Abstract
The aim of this work was to study the ability of β-cyclodextrin (β-CD) or hydroxypropyl β-cyclodextrin (HP-β-CD) to ameliorate the induction of gastric ulcers by a nonsteroidal anti-inflammatory drug, indomethacin or piroxicam, in rats exposed to restraint and hypothermic stress at 4 °C. Using oral gavage, rats fasted for 72 h were administered the equivalent of a 100 mg/kg dose of the assigned drug, alone or with the designated cyclodextrin (CD). The rats were placed in suitable rodent restrainers and then placed inside a ventilated refrigerator maintained at a temperature of 4 °C. Six hours later, each animal was removed, anaesthetized with ether, and the abdomen opened. Each stomach was removed, opened along the greater curvature and gently rinsed with isotonic saline solution. The induced gastric ulcers were examined and assessed with the help of a 10x binocular magnifier. Pronounced and marked gastric ulceration with complete loss of the mucosa, extensive deposition of fibrin and dense neutrophilic infiltrate were observed in rats treated with each of the drugs alone. Treatment with indomethacin or piroxicam alone induced ulcer indices of 26 ± 2.3 or 14 ± 1.8, respectively. However, β-CD and HP-β-CD each significantly suppressed ulceration due to restraint and cold stress. Rats treated with indomethacin or piroxicam in the presence of either β-CD or HP-β-CD exhibited normal tissues. Therefore, β-CD and HP-β-CD act as protective agents against gastrointestinal disorders produced by restraint and cold stress, even with the added stress from administration of either indomethacin or piroxicam.
Keywords: β-cyclodextrin, hydroxypropyl β-cyclodextrin, indomethacin, piroxicam, gastric ulcers, histological examination
Introduction
Although oral nonsteroidal anti-inflammatory drugs (NSAIDs) are effective in the treatment of a variety of acute and chronic pain conditions, their use has been associated with the induction of gastric injury, which is the most common adverse effect 1. Physical and psychological stresses are triggers or modifiers of the clinical course of gastrointestinal disorders, such as peptic ulcer, irritable bowel syndrome, or inflammatory bowel disease 2. Stress can act synergistically with other pathogenic factors, such as Helicobacter pylori, nonsteroidal anti-inflammatory drugs, or colitis-inducing chemicals to produce gastrointestinal disease 2. It has been demonstrated that restraint and cold (4 oC), as well as indomethacin 3-5, can induce ulceration.
The principal mechanism of action of all NSAIDs is the inhibition of the biosynthesis of prostaglandins by blocking the activity (selectively and/or non-selectively) of two isoforms of cyclooxygenase enzyme (COX-1 and COX-2) 6-8. With the exception of aspirin, NSAIDs inhibit synthesis of prostaglandins (mediators of inflammation) via competition with arachidonic acid for the two isoforms. Generally, in the absence of any pathological conditions, continuous release of prostaglandins usually contributes to certain physiological functions in both humans and animals. These include inhibition of gastric acid secretion, regulation of vascular tone, regulation of renal blood flow, and regulation of platelet aggregation 11,12.
The gastric ulcerogenic action of NSAIDs is believed to occur mainly due to their local inhibitory effect on gastric prostaglandin E2 (PGE2) and prostaglandin I2 (PGI2) that are the main inhibitors of gastric acid secretion 13,14. The major contribution of the local ulcerogenic action of NSAIDs can be appreciated from the decreased incidence of ulcers following the use of NSAIDs enteric coated tablets.
CDs are non-reducing, water-soluble oligosaccharides with nonpolar cavities that allow complexation with the nonpolar portion of many drug molecules 13. Inclusion complexes of poorly soluble drugs with CDs present more solubilized drug, a greater rate of dissolution, and a more efficient absorption after oral administration, compared to uncomplexed drug 15,16. Toxicity tests have demonstrated that orally administered CDs are essentially nontoxic, largely because they are not absorbed from the gastrointestinal tract 17. Gastric ulceration induced by oral administration of flurbiprofen was significantly decreased by the presence of CDs, in particular by β-CD 18.
Indomethacin is one of the first anti-inflammatory drugs introduced for closure of patent ductus arteriosus of newborns and treatment of various inflammatory conditions 13. The complex of indomethacin with CDs has been well studied 19-22 in solution and in the solid state 13,19,20. It accomplishes a 1:1 inclusion complex with β-CD or HP-β-CD 22 by inserting either the p-chlorobenzoic portion or the indole unit of the molecule into the CD cavity 23-25. In addition, inclusion complexes of indomethacin with β-CD or HP-β-CD were prepared and evaluated in vivo 21,22. These inclusion complexes have the ability to reduce gastrointestinal adverse effects 23,24.
The oxicams are a class of NSAIDs that have made a considerable impact since their introduction. However, some concern has been expressed about the apparent high risk of gastrointestinal side effects associated with the use of some oxicams 26. As a result, research efforts have been directed toward identifying a chemical that combines the potency of the oxicam class with a reduced risk of unwanted effects.
Piroxicam is a potent NSAID that is used in the treatment of rheumatoid arthritis, osteoarthritis, traumatic contusions, and different regional inflammatory disorders 27. It was reported that the inclusion complex of piroxicam with β-cyclodextrin (β-CD) in solution and solid states was obtained with a 1:1 stoichiometry 18,28,29. Moreover, the inclusion behaviors of piroxicam with β-CD, HP-β-CD, and carboxymethyl β-CD were investigated and the highest inclusion capacity was obtained with β-CD 30.
Since gastric ulceration induced by flurbiprofen (log P = 4.24) 31 was substantially reduced by β-CD, it would be appropriate to investigate the effect of CDs as protective agents against ulceration induced by more hydrophilic NSAIDs, namely indomethacin (log P = 3.8) 31,32 and piroxicam (log P = 0.59) 33, where CD inclusion becomes less important for solubilization.
The aim of the study is to compare the magnitude of gastric ulcers of rats under cold stress (4 ºC) following oral treatment with indomethacin and piroxicam each alone and after complexation of each with β-CD or HP-β-CD.
Materials and Methods
β-Cyclodextrin was purchased from Acros Organics (Morris Plains, New Jersey, USA). Indomethacin and hydroxypropyl β-cyclodextrin with a 0.6 degree of substitution were purchased from Fluka Chemical Company (Chemie GmbH, Buchs, Switzerland). CDs were used as received. Piroxicam was obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). All remaining chemicals were analytical grade.
Preparation of CD Solutions
An aqueous solution of each CD was prepared by dissolving accurately weighed CDs (180 mg of β-CD and 300 mg of HP-β-CD) in 10 ml of distilled water with the aid of a UP100H ultrasonic device, (100W, 30kHz, Hielscher Ultrasonics, Teltow, Berlin, Germany) for 5 min. In the case of β-CD, a concentration of 1.8 % w/v (16 mM) was prepared. A 30 % w/v (217.4 mM) solution of HP β-CD was also prepared. These aqueous CDs solutions in the same concentrations were used to dose the rats with the drugs.
Preparation of Piroxicam and Indomethacin Suspensions
An aqueous suspension of piroxicam or indomethacin was prepared by spreading 250 mg of each drug into 10 ml distilled water. In addition, a suspension of each drug was prepared in the presence of either β-CD or HP β-CD by sprinkling 250 mg of each drug into 10 ml of aqueous CD solutions with the same concentrations (1.8% of β-CD and 30% of HP β-CD) as described in the previous section. Ultrasonication was utilized to obtain a well-dispersed suspension of each drug. The suspension of each drug in the presence of CDs was considered to contain both the soluble drug complex with CD and excess insoluble suspended particles of drug.
Solubility Study
The phase-solubility experiments of each drug (piroxicam or indomethacin) with either β-CD or HP-β-CD were carried out by the method reported by Higuchi and Connors 34 in distilled water. A series of volumetric flasks each containing successively increasing quantities of either β-CD or HP-β-CD were prepared. Excess of each drug was added into each flask to maintain saturated conditions. All flasks were sonicated for 30 min at 37±1 °C. Following equilibrium, each supernatant phase was removed, filtered, diluted, and assayed for the total solubilized drug content by UV analysis. The binding constants of each drug with each CD were calculated from the phase-solubility slope according to the following equation:
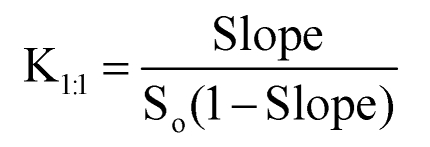 |
where So is the solubility of the drug in the absence of the CD, and K1:1 is the binding constant.
Determination of Complexed Drug with CD
The amount of complexed drug (piroxicam or indomethacin) with either β-CD or HP-β-CD, was determined by its distribution coefficient (partition coefficient) in order to estimate the ratio of free-to-complexed drug. An accurate concentration of piroxicam (1.5 x 10-4 M) or indomethacin (1.1 x 10-4 M) in 0.1 N HCl was prepared from which 5 ml was added to 5 ml n-octanol in volumetric flasks. These two immiscible liquids were sonicated for 15 min at 37 ± 1 °C, set aside for 1.5 h, and then the aqueous phase was separated using a separating funnel. The concentration of each drug was determined in the aqueous phase spectrophotometrically at 338 nm and 267 nm for piroxicam and indomethacin, respectively. The procedures were repeated in the presence of either β-CD (0.016 M) or HP-β-CD (0.030 M) in the aqueous phase. The total concentration of free and complexed drug in the presence of CDs was measured, and the ratio of the free-to-complexed amount was calculated. Due to the low concentration of drug in each phase, the partition coefficients of each drug in the absence and presence of CDs could be estimated by the ratio of the drug concentration in the octanol phase to the concentration in the aqueous phase.
Experimental Animals
Male Wistar rats (250 g body weight) were provided by the Experimental Animals Care Center (College of Pharmacy, King Saud University, Riyadh, Saudi Arabia). The animals were maintained at 22 ± 1 °C on a 12 h light-dark cycle and allowed rat chow and water ad libitum. Nine groups of Wistar rats (n = 4 animals per group) were used. The allocation of the animals to the groups was randomized using a standard random table. Experimental protocols were approved by the Animal Care and Use Committee and were in accordance with the recommendations in the University Guide for the Care and Use of Laboratory Animals.
Before the start of the experiments, food was withdrawn for 72 h but water was allowed ad libitum 13. Preliminary results have revealed that the pre-fasting condition alone doesn't induce ulcers, as evidenced by the absence of ulcers in some of the treated groups. It was found that the minimum experimental conditions for gastric induction in the Wistar rats used in this study were: fasting (72 h), hypothermic restraint stress exposure, and the administration of specified doses of the two NSAIDs.
As described in the studies by Bhargava et al. 14, Schmassmann et al. 35 and Brzozowski et al. 36, on the morning of the experiments each fasted rat was administered the assigned drug by oral gavage in a dose equivalent to 100 mg indomethacin or piroxicam per kg, then restrained in an appropriate rodent restrainer (Harvard Apparatus, Holliston, Massachusetts, USA) and placed inside a ventilated refrigerator, model 1852G (Norfolk Marine, Ltd., Norwich, Norfolk, UK), maintained at a temperature of 4 °C. Six hours later 37, each animal was removed, anaesthetized with ether, and the abdomen was opened. Each stomach was removed, opened along the greater curvature, and gently rinsed with isotonic saline solution. Each stomach was pinned out on a flat surface with the mucosal surface uppermost.
Examination of Gastric Ulcers
The induced gastric ulcers were examined and assessed with the help of a 10x binocular magnifier. To quantify the induced ulcers in each stomach, a modification of the scoring systems described in the literature 35,38-41 was employed. The induced ulcers in these experiments were small spots and thus each was given a score between 1 and 4. Ulcers of 0.5 mm diameter were given a score of 1 whereas ulcers of diameters 1 and 2 mm were given scores of 2 and 4, respectively. For each stomach, an ulcer index was calculated as the sum of the total score of ulcers. The cumulative ulcer index is presented as the mean (n = 4) ± standard deviation (s.d.).
Statistical Analysis
All data are presented as the mean ± the standard deviation (s.d.). Significant differences between the different treatment groups were determined by one-way analysis of variance (ANOVA) using the SPSS® statistical package (version 10, 1999, SPSS Inc., Chicago, IL). Statistical differences yielding P ≤ 0.05 were considered significant. Tukey's multiple comparison post hoc test was applied when necessary.
Histological Examination
For histological examination, all samples (stomachs of the rats) were removed and fixed overnight in 10 % w/v formalin. Each specimen was sectioned and submitted totally. They were processed overnight and then embedded in paraffin. The paraffin blocks were sectioned and the slides were stained with a standard haematoxylin and eosin stain and photographed under 20x magnification using a Nikon Eclipse 80i light microscope (Nikon Corporation, Japan) equipped with a digital DS-Ri1 camera.
Results and Discussion
The binding constants of piroxicam with β-CD and HP-β-CD were found to be 105 M-1 and 143 M-1, respectively with 1:1 stiochiometry. The amounts of complexed piroxicam with β-CD and HP-β-CD evaluated by distribution coefficient method were 20.6% and 44.4%, respectively. The ratio of free-to complexed piroxicam was 4:1 in case of β-CD and 5:4 in case of HP-β-CD. The binding constant of indomethacin with β-CD was also determined by the solubility method in water and calculated as 278 M-1. These results were in agreement with those constants reported by Uekama and Otagiri 18. The amount of complexed indomethacin with β-CD and HP-β-CD was evaluated by distribution coefficient between n-octanol and 0.1 N HCl and was found as 8.3% and 26.6%, respectively. The ratio of free-to-complexed indomethacin was 11:1 in the case of β-CD, and 3:1 for HP-β-CD. The lower partition coefficient of piroxicam in the presence of HP-β-CD, as compared to β-CD, is likely due to the relative complexation of the drug with the CDs. A similar effect of the CDs on the partition coefficient of indomethacin, albeit to a lesser extent, was observed.
Initial experiments using 10 or 50 mg/kg doses of either indomethacin or piroxicam in the absence of cold stress did not produce ulcerations whereas 100 mg/kg doses induced few and insignificant ulcers. However, if doses of 100 mg/kg given by oral gavage were coupled with restraint and hypothermic stress at 4 °C, a greater number of ulcers were produced. It should be noted that previous studies have pointed out that cold stress acts synergistically with NSAIDs to induce gastric ulceration 2. This last approach was used in this study to induce gastric ulceration. The diameters of the induced ulcers ranged from 0.5 to 2 mm.
Treatment with indomethacin and piroxicam alone induced ulcer indices of 26 ± 2.3 and 14 ± 1.8, respectively. Complexation of each of the non-steroidal drugs with β-CD or HP-β-CD significantly suppressed the induced ulcers. Rats treated with either β-CD or HP-β-CD alone and exposed to restraint and hypothermic stress gave ulcer indices of 5.75 ± 0.9 and 2.75 ± 0.4, respectively. The cumulative results of these studies are shown in Table 1. The mean percentage reductions of the induced ulcers in the case of indomethacin were 75 and 86.5% following complexation with β-CD and HP-β-CD, respectively. The corresponding decreases in the case of piroxicam were 89.2 and 80.3%, respectively.
Table 1.
Effect of β-CD and HP-β-CD on indomethacin and piroxicam induced ulcers
| Treatment | Ulcer Index |
|---|---|
| Indomethacin | 26 ± 2.3 |
| Indomethacin + β-CD | 6.5 ± 0.9* |
| Indomethacin + HP-β-CD | 3.5 ± 0.3* |
| Piroxicam | 14 ± 1.8 |
| Piroxicam + β-CD | 1.5 ± 0.2* |
| Piroxicam + HP-β-CD | 2.75 ± 0.3* |
| β-CD | 5.75 ± 0.9 |
| HP-β-CD | 2.75 ± 0.4 |
| Cold stress | 3.8 ± 0.4 |
* Each of the drug and CD treatments are significantly different from the treatment with drug alone (P < 0.01, n = 4).
In this study, administration of each of the two NSAIDs coupled with hypothermic and restraint stress clearly induced ulcers. Chemically induced ulcers are believed to begin by inhibition of gastric prostaglandins derived via both COX-1 and COX-2 enzymes 35, 42 and increased free radical formation 43,44. On the other hand, the stress-induced ulcers are believed to result from an increase in the formation of free radicals 45,46, an increase in gastric acid secretion 45,47, reduction in gastric glutathione content 48,49 and a decrease in gastric PGE2 production 50,51. CDs are not absorbed in the gastrointestinal tract; they only enhance the absorption of drugs after oral administration. Thus, it seems that complexing NSAIDs with β-CD or HP-β-CD acts to interfere or prevent the local actions of the NSAIDs that induce gastric ulcers through local inhibition of gastric prostaglandins, increased gastric acid secretion, reduction of gastric glutathione, and local production of free radicals 15,16.
Histological Observations
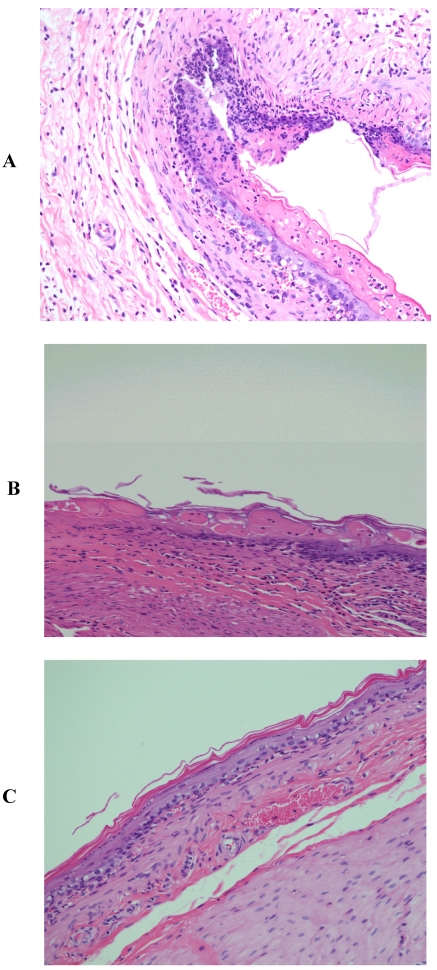
The histological pattern of the mucosal specimens was studied by examining the histology of the treated and control samples. Stomach tissue with mucosal ulceration was observed in indomethacin treated rats (Fig. 1A) with a complete loss of the mucosa and neutrophil infiltration and fibrin deposition. At the periphery of the ulcer, the adjacent squamous epithelium shows regenerative changes. On the other hand, the mucosal ulceration in groups treated with indomethacin and β-CD (Fig. 1B) was focal with a complete loss of mucosa with fibrin deposition and mild neutrophil infiltration. The specimens from rats treated with indomethacin and HP-β-CD (Fig. 1C) revealed no significant ulcerations and the tissues were almost intact.
Figure 1.
Histopathological photographs of rat stomach specimens stained with hematoxylin and eosin A: from rats treated with indomethacin alone; B: from rats treated with indomethacin and β-CD; C: from rats treated with indomethacin and HP-β-CD.
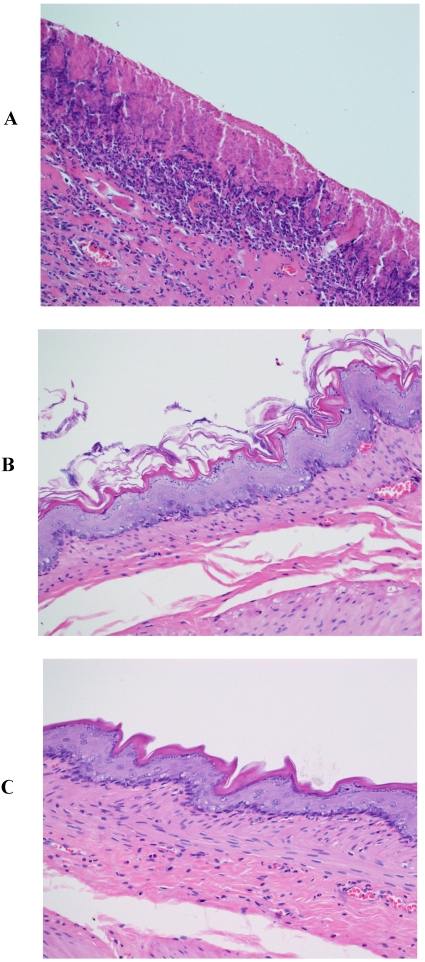
Pronounced and marked stomach ulceration with complete loss of the mucosa, extensive deposition of fibrin and dense neutrophil infiltrate was observed with rats treated with piroxicam (Fig. 2A). As with indomethacin, at the periphery of the ulcer, the adjacent squamous epithelium showed regenerative changes. However, rats treated with piroxicam and β-CD or HP-β-CD exhibited normal tissues (Figs. 2B and 2C).
Figure 2.
Histopathological photographs of rat stomach specimens stained with hematoxylin and eosin A: from rats treated with piroxicam alone; B: from rats treated with piroxicam and β-CD; C: from rats treated with piroxicam and HP-β-CD.
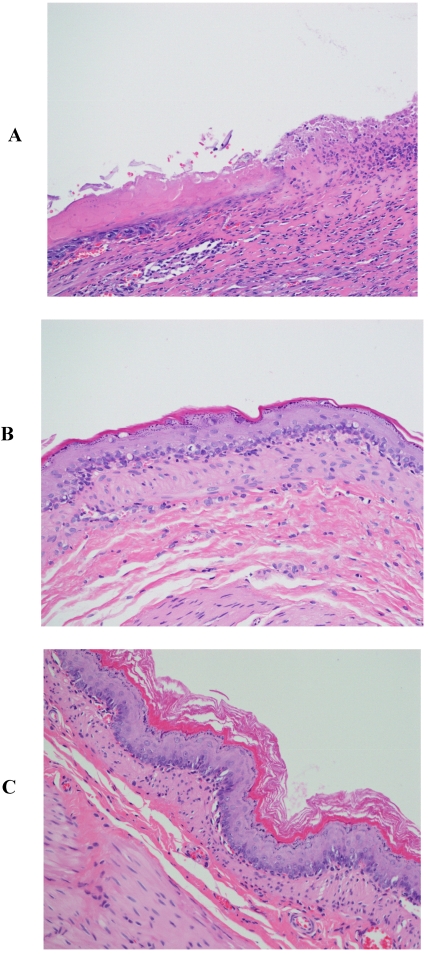
Those rats that served as the controls experienced the stress from restraint and cold, but not from treatment with an NSAID. Nevertheless, the specimens from these rats exhibited the same results seen with the additional treatment with indomethacin or piroxicam (Fig. 3A). Specimens from the control rats treated with β-CD (Fig. 3B) or HP-β-CD alone (Fig 3C) revealed that there were no significant ulcerations and the tissues were almost intact. The images for specimens from rats treated with either β-CD or HP-β-CD alone provide new evidence of their safety when orally administered. Perhaps more importantly, these CDs alone are capable of providing protection from ulceration due to restraint and cold stress.
Figure 3.
Histopathological photographs rat stomach specimens stained with hematoxylin and eosin A: from rats experiencing restraint and cold stress alone (X200); B: from rats experiencing restraint and cold stress, and treated with β-CD; C: from rats experiencing restraint and cold stress, and treated with HP-β-CD.
Both β-CD and HP-β-CD are practically insensitive to salivary α-amylase as well as stomach acid. They are poorly digested by pancreatic α-amylase in the human small intestine but might be degraded by the colonic flora. When either β-CD or HP-β-CD is administered orally, absorption is low and intact CDs are excreted in the feces 20. Hence, their protection against gastric ulceration could be attributed to competitive inclusion complexation with prostaglandins or mucin. Gastric ulcer prevention was evident from the appearance of normal tissues in rats treated with β-CD or HP-β-CD alone (Figs. 3B and 3C, respectively). On the other hand, untreated control rats exhibited marked ulceration with complete loss of mucosa (Figure 3A). This means that both CDs could be counteracting the stress-induced ulcers by inclusion complexation of prostaglandins or mucin. These proposed mechanisms are supported by the reported data for complexation of CDs with prostaglandins E1, E2, and F2,a in aqueous solutions 8,52-54.
Conclusions
It is clear that β-CD and HP-β-CD are safe for oral use. They act as protective agents against gastrointestinal disorders initiated by restraint and cold stress, or by restraint and cold stress and the co-administration of either indomethacin or piroxicam. Consequently, each of these CDs can be utilized in the formulation of oral preparations of indomethacin or piroxicam to avoid the typical ulceration side effects.
References
- 1.Bulbena O, Escolar G, Navarro C. et al. Gastroprotective effect of zinc acexamate against damage induced by nonsteroidal anti-inflammatory drugs: A morphological study. Dig Dis Sci. 1993;38:730–39. doi: 10.1007/BF01316807. [DOI] [PubMed] [Google Scholar]
- 2.Caso JR, Leza JC, Menchén L. The effects of physical and psychological stress on the gastro-intestinal tract: lessons from animal models. Curr Mol Med. 2008;8:299–312. doi: 10.2174/156652408784533751. [DOI] [PubMed] [Google Scholar]
- 3.Kleiman-Wexler RL, Adair CG, Ephgrave KS. Pharmacokinetics of naloxone: an insight into the locus of effect on stress-ulceration. J Pharmacol Exp Ther. 1989;251:435–8. [PubMed] [Google Scholar]
- 4.Savran B, Görgün CZ, Zengil H. Circadian reactivity rhythm of rat gastric mucosa to restraint-cold stress and indomethacin: Temporal variation in the protective effect of iloprost. Chronobio Int. 1997;14:575–583. doi: 10.3109/07420529709001448. [DOI] [PubMed] [Google Scholar]
- 5.Waisman Y, Dinari G, Marcus H. et al. Naloxone is protective against indomethacin-induced intestinal ulceration in the rat. Gastroenterology. 1985;89:86–91. doi: 10.1016/0016-5085(85)90748-6. [DOI] [PubMed] [Google Scholar]
- 6.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin like drugs. Nature. 1971;231:232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 7.Vane JR. Mechanism of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:25–85. doi: 10.1016/s0002-9343(97)00203-9. [DOI] [PubMed] [Google Scholar]
- 8.Simon LS, Lanza FL, Lipsky PE. et al. Preliminary study of the safety and efficacy of SC-58635, a novel cyclooxygenase 2 inhibitor: efficacy and safety in two placebo-controlled trials in osteoarthritis and rheumatoid arthritis, and studies of gastrointestinal and platelet effects. Arthritis Rheum. 1998;41:1591–602. doi: 10.1002/1529-0131(199809)41:9<1591::AID-ART9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Vane JR, Botting RM. New insights into the mode of action of anti-inflammatory drugs. Inflamm Res. 1995;44:1–10. doi: 10.1007/BF01630479. [DOI] [PubMed] [Google Scholar]
- 10.Botting RM. Inhibitors of cyclooxygenases: mechanisms, selectivity and uses. J Physiol Pharmacol. 2006;57:113–24. [PubMed] [Google Scholar]
- 11.El-Bayer H, Steel L, Montcalm E. et al. The role of endogenous prostaglandins in the regulation of gastric secretion in rhesus monkeys. Prostaglandins. 1985;30:401–17. doi: 10.1016/0090-6980(85)90115-7. [DOI] [PubMed] [Google Scholar]
- 12.Wallace JL, Whittle BJ, Boughton-Smith NK. Prostaglandin protection of rat colonic mucosa from damage induced by ethanol. Dig Dis Sci. 1985;30:866–76. doi: 10.1007/BF01309518. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro-Rama AC, Figueiredo IV, Veiga F. et al. Evaluation of gastric toxicity of indomethacin acid, salt form and complexed forms with hydroxypropyl-β-cyclodextrin on Wistar rats: Histopathologic analysis. Fund Clin Pharmacol. 2009;23:747–55. doi: 10.1111/j.1472-8206.2009.00718.x. [DOI] [PubMed] [Google Scholar]
- 14.Bhargava KP, Gupta MB, Tangri KK. Mechanism of ulcerogenic activity of indomethacin and oxyphenbutazone. Eur J Pharmacol. 1973;22:191–5. doi: 10.1016/0014-2999(73)90012-5. [DOI] [PubMed] [Google Scholar]
- 15.Szejtli J. Cyclodextrin properties and applications. Drug Invest. 1990;2:11–21. [Google Scholar]
- 16.Uekama K, Irie T. New perspectives in cyclodextrin pharmaceutical applications: Cyclodextrin derivatives as new drug carriers. Drug Invest. 1990;2:22–8. [Google Scholar]
- 17.Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci. 1997;86:147–62. doi: 10.1021/js960213f. [DOI] [PubMed] [Google Scholar]
- 18.Uekama K, Otagiri M. Cyclodextrins in drug carrier systems. Crit Rev Ther Drug Carrier Systems. 1987;3:1–40. [PubMed] [Google Scholar]
- 19.Lin SZ, Wouessidjewe D, Poelman MC. et al. Indomethacin and CDs complexes. Int J Pharm. 1991;69:211–9. [Google Scholar]
- 20.Iohara D, Hirayama F, Ishiguro T. et al. Preparation of amorphous indomethacin from aqueous 2,6-di-O-methyl- β-CD solution. Int J Pharm. 2008;354:70–6. doi: 10.1016/j.ijpharm.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Lin SZ, Wouessidjewe D, Poelman MC. et al. In vivo evaluation of indomethacin/cyclodextrin complexes gastrointestinal tolerance and dermal antiiflammatory activity. Int J Pharm. 1994;106:63–7. [Google Scholar]
- 22.Jambhekar S, Casella R, Maher T. The physicochemical characteristics and bioavailability of indomethacin from β-CD, hydroxyethyl β-CD and HP-β-CD complexes. Int J Pharm. 2004;270:149–66. doi: 10.1016/j.ijpharm.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Backensfeld T, Müller BW, Wiese M. et al. Effect of cyclodextrin derivatives on indomethacin stability in aqueous solution. Pharm Res. 1990;7:484–90. doi: 10.1023/a:1015860531565. [DOI] [PubMed] [Google Scholar]
- 24.Djedaini F, Lin SZ, Perly B. et al. High field nuclear magnetic resonance techniques for the investigation of a β-cyclodextrin:indomethacin inclusion complex. J Pharm Sci. 1990;79:643–6. doi: 10.1002/jps.2600790721. [DOI] [PubMed] [Google Scholar]
- 25.Redenti E Szente L, Szejtli J. Cyclodextrin complexes of salts of acidic drugs: Thermodynamic properties, structural features, and pharmaceutical applications. J Pharm Sci. 2001;90:979–86. doi: 10.1002/jps.1050. [DOI] [PubMed] [Google Scholar]
- 26.Goodman LS, Gilman A, Rall TW, The pharmacological basis of therapeutics. Toronto, Canada: McGraw-Hill; 1992. pp. 498–501. [Google Scholar]
- 27.Hobbs DC. Pharmacokinetics of piroxicam in man. Eur J Rheumatol Inflamm. 1983;6:46–55. [PubMed] [Google Scholar]
- 28.Jug M, Becirevic-Lacan M. Multicomponent complexes of piroxicam with cyclodextrins and HPMC. Drug Dev Ind Pharm. 2004;30:1051–60. doi: 10.1081/ddc-200040245. [DOI] [PubMed] [Google Scholar]
- 29.Jug M, Becirevic-Lacan M, Kwokal A. et al. Influence of cyclodextrin complexation on piroxicam gel formulations. Acta Pharma. 2005;55:223–36. [PubMed] [Google Scholar]
- 30.Xiliang G, Yu Y, Guoyan Z. et al. Study of inclusion interaction of piroxicam with β-CD derivatives. Spectrochimica Acta Part A Mol Biomol Spectrosc. 2003;59:3379–86. doi: 10.1016/s1386-1425(03)00164-1. [DOI] [PubMed] [Google Scholar]
- 31.Qing L, Hiroyuki T, Yukio K. et al. Characterization of the transdermal transport of flurbiprofen and indomethacin. J Control Rel. 2006;110:542–556. doi: 10.1016/j.jconrel.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 32.Fujii M, Hori N, Shiozawa K, Wakabayashi K, Kawahara E, Matsumoto M. Effect of fatty acid esters on permeation of ketoprofen through hairless rat skin. Int J Pharm. 2000;205:117–125. doi: 10.1016/s0378-5173(00)00496-8. [DOI] [PubMed] [Google Scholar]
- 33.Cheong H-A, Choi H-K. Enhanced percutaneous absorption of piroxicam via salt formation with ethanolamines. Pharm Res. 2002;19:1375–80. doi: 10.1023/a:1020367212307. [DOI] [PubMed] [Google Scholar]
- 34.Higuchi T, Connors KA. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965;4:117–212. [Google Scholar]
- 35.Schmassmann A, Peskar BM, Selter C. et al. Effects of inhibition of prostaglandin endoperoxide synthase-2 in chronic gastro-intestinal ulcer models in rats. Br J Pharmacol. 1998;123:795–804. doi: 10.1038/sj.bjp.0701672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brzozowski T, Konturek PC, Konturek SJ. et al. Classic NSAID and selective cyclooxygenase (COX)-1 and COX-2 inhibitors in healing of chronic gastric ulcers. Microsc Res Tech. 2001;1:343–53. doi: 10.1002/jemt.1102. [DOI] [PubMed] [Google Scholar]
- 37.Chandranath SI, Bastaki SM, Singh JA. A comparative study on the activity of lansoprazole, omeprazole and PD-136450 on acidified ethanol- and indomethacin-induced gastric lesions in the rat. Clin Exp Pharmacl Physiol. 2002;29:173–80. doi: 10.1046/j.1440-1681.2002.03626.x. [DOI] [PubMed] [Google Scholar]
- 38.Stroff T, Plate S, Ebrahim SJ. et al. Tachykinin-induced increase in gastric mucosal resistance: role of primary afferent neurons, CGRP, and NO. Am J Physiol. 1996;271:G1017–27. doi: 10.1152/ajpgi.1996.271.6.G1017. [DOI] [PubMed] [Google Scholar]
- 39.Peskar BM. Neural aspects of prostaglandin involvement in gastric mucosal defense. J Physiol Pharmacol. 2001;52:555–68. [PubMed] [Google Scholar]
- 40.Gretzer B, Ehrlich K, Maricic N. et al. Selective cyclo-oxygenase-2 inhibitors and their influence on the protective effect of a mild irritant in the rat stomach. Br J Pharmacol. 1998;123:927–35. doi: 10.1038/sj.bjp.0701673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peskar BM, Ehrlich K, Peskar BA. Interaction of cyclooxygenase-2 inhibitors and salicylate in gastric mucosal damage. Eur J Pharmacol. 2002;434:65–70. doi: 10.1016/s0014-2999(01)01524-2. [DOI] [PubMed] [Google Scholar]
- 42.Wallace JL, Whittle BJR. Role of mucus in the repair of gastric epithelial damage in rat. Gastroenterology. 1985;91:611–30. doi: 10.1016/0016-5085(86)90629-3. [DOI] [PubMed] [Google Scholar]
- 43.Rainsford KD. Relations between gastric irritancy/ulcerogenicity and anti-oedemic activity of non-steroidal anti-inflammatory drugs. J Pharm Pharmacol. 1985;37:678–9. doi: 10.1111/j.2042-7158.1985.tb05115.x. [DOI] [PubMed] [Google Scholar]
- 44.Shoman ME, Abdel-Aziz M, Aly OM. et al. Synthesis and investigation of anti-inflammatory activity and gastric ulcerogenicity of novel nitric oxide-donating pyrazoline derivatives. Eur J Med Chem. 2009;44:3068–76. doi: 10.1016/j.ejmech.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Morsy M, Ashour O, Amin E. et al. Gastroprotective effects of telmisartan on experimentally-induced gastric ulcers in rats. Pharmazie. 2009;64:590–4. [PubMed] [Google Scholar]
- 46.Tandon R, Khanna HD, Dorababu M. et al. Oxidative stress and antioxidants status in peptic ulcer and gastric carcinoma. Indian J Physiol Pharmacol. 2004;48:115–8. [PubMed] [Google Scholar]
- 47.Tanaka A, Hatazawa R, Takahira Y. et al. Preconditioning stress prevents cold restraint stress-induced gastric lesions in rats: roles of COX-1, COX-2, and PLA2. Dig Dis Sci. 2007;52:478–87. doi: 10.1007/s10620-006-9394-8. [DOI] [PubMed] [Google Scholar]
- 48.Konturek PK, Brzozowski T, Konturek SJ. et al. Role of epidermal growth factor, prostaglandin, and sulfhydryls in stress-induced gastric lesions. Gastroenterology. 1990;99:1607–15. doi: 10.1016/0016-5085(90)90464-c. [DOI] [PubMed] [Google Scholar]
- 49.Das D, Banerjee RK. Effect of stress on the antioxidant enzymes and gastric ulceration. Mol Cell Biochem. 1993;125:115–25. doi: 10.1007/BF00936440. [DOI] [PubMed] [Google Scholar]
- 50.Moody FG, Cheung LY. Stress ulcers: their pathogenesis, diagnosis, and treatment. Surg Clin North Am. 1976;56:1469–78. doi: 10.1016/s0039-6109(16)41099-6. [DOI] [PubMed] [Google Scholar]
- 51.Feliciano DV. Do perforated duodenal ulcers need an acid-decreasing surgical procedure now that omeprazole is available? Surg Clin North Am. 1992;72:369–80. doi: 10.1016/s0039-6109(16)45684-7. [DOI] [PubMed] [Google Scholar]
- 52.Simon LS, Weaver AL, Graham DY. et al. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA. 1999;282:1921–8. doi: 10.1001/jama.282.20.1921. [DOI] [PubMed] [Google Scholar]
- 53.Clemett D, Goa KL. Celecoxib: a review of its use in osteoarthritis, rheumatoid arthritis and acute pain. Drugs. 2000;59:957–80. doi: 10.2165/00003495-200059040-00017. [DOI] [PubMed] [Google Scholar]
- 54.Silverstein FE, Faich G, Goldstein JL. et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–55. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]