosphatase activity and calcium phosphate deposition. Also, MSCs derived from osteoporotic donors may have a diminished ability to differentiate into the osteogenic lineage concomitant with an increased ability to differentiate to others cell phenotypes, like adipocytes [9, 21, 23, 24]. Thus, clinical and in vitro observations document an inverse relationship between adipocyte and osteoblast. In osteoporotic patients, increase bone marrow adipose tissue correlates with decreased trabecular bone volume [12]. The results of our study revealed that the MSCs obtained from the AIS patients had decreased osteogenic differentiation ability and alkaline phosphatase activity compared with the cells obtained from the control subjects, but the ability of the MSCs from the patients with AIS to undergo adipogenic differentiation was similar to that of the control subjects. The LSBMD in AIS patients was decreased compared with that in control subjects. The osteogenic differentiation ability was positively correlated to alkaline phosphatase activity in the AIS group. However, the osteogenic and adipogenic differentiation abilities were not correlated to LSBMD and FNBMD in both groups. These results might impose the assumptions that the decreased osteogenic differentiation ability of MSC might be one of the possible mechanisms leading to low bone mass in AIS.
Some potential limitation of this study should be considered. Firstly, the number of patients included was relatively small. To reflect the true levels of differentiation abilities of MSCs in BM, studies with larger patient numbers should be performed. Secondly, it is well known that the decreased osteogenic differentiation and proliferation abilities are shown in osteoporotic woman. However, these levels are not specific for delineation of disease conditions. In the current study, the AIS patients revealed the reduction of osteogenic differentiation ability. Nevertheless, with these changes of the osteogenic differentiation ability, it might be difficult to distinguish the AIS from other disease conditions. Thirdly, the results of our study revealed that the osteogenic differentiation ability of MSCs and LSBMD in AIS patients were lower than in controls. The positive correlation between osteogenic differentiation ability of MSCs and LSBMD is required to support the pathogenesis and cause of low bone mass in AIS patients, but the osteogenic differentiation ability of MSCs was not correlated to LSBMD in this study.
In summary, we attempted to determine the low BMD, to measure the MSCs activities, and to find the correlation between BMD and MSCs activities in AIS. The MSCs obtained from the AIS patients had decreased osteogenic differentiation ability and alkaline phosphatase activity compared with the cells obtained from the control subjects but ostoegenic differentiation ability did not tend to be related to BMD. Our data suggest that the decreased osteogenic differentiation ability of MSC might be one of the possible mechanisms leading to low bone mass in AIS. However, we did not determine definite mechanisms of low bone mass in AIS. Therefore, further study with large scale will be needed to identify the mechanism involved.
References
- 1.Ahn UM, Ahn NU, Nallamshetty L, Buchowski JM, Rose PS, Miller NH, Kostuik JP, Sponseller PD. The etiology of adolescent idiopathic scoliosis. Am J Orthop. 2002;31:387–395. [PubMed] [Google Scholar]
- 2.Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996;11:568–577. doi: 10.1002/jbmr.5650110504. [DOI] [PubMed] [Google Scholar]
- 3.Burner WL, Badger VM, Sherman FC. Osteoporosis and acquired back deformities. J Pediatr Orthop. 1982;2:383–385. doi: 10.1097/01241398-198210000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Cheng JC, Guo X. Osteopenia in adolescent idiopathic scoliosis. A primary problem or secondary to the spinal deformity? Spine. 1997;22:1716–1721. doi: 10.1097/00007632-199708010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Cheng JC, Guo X, Sher AH. Persistent osteopenia in adolescent idiopathic scoliosis. A longitudinal follow up study. Spine. 1999;24:1218–1222. doi: 10.1097/00007632-199906150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Cheng JC, Qin L, Cheung CS, Sher AH, Lee KM, Ng SW, Guo X. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res. 2000;15:1587–1595. doi: 10.1359/jbmr.2000.15.8.1587. [DOI] [PubMed] [Google Scholar]
- 7.Cheng JC, Tang SP, Guo X, Chan CW, Qin L. Osteopenia in adolescent idiopathic scoliosis: a histomorphometric study. Spine. 2001;26:E19–E23. doi: 10.1097/00007632-200104150-00023. [DOI] [PubMed] [Google Scholar]
- 8.Cook SD, Harding AF, Morgan EL, Nicholson RJ, Thomas KA, Whitecloud TS, Ratner ES. Trabecular bone mineral density in idiopathic scoliosis. J Pediatr Orthop. 1987;7:168–174. doi: 10.1097/01241398-198703000-00011. [DOI] [PubMed] [Google Scholar]
- 9.D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 10.Ford DM, Bagnall KM, Clements CA, McFadden KD. Muscle spindles in the paraspinal musculature of patients with adolescent idiopathic scoliosis. Spine. 1988;13:461–465. doi: 10.1097/00007632-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ford DM, Bagnall KM, McFadden KD, Greenhill BJ, Raso VJ. Paraspinal muscle imbalance in adolescent idiopathic scoliosis. Spine. 1984;9:373–376. doi: 10.1097/00007632-198405000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421–428. doi: 10.1016/S8756-3282(96)00258-X. [DOI] [PubMed] [Google Scholar]
- 13.Herman R, Mixon J, Fisher A, Maulucci R, Stuyck J. Idiopathic scoliosis and the central nervous system: a motor control problem [The Harrington lecture 1983]. Scoliosis Research Society. Spine. 1985;10:1–14. doi: 10.1097/00007632-198501000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Ho S, Wong E, Chan SG, Lau J, Chan C, Leung PC. Determinants of peak bone mass in Chinese women aged 21–40 years. III. Physical activity and bone mineral density. J Bone Miner Res. 1997;12:1262–1271. doi: 10.1359/jbmr.1997.12.8.1262. [DOI] [PubMed] [Google Scholar]
- 15.Johnston CC, Jr, Miller JZ, Slemenda CW, Reister TK, Hui S, Christian JC, Peacock M. Calcium supplementation and increase in bone mineral density in children. N Engl J Med. 1992;327:82–87. doi: 10.1056/NEJM199207093270204. [DOI] [PubMed] [Google Scholar]
- 16.Kindsfater K, Lowe T, Lawellin D, Weinstein D, Akmakjian J. Levels of platelet calmodulin for the prediction of progression and severity of adolescent idiopathic scoliosis. J Bone Joint Surg. 1994;76-A:1186–1192. doi: 10.2106/00004623-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J, Toriyama S. Pathogenesis of idiopathic scoliosis: SEPs in chicken with experimentally induced scoliosis and in patients with idiopathic scoliosis. J Pediatr Orthop. 1994;14:329–335. doi: 10.1097/01241398-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15:546–557. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- 19.McCarrey JR, Abbott UK, Benson DR, Riggins RS. Genetics of scoliosis in chickens. J Hered. 1981;72:6–10. doi: 10.1093/oxfordjournals.jhered.a109428. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson BE, Westlin NE. Bone density in athletes. Clin Orthop. 1971;77:179–182. [PubMed] [Google Scholar]
- 21.Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res. 1998;13:371–382. doi: 10.1359/jbmr.1998.13.3.371. [DOI] [PubMed] [Google Scholar]
- 22.Pignolo RJ, Suda RK, McMillan EA, Shen J, Lee SH, Choi Y, Wright AC, Johnson FB. Defects in telomere maintenance molecules impair osteoblast differentiation and promote osteoporosis. Aging Cell. 2008;7:23–31. doi: 10.1111/j.1474-9726.2007.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez JP, Astudillo P, Rios S, Pino AM. Involvement of adipogenic potential of human bone marrow mesenchymal stem cells (MSCs) in osteoporosis. Curr Stem Cell Res Ther. 2008;3:208–218. doi: 10.2174/157488808785740325. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez JP, Garat S, Gajardo H, Pino AM, Seitz G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999;75:414–423. doi: 10.1002/(SICI)1097-4644(19991201)75:3<414::AID-JCB7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 25.Slemenda CW, Peacock M, Hui S, Zhou L, Johnston CC. Reduced rates of skeletal remodeling are associated with increased bone mineral density the development of peak skeletal mass. J Bone Miner Res. 1997;12:676–682. doi: 10.1359/jbmr.1997.12.4.676. [DOI] [PubMed] [Google Scholar]
- 26.Smith FM, Latchford G, Hall RM, Millner PA, Dickson RA. Indications of disordered eating behaviour in adolescent patients with idiopathic scoliosis. J Bone Joint Surg. 2002;84-B:392–394. doi: 10.1302/0301-620X.84B3.12619. [DOI] [PubMed] [Google Scholar]
- 27.Stilwell DL., Jr Structural deformities of vertebrae: bone adaptation and modeling in experimental scoliosis and kyphosis. J Bone Joint Surg. 1962;44-A:611–634. [PubMed] [Google Scholar]
- 28.Suh KT, Lee SS, Hwang SH, Kim SJ, Lee JS. Elevated soluble receptor activator of nuclear factor-κB ligand and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J. 2007;16:1563–1569. doi: 10.1007/s00586-007-0390-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suh KT, Lee SS, Kim SJ, Kim YK, Lee JS. Pineal gland metabolism in patients with adolescent idiopathic scoliosis. J Bone Joint Surg. 2007;89-B:66–71. doi: 10.1302/0301-620X.89B1.18058. [DOI] [PubMed] [Google Scholar]
- 30.Thomas KA, Cook SD, Skalley TC, Renshaw SV, Makuch RS, Gross M, Whitecloud TS, 3rd, Bennett JT. Lumbar spine and femoral neck bone mineral density in idiopathic scoliosis: a follow up study. J Pediatr Orthop. 1992;12:235–240. doi: 10.1097/01241398-199203000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Wang ED, Drummond DS, Dormans JP, Moshang T, Davidson RS, Gruccio D. Scoliosis in patients treated with growth hormone. J Pediatr Orthop. 1997;17:708–711. doi: 10.1097/00004694-199711000-00003. [DOI] [PubMed] [Google Scholar]