Abstract
Adolescent idiopathic scoliosis (AIS) is a progressive growth disease that affects spinal anatomy, mobility, and left-right trunk symmetry. Consequently, AIS can modify human locomotion. Very few studies have investigated a simple activity like walking in a cohort of well-defined untreated patients with scoliosis. The first goal of this study is to evaluate the effects of scoliosis and scoliosis severity on kinematic and electromyographic (EMG) gait variables compared to an able-bodied population. The second goal is to look for any asymmetry in these parameters during walking. Thirteen healthy girls and 41 females with untreated AIS, with left thoracolumbar or lumbar primary structural curves were assessed. AIS patients were divided into three clinical subgroups (group 1 < 20°, group 2 between 20 and 40°, and group 3 > 40°). Gait analysis included synchronous bilateral kinematic and EMG measurements. The subjects walked on a treadmill at 4 km/h (comfortable speed). The tridimensional (3D) shoulder, pelvis, and lower limb motions were measured using 22 reflective markers tracked by four infrared cameras. The EMG timing activity was measured using bipolar surface electrodes on quadratus lumborum, erector spinae, gluteus medius, rectus femoris, semitendinosus, tibialis anterior, and gastrocnemius muscles. Statistical comparisons (ANOVA) were performed across groups and sides for kinematic and EMG parameters. The step length was reduced in AIS compared to normal subjects (7% less). Frontal shoulder, pelvis, and hip motion and transversal hip motion were reduced in scoliosis patients (respectively, 21, 27, 28, and 22% less). The EMG recording during walking showed that the quadratus lumborum, erector spinae, gluteus medius, and semitendinosus muscles contracted during a longer part of the stride in scoliotic patients (46% of the stride) compared with normal subjects (35% of the stride). There was no significant difference between scoliosis groups 1, 2, and 3 for any of the kinematic and EMG parameters, meaning that severe scoliosis was not associated with increased differences in gait parameters compared to mild scoliosis. Scoliosis was not associated with any kinematic or EMG left–right asymmetry. In conclusion, scoliosis patients showed significant but slight modifications in gait, even in cases of mild scoliosis. With the naked eye, one could not see any difference from controls, but with powerful gait analysis technology, the pelvic frontal motion (right–left tilting) was reduced, as was the motion in the hips and shoulder. Surprisingly, no asymmetry was noted but the spine seemed dynamically stiffened by the longer contraction time of major spinal and pelvic muscles. Further studies are needed to evaluate the origin and consequences of these observations.
Keywords: Scoliosis, Gait, Asymmetry, Electromyography
Introduction
Human gait is the most common of all human movements since evolutionary aspects of the spine, pelvis, hip, and knee have been modified in order to make bipedal locomotion the most efficient and functional [23–25]. In locomotion, the legs execute the primary propulsive movement, whereas the trunk assists in the maintenance of equilibrium and interacts with the limb movements to achieve efficient locomotion [41]. Indeed, the shoulder and pelvis girdles rotate to the opposite side in order to allow the head position to be maintained in the axis of progression. This opposing rotation is allowed by spine segmental movement [14]. In normal walking, an appropriated activation pattern of spinal and lower limb muscles is also designed to control segmental mobility, and trunk balance during each step [28, 43].
Adolescent idiopathic scoliosis (AIS) affects spinal mobility [9] and trunk balance [21, 38], altering human locomotion. In AIS, walking analysis has shown a side-to-side asymmetry of trunk kinematics [19] and spinal electromyographic (EMG) activity [1, 16, 34]. This conventional gait analysis, focusing on trunk, pelvis, and lower limb motion and on muscular activity necessary to move these joints, could help to explain the physiopathogenesis of AIS. Even though AIS affects about 2–4% of children [18, 36] with a maximum risk of curve progression at the adolescent growth spurt, its aetiology remains unclear [4, 26, 31]. There are several relevant hypotheses regarding underlying mechanisms focusing on genetic, biochemical, mechanic, neurological, muscular, and hormonal factors. For a better understanding of the aetiopathogenesis of AIS, it may prove informative to compare the walk of healthy subjects with that of patients with scoliosis curve angles at different levels of severity, before any treatment. We therefore hoped to gain valuable insight as to the aetiopathogenesis of AIS and the functional effects of scoliosis severity from a dynamic perspective using human locomotion.
Our first goal was to compare able-bodied subjects and AIS patients in order to assess the effects of the severity of scoliosis curves on kinematic and electromyographic gait variables.
Secondly, we hypothesized that in AIS patients with more severe spinal deformations, we would detect more of an asymmetric phenomenon.
Materials and methods
Study population
Fifty-four female adolescents were included in the study. This sample consisted of two groups: 13 healthy girls (mean age 16.5 years, range 16–17 years) without any known spinal deformation or disease that would affect gait and 41 girls with untreated adolescent idiopathic scoliosis (mean age 14.5 years, range 12–17 years) attending our outpatient clinic (Table 1). The scoliosis patient group was divided into three subgroups according to clinical outcome: group 1 (Cb ≤ 20°, n = 12), group 2 (20 < Cb < 40°, n = 13) and group 3 (Cb ≥ 40°, n = 16). All patients had a left thoracolumbar or lumbar primary structural curve (types 5 and 6) according to Lenke classification [20]. Patients with leg length discrepancies higher than 1 cm, any locomotor disorders, low back pain, neurological abnormalities observed on clinical examination or with any previous treatment for their scoliosis were not included in this study. Every subject signed on and participated freely in the study, approved by the local ethics board. All subjects were submitted to a clinical, radiological, and gait assessment.
Table 1.
Result of ANOVA on demographic data in 54 female subjects
| Control mean (±SD) |
Scoliosis patients mean (±SD) |
P value | |||
|---|---|---|---|---|---|
| (n = 13) | Group 1 (n = 12) | Group 2 (n = 13) | Group 3 (n = 16) | ||
| Height (cm) | 164.3 (4) | 162.4 (7.6) | 157 (7.7) | 163.4 (8.5) | NS |
| Weight (kg) | 54.7 (2.9) | 49.1 (8.8) | 48.5 (8.4) | 50.2 (7.1) | NS |
| BMI (kg m−2) | 20.2 (1) | 18.4 (1.9) | 19.5 (1.9) | 18.8 (2.1) | NS |
| Age (years)$* | 16 [15, 16] | 14 [13, 14]* | 14 [12–15]* | 16 [14–17] | <0.001 |
| Menarchal status | Yes | Yes | Yes | Yes | |
Significant differences are typed in bold and are accepted for P value ≤0.05
NS not significant, i.e. P value >0.05
* Significantly different from the control group
$ Median and quartile [25–75%]
Demographic and radiological assessment
Demographic data included height (cm), weight (kg), body mass index (BMI) computed as weight/height2 and expressed in kg m−2, and chronological age (years). A standard anteroposterior standing full spine and a left hand X-ray assessment were performed. On digital photographs, some parameters were evaluated: the main Cobb angle curve [8], the frontal body balance [21], the Risser sign [35] and the apical vertebral rotation (with the Perdriolle technique) [33]. The bone age was assessed on the left hand X-ray with the Greulich and Pyle table [33, 35].
Instrumented gait analysis
Gait was assessed using a three-dimensional analysis, including synchronous kinematic and EMG measurements.
Segmental kinematics were measured with the Elite system (BTS, Italy). Six infrared cameras measured at 100 Hz, co-ordinates in the three spatial planes (3D) of 22 reflective markers positioned on specific anatomical landmarks (Fig. 1). These measurements allowed computation of the 3D angular displacement and angular speed of shoulder, pelvis, hip, knee, and ankle [10]. On each segmental angular displacement and speed curve expressed as a function of normalized stride (%), the maximum and minimum angular positions were measured, as well as the motion of these segments, evaluated as maximum angular position minus minimum angular position.
Fig. 1.
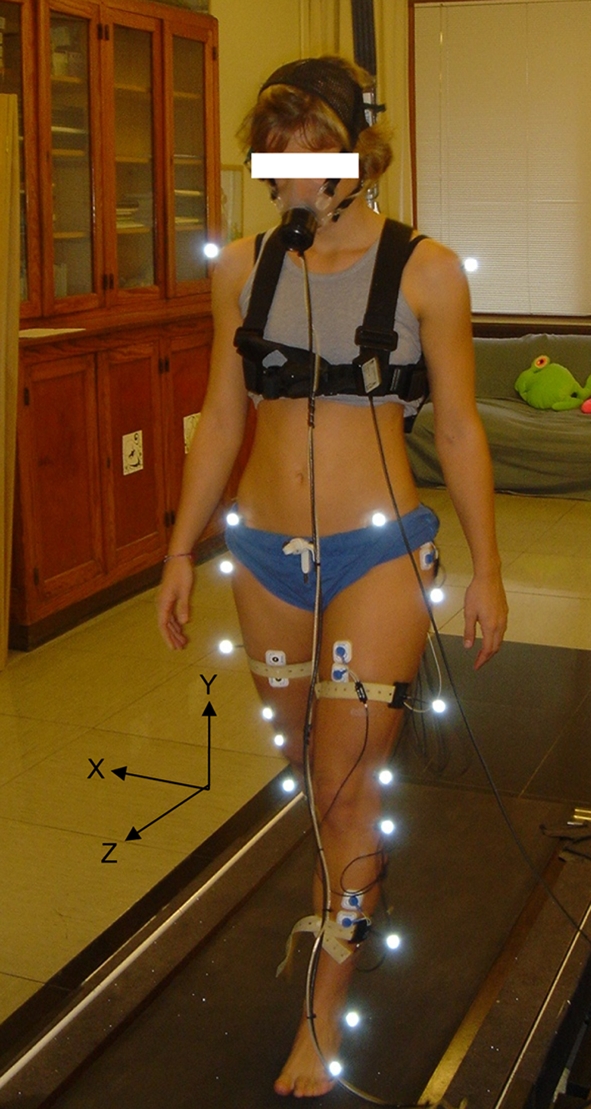
Frontal viewing of a patient equipped with the cutaneous markers, the bipolar muscular electrodes, and the mask linked to the ergospirometer. The patient is walking on the motor-driven treadmill, suspended from the ceiling by a harness. The three solid black arrows represent the X–Y–Z axis in the three spatial planes. These axes served as reference to allow the computation of the 3D angular displacement and angular speed of shoulder, pelvis, hip, knee, and ankle
Spatio-temporal parameters such as step length, step frequency, and stance phase duration were also computed from the 3D Kinematics. The electrical bilateral activity (EMG) of Quadratus Lumborum (QL), Erector Spinae (ES), Gluteus Medius (GM), Rectus Femoris (RF), Semitendinosus (ST), Tibialis Anterior (TA) and Gastrocnemius (G) muscles was recorded by a telemetry EMG system (Telemg, BTS, Italy) with surface electrodes (Medi-Trace, Graphic Controls Corporation, NY, USA). The signal was digitised at 1,000 Hz, full-wave rectified, and filtered (bandwidth 25–300 Hz). The onset and cessation of muscle activity were determined as described by Van Boxtel et al. [42].
Kinematic and EMG data were normalized to 100% of the time of the stride, with 0% corresponding to the initial contact of the left foot.
Protocol
All subjects wore a harness that suspended them from the ceiling to avoid falling when walking on the treadmill. The sessions began with a rest period, in which the subjects stood barefoot on the motor-driven treadmill (Mercury LTmed, HP Cosmos®, Germany) [13] for the static calibration of kinematic variables (Fig. 1). Thereafter, the subjects were asked to walk at a constant speed of 4 km h−1 for a few minutes until a steady state was reached. Then, all variables were simultaneously recorded for 20 s and averaged for ten successive strides. The mean value for each variable was used for statistical analysis.
Statistical analysis
The statistical analysis was performed using the software SigmaStat version 2.0 (SPSS Sciences Software GmbH, Erkrath, Germany). All parametric variables are presented as mean (±SD) and nonparametric variables as median and quartile [25–75%]. Homoscedasticity (normal distribution and equal variance) was verified for all comparisons.
A two-way repeated measures ANOVA was performed on all parametric gait variables with as factors side (convex vs. concave) and each scoliosis subgroups.
A one way analysis of variance (ANOVA) or a Kruskal-Wallis one way analysis of variance on ranks (ANOVA on ranks) in nonparametric conditions was performed on all the gait variables to compare the able-bodied subjects and the three scoliotic groups. A post-hoc test with a Bonferroni correction completed the analysis to determine which groups were significantly different. The accepted significance level was <0.05 for all analyses.
Results
Radiology
The radiological results (Table 2) showed significant differences among the four groups for the frontal Cobb angle curves (P < 0.001), the apical rotation (P < 0.001) and the frontal body balance (P < 0.001). The bone age, assessed by both the Risser sign and the Greulich and Pyle table, was significantly lower in scoliosis groups 1 and 2 (P < 0.001).
Table 2.
Result of one way ANOVA on clinical data in 54 female subjects
| Control group median [25–75%] |
Scoliosis patients median [25–75%] |
P value | |||
|---|---|---|---|---|---|
| (n = 13) | Group 1 (n = 12) | Group 2 (n = 13) | Group 3 (n = 16) | ||
| Cb (°)$ | 0.8 (1.6) | 15.3 (5)* | 27.8 (6)* | 44.3 (8.1)* | <0.001 |
| Balance (mm) | 0 [0–0.5] | 11 [1.5–13.5]* | 11 [0–20]* | 27.5 [19–30.5]* | <0.001 |
| Risser | 4 [3–4] | 2 [1–2]* | 2 [2–3] | 3 [3–4] | <0.001 |
| Apical rotation (°) | 0 [0–0] | 10 [5–20]* | 20 [13.7–30]* | 30 [22.5–40]* | <0.001 |
| Greulich and Pyle (years)$ | 16 (1) | 13 (1)* | 13 (1)* | 15 (2) | <0.001 |
Significant differences are typed in bold and are accepted for P value ≤0.05
Cb Lumbar/thoracolumbar Cobb angle range
* Significantly different from the control group
$ Mean (±SD)
Comparisons of side effects (Figs. 2, 3)
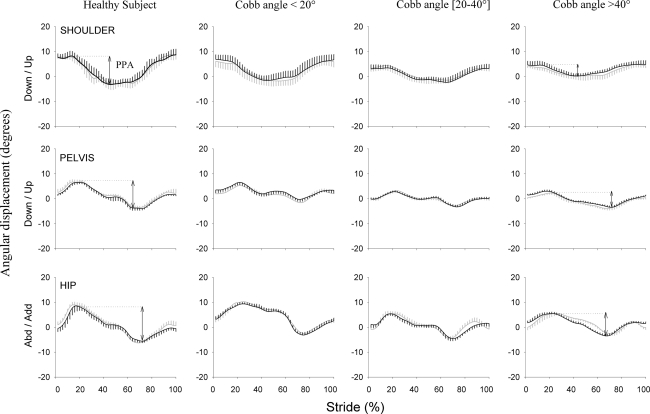
Fig. 2.
Typical trace of shoulder (upper graphs), pelvis (middle graphs), and hip (lower graphs) kinematics in the frontal plane for a normal subject and a patient with idiopathic scoliosis selected from each subgroup. Angular displacements (°) are expressed as a function of the normalized gait cycle (%). The mean (±SD vertical bars—n = 10 consecutive strides) angular displacement is plotted in black for the left side of the normal subject and the convex side of patients. The mean (±SD vertical bars) angular displacement is plotted in thick gray for the right side of the normal subject and concave side of patients. The arrow represents the peak-to-peak amplitude of each joint, i.e. the maximum minus the minimum position
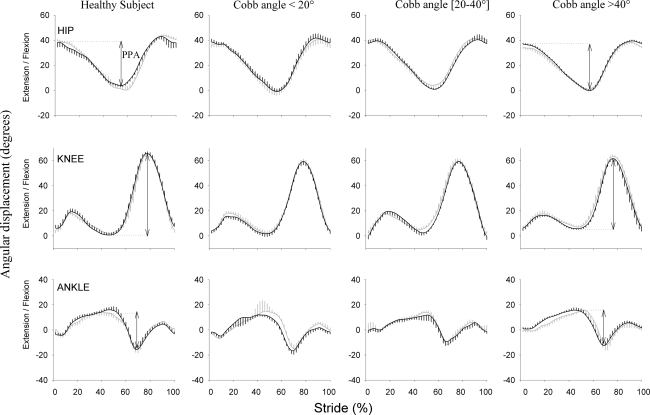
Fig. 3.
Typical trace of hip (upper graphs), knee (middle graphs), and ankle (lower graphs) kinematics in the sagittal plane for a normal subject and a patient with idiopathic scoliosis selected from each subgroup. Angular displacements (°) are expressed as a function of the normalized gait cycle (%). The mean (±SD vertical bars—n = 10 consecutive strides) angular displacement is plotted in black for the left side of the normal subject and convex side of patients. The mean (±SD vertical bars) angular displacement is plotted in thick gray for the right side of the normal subject and concave side of patients. The arrow represents the peak-to-peak amplitude of each joint, i.e. the maximum minus the minimum position
No significant effect was observed for any bilateral gait variables between the two sides in each scoliosis group (Table 3). Therefore the results were presented arbitrarily for the convex side for the scoliosis patients and the left side for the healthy subjects.
Table 3.
Results of two ways repeated measures ANOVA on gait variables: side effect in each AIS group
| Scoliosis patients mean (±SD) |
P value | ||||||
|---|---|---|---|---|---|---|---|
| Group 1 (n = 12) | Group 2 (n = 13) | Group 3 (n = 16) | |||||
| Convex | Concave | Convex | Concave | Convex | Concave | ||
| Segmental kinematic variables | |||||||
| Frontal pelvis motion (°) | 6.2 (1.5) | 6.3 (1.3) | 6 (1.1) | 6.3 (1.7) | 6.2 (1.4) | 6.5 (2.1) | NS |
| Sagittal pelvis motion (°) | 3.2 (0.8) | 3.2 (1) | 2.7 (0.7) | 2.8 (1) | 2.9 (0.7) | 3 (0.9) | NS |
| Transversal pelvis motion (°) | 8.1 (2.4) | 7.4 (2.6) | 7.6 (2) | 6.9 (2.6) | 7.2 (1.7) | 6.7 (1.5) | NS |
| Frontal hip motion (°) | 10.2 (1.8) | 9.5 (1.9) | 9.6 (1.6) | 10.6 (2.7) | 8.4 (1.1) | 10.3 (2.5) | NS |
| Sagittal hip motion (°) | 40.8 (1.8) | 41.2 (3) | 42.2 (4) | 41 (3) | 41.5 (4.2) | 40.2 (3.9) | NS |
| Transversal hip motion (°) | 13.3 (3.1) | 14.1 (2.6) | 12.5 (2.6) | 15.2 (5) | 14.5 (2.4) | 16.6 (0.9) | NS |
| Frontal shoulder motion (°) | 7.3 (1.6) | 7.6 (2.2) | 7 (1.8) | 7.6 (2.2) | 7.7 (0.8) | 8.7 (2) | NS |
| Sagittal shoulder motion (°) | 2.7 (1.3) | 2.7 (5) | 3.8 (1.4) | 3.8 (3.6) | 3.9 (3) | 3.1 (5.1) | NS |
| Transversal shoulder motion (°) | 3.5 (1.5) | 3.5 (1.5) | 4.8 (2.4) | 4.6 (2.3) | 4.5 (1.8) | 4.2 (1.7) | NS |
| Sagittal knee motion (°) | 56.3 (6.6) | 55.1 (4.2) | 56.8 (6.8) | 58.6 (4.7) | 55.6 (7) | 56.6 (2.6) | NS |
| Sagittal ankle motion (°) | 26.9 (5.7) | 27.3 (5.1) | 27.5 (7.1) | 26.8 (9.4) | 26.9 (5.7) | 27 (7.5) | NS |
| Transversal ankle motion (°) | 14.7 (3.3) | 15 (3) | 16.2 (2.3) | 15.8 (3.2) | 15.1 (3.9) | 15 (3.8) | NS |
| EMG variables | |||||||
| QL duration (%) | 50.5 (8.2) | 43.9 (8.9) | 43.8 (9) | 47.8 (7.4) | 42.8 (9) | 48.8 (9.8) | NS |
| ES duration (%) | 50.8 (11) | 43.3 (6.7) | 42.9 (10) | 42.6 (9.5) | 40 (8.8) | 42.7 (6.9) | NS |
| GM duration (%) | 49 (4.3) | 46.2 (3.2) | 48 (4) | 49.2 (4.9) | 47.4 (3.5) | 50.7 (3.9) | NS |
| RF duration (%) | 41.1 (12.8) | 40.7 (9.5) | 43.4 (13.4) | 43.7 (10.6) | 41.4 (11.4) | 44.3 (13.7) | NS |
| ST duration (%) | 52.3 (5.1) | 50.1 (6.7) | 46.1 (7.3) | 46.8 (8.4) | 41.4 (3) | 44.9 (6.4) | NS |
| TA duration (%) | 52.4 (10.9) | 48.8 (8.1) | 55.8 (9.6) | 55.5 (15.2) | 47.4 (7.4) | 46.7 (8.6) | NS |
| G duration (%) | 34.5 (2.5) | 34.1 (5.2) | 36.3 (3.1) | 35.2 (3.9) | 38.5 (6.2) | 38.2 (6.6) | NS |
| Angular sagittal speed | |||||||
| Ankle (° s−1) | 295 (48) | 303 (43) | 293 (88) | 303 (96) | 292 (58) | 290 (90) | NS |
| Knee (° s−1) | 566 (61) | 587 (60) | 583 (54) | 613 (78) | 586 (47) | 613 (76) | NS |
| Hip (° s−1) | 287 (17) | 280 (27) | 288 (20) | 283 (42) | 277 (14) | 274 (37) | NS |
| Pelvis (° s−1) | 33 (8) | 32 (8) | 32 (11) | 31 (11) | 33 (12) | 34 (15) | NS |
NS not significant, i.e. P value >0.05
Spatio-temporal parameters
The step length and the stance phase were slightly but significantly (P < 0.001) reduced in all scoliosis patient groups compared to normal subjects, from 7 and 2%, respectively. No significant difference was observed for cadence (Table 4).
Table 4.
Results of one way ANOVA on gait variables for control subjects and scoliosis subgroups patients
| Control group mean (±SD) |
Scoliosis patients mean (±SD) | P value | |||
|---|---|---|---|---|---|
| (n = 13) | Group 1 (n = 12) | Group 2 (n = 13) | Group 3 (n = 16) | ||
| Spatio-temporal variables | |||||
| Speed (km h−1) | 4 | 4 | 4 | 4 | |
| Step length (m) | 0.7 (0.02) | 0.67 (0.02)* | 0.64 (0.02)* | 0.65 (0.02)* | <0.001 |
| Cadence (step min−1) | 111 (7) | 109 (6) | 117 (8) | 114 (5) | NS |
| Stance phase (%) | 64.9 (1) | 63.7 (0.6)* | 63.3 (0.8)* | 64 (0.9)* | <0.001 |
| Segmental kinematic variables | |||||
| Frontal pelvis motion (°) | 8.4 (1.5) | 6.2 (1.5)* | 6 (1.1)* | 6.2 (1.4)* | <0.001 |
| Sagittal pelvis motion (°) | 2.6 (0.9) | 3.2 (0.8) | 2.7 (0.7) | 2.9 (0.7) | NS |
| Transversal pelvis motion (°) | 7.3 (1.1) | 8.1 (2.4) | 7.6 (2) | 7.2 (1.7) | NS |
| Frontal hip motion (°) | 13 (1.7) | 10.2 (1.8)* | 9.6 (1.6)* | 8.4 (1.1)* | <0.001 |
| Sagittal hip motion (°) | 42.4 (3.3) | 40.8 (1.8) | 42.2 (4) | 41.5 (4.2) | NS |
| Transversal hip motion (°) | 17.3 (2.7) | 13.3 (3.1)* | 12.5 (2.6)* | 14.5 (2.4)* | <0.001 |
| Frontal shoulder motion (°) | 9.3 (1.3) | 7.3 (1.6)* | 7 (1.8)* | 7.7 (0.8)* | 0.007 |
| Sagittal shoulder motion (°) | 3.1 (1) | 2.7 (1.3) | 3.8 (1.4) | 3.9 (3) | NS |
| Transversal shoulder motion (°) | 4.7 (1.9) | 3.5 (1.5) | 4.8 (2.4) | 4.5 (1.8) | NS |
| Sagittal knee motion (°) | 62.2 (3.8) | 56.3 (6.6) | 56.8 (6.8) | 55.6 (7)* | 0.03 |
| Sagittal ankle motion (°) | 31.4 (6.1) | 26.9 (5.7) | 27.5 (7.1) | 26.9 (5.7) | NS |
| Transversal ankle motion (°) | 16 (4) | 14.7 (3.3) | 16.2 (2.3) | 15.1 (3.9) | NS |
| EMG variables | |||||
| QL duration (%) | 34.5 (7.1) | 50.5 (8.2)* | 43.8 (9)* | 42.8 (9)* | <0.001 |
| ES duration (%) | 31.4 (6.7) | 50.8 (11)* | 42.9 (10)* | 40 (8.8)* | <0.001 |
| GM duration (%) | 40.4 (5.2) | 49 (4.3)* | 48 (4)* | 47.4 (3.5)* | <0.001 |
| RF duration (%) | 34.6 (11.9) | 41.1 (12.8) | 43.4 (13.4) | 41.4 (11.4) | NS |
| ST duration (%) | 36.1 (3.9) | 52.3 (5.1)* | 46.1 (7.3)* | 41.4 (3)* | <0.001 |
| TA duration (%) | 50.1 (7.5) | 52.4 (10.9) | 55.8 (9.6) | 47.4 (7.4) | NS |
| G duration (%) | 35.1 (3.2) | 34.5 (2.5) | 36.3 (3.1) | 38.5 (6.2) | NS |
| Angular sagittal speed | |||||
| Ankle (° s−1) | 361 (68) | 295 (48)* | 293 (88)* | 292 (58)* | 0.02 |
| Knee (° s−1) | 656 (33) | 566 (61)* | 583 (54)* | 586 (47)* | <0.001 |
| Hip (° s−1) | 294 (9) | 287 (17) | 288 (20) | 277 (14) | NS |
| Pelvis (° s−1) | 26 (9) | 33 (8) | 32 (11) | 33 (12) | NS |
Significant differences are typed in bold and are accepted for P value ≤0.05
* Significantly different from the control group
Kinematics
Scoliosis patients had significantly reduced pelvis (P = 0.001), hip (P < 0.001), and shoulder (P < 0.001) motion in the frontal plane and hip (P < 0.001) transversal motion when compared to normal subjects (21, 27, 28, and 22%, respectively) (Figs. 2, 3).
The knee sagittal motion was reduced in all scoliosis patient groups (P < 0.03).
The angular speed of the ankle (P = 0.02) and the knee (P < 0.001) was significantly reduced in each scoliosis group compared to healthy subjects. However, the post-hoc test did not show any significant difference between the three scoliosis groups for all the kinematic variables (Table 4).
Electromyographics
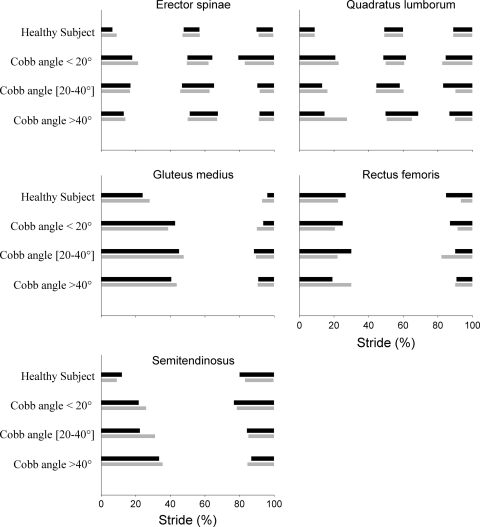
The electrical activity (EMG) duration of QL, ES, GM, and S muscles was bilaterally increased in the three scoliosis groups (P < 0.001) compared to normal subjects (46% of the stride in scoliosis patients vs. 35% of the stride in healthy subjects) (Table 4; Fig. 4). The post-hoc test did not show any significant difference between the three scoliosis groups for the EMG variables.
Fig. 4.
Typical trace of electromyographic activity of Quadratus Lumborum (QL), Erector Spinae (ES), Gluteus Medius (GM), and Semitendinosus (S) muscles for a scoliosis patient from each scoliosis group compared to a normal subject, expressed as a function of normalized stride (expressed in %). The horizontal black bars represent the phasic activity of the muscles for the left side of the normal subject and convex side of patients. The horizontal gray bars represent the phasic activity of the muscles for the right side of the normal subject and concave side of patients
Discussion
The goals of this study were to detect the effects of the severity of the scoliosis curves on gait variables, as compared to an able-bodied population and an asymmetric phenomenon more pronounced with severe spinal deformations. We chose to limit our study to the AIS patients with thoraco-lumbar and lumbar primary curves because deformities at these levels are anatomically related to the pelvis [7, 15, 27, 32], an important determinant of gait that would be primarily affected [11, 40]. This study group was well-defined with the Lenke classification [20] and comprised one of the largest series on untreated scoliosis assessed by a technically validated gait analysis. To our knowledge, it was the first study where a control group of healthy subjects has been compared to a sample of untreated scoliosis patients from low to high severity. The scoliosis patients in groups 1 and 2 were younger, which was associated with lower Risser and Greulich and Pyle signs. This was due to the fact that the studied patients were enrolled at the time of the first clinical exploration. However, their height and weight did not differ significantly since, even for scoliosis group 1, observation occurred after the growth spurt (>14 years). Moreover, gait is mature before adolescence, as observed by Dierick et al. [12]. Our radiological results showed significant increase for Cobb angle curves, apical rotation and frontal body imbalance between the three scoliosis groups, allowing discrimination of each scoliosis group on the basis of medical outcome. This study was conducted at a constant speed of 4 km h−1 first to eliminate any effect on the kinematics and EMG [39], and second to correspond to the most economic self-selected speed [5] and to previous studies [21, 29].
The results of this study showed, on AIS patients, a frontal pelvis, hip, and shoulder, transversal hip and sagittal knee motion restriction with bilaterally prolonged activation timing of QL, ES, GM, and S muscles.
This restriction of motion was small, between 1.6° and 4.6° for the shoulder, pelvis, and hip motion and 6.6° in mean for the knee motion. The step length was reduced by 6 cm and the stance phase duration by only 2%. All these results indicated an almost physiological walk, even for those patients with severe scoliosis.
The absence of differences in side-to-side comparison (convex vs. concave) for all kinematic data in AIS may be explained as a global phenomenon characterized by bilateral disturbances. These results agreed with others studies of lower limb motion [6, 19, 21]. But Kramers-de-Quervain et al. [19] had showed a ‘‘torsional offset’’ in left-lumbar and right-thoracic idiopathic scoliosis during walking. This was characterized by an asymmetrical trunk rotation correlated with the degree of the thoracic component, with a lack of rotation on the convex side of thoracic scoliosis curves, most likely caused by the structural deformity. In comparison, this ‘‘torsional offset’’ computed in our study group was present in all groups (normal and scoliosis) without any significant difference between groups. However, our sample did not involve main thoracic curves. It therefore seems that the thoraco-lumbar and lumbar main curves do not induce asymmetric trunk kinematics, such as thoracic ones.
The motion restriction found in our study can be interpreted in several different ways. First, as reported by several authors, it could be due to the 3D structural changes of spine [9, 19, 21, 22, 30], pelvis [15, 29, 32], and hip components [3, 37, 38], which provide a clinically observed stiffness of the human body. However, we did not have any hypothesis for the hip transversal restriction, except as a consequence of the prolonged contraction of the GM and S muscles. Second, this reduction of motion could be considered as a compensation mechanism to limit the progression of frontal upper body imbalance [6].
Third, this reduction of motion could be the consequence of the prolonged bilateral electrical activity (EMG) duration of the muscles that are connected to the pelvis. This bilateral increase in EMG duration does not agree with data from other studies, which have shown asymmetry of back muscle activity between concave and convex sides of the spine with scoliosis.
Allenbach and Wiest [1] and Riddle and Roaf [34] obtained a higher paravertebral EMG on the convex side of the curvature when patients were examined in the static position, reflecting stronger contraction. In both static and dynamic EMG measurements, asymmetric activity has been reported by Güth et al. [16] in non-progressive scoliosis. More, in scoliosis patients wearing a Milwaukee brace, the EMG activity of the erector muscles decreased on the convex side when walking with the brace. This muscular asymmetry may be expressed as a biomechanical consequence of the scoliotic curvature rather than its cause. Also, Hopf et al. [17] showed a significant decrease in paravertebral muscle activity on the convex side but not on the concave side after spinal arthrodesis surgery. They concluded that the asymmetrical muscle activity results from the body deformations that produce asymmetries in the orientation of biomechanical forces linked to various movements and body postures, as opposed to neurological etiological factors. But as explained by Basmajan [2], it is difficult to evaluate the force of the muscles in dynamic situations such as locomotion. Therefore, our study was based on the timing and duration of EMG muscular contraction expressed as a percentage of the gait cycle, despite the fact that most EMG studies analysed muscle strength. Our results are in agreement with our previous study that was limited to AIS patients with mild Cobb angle curves (less than 30°) [29] showing an increase of muscle duration by 21–61%. Therefore, we think that these appreciable muscular changes are responsible for the dynamically stiff behaviour of the lombo-pelvic segment. These muscles contracted for a long time, which raised several hypotheses. First, it could be a compensation reaction for the imbalances due to the tridimensional spino-pelvic deformities. However, even for mild scoliosis and for a small radiological frontal imbalance, the alterations of kinematics and electromyography of studied muscles were already obvious. Another hypothesis would be either a muscular dysfunction or the expression of a disease linked to motor control activity.
It would be expected that the correction of the imbalance with a conservative or surgical treatment could reduce the excessive electrical activity timing of these paravertebral and pelvi-femoral muscles as a sort of compensation phenomenon, as already observed for muscle strength by Guth et al. [16] and Hopf et al. [17]. However, if there was no change, it would be very interesting to analyse, from even the smallest scoliosis curves, the properties of the muscles surrounding the scoliosis curves and the pelvis with the aim of investigating the possibility of a systemic disease.
In conclusion, our study, performed on thoraco-lumbar and lumbar AIS during gait, has shown a frontal pelvis, hip, and shoulder and transversal hip motion restriction. This occurred even for mild scoliosis curves (Cb < 20°). It may be explained either by the stiffness of the spinal deformity or by the bilateral prolonged activation timing of the lumbar and pelvic muscles. These findings should be taken into account in research on the aetiopathogenesis of AIS.
More studies are necessary to research the causes of this excessive scoliosis muscles activity, the effects of current orthopaedic and surgical treatments and the consequence of these alterations on the mechanics and the energetics of human locomotion.
Acknowledgment
This work was supported by the Orthopedie Van Haesendonk firm.
References
- 1.Allenbach E, Wiest E. Condition of the paravertebral musculature in idiopathic scolioses before and after grafting. Rev Chir Orthop Repar Appar Mot. 1953;39:588–590. [PubMed] [Google Scholar]
- 2.Basmajian J (1979) Muscles alive. Their functions revealed by electromyography. Baltimore, USA
- 3.Burwell RG, Aujla RK, Freeman BJ, Dangerfield PH, Cole AA, Kirby AS, Pratt RK, Webb JK, Moulton A. Patterns of extra-spinal left-right skeletal asymmetries in adolescent girls with lower spine scoliosis: relative lengthening of the ilium on the curve concavity and of right lower limb segments. Stud Health Technol Inform. 2006;123:57–65. [PubMed] [Google Scholar]
- 4.Byrd JA 3rd (1988) Current theories on the etiology of idiopathic scoliosis. Clin Orthop Relat Res 229:114–119 [PubMed]
- 5.Cavagna GA, Thys H, Zamboni A. The sources of external work in level walking and running. J Physiol. 1976;262:639–657. doi: 10.1113/jphysiol.1976.sp011613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen PQ, Wang JL, Tsuang YH, Liao TL, Huang PI, Hang YS. The postural stability control and gait pattern of idiopathic scoliosis adolescents. Clin Biomech (Bristol, Avon) 1998;13:S52–S58. doi: 10.1016/S0268-0033(97)00075-2. [DOI] [PubMed] [Google Scholar]
- 7.Cheung KM, Wang T, Poon AM, Carl A, Tranmer B, Hu Y, Luk KD, Leong JC. The effect of pinealectomy on scoliosis development in young nonhuman primates. Spine. 2005;30:2009–2013. doi: 10.1097/01.brs.0000179087.38730.5d. [DOI] [PubMed] [Google Scholar]
- 8.Cobb J. Outline for study of scoliosis. Am Acad Orthop Surg. 1948;5:261–275. [Google Scholar]
- 9.Danielsson AJ, Romberg K, Nachemson AL. Spinal range of motion, muscle endurance, and back pain and function at least 20 years after fusion or brace treatment for adolescent idiopathic scoliosis: a case–control study. Spine. 2006;31:275–283. doi: 10.1097/01.brs.0000197652.52890.71. [DOI] [PubMed] [Google Scholar]
- 10.Davis RBOS, Tyburski D, Gage JR. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10:575–587. doi: 10.1016/0167-9457(91)90046-Z. [DOI] [Google Scholar]
- 11.Della Croce U, Riley PO, Lelas JL, Kerrigan DC. A refined view of the determinants of gait. Gait Posture. 2001;14:79–84. doi: 10.1016/S0966-6362(01)00128-X. [DOI] [PubMed] [Google Scholar]
- 12.Dierick F, Lefebvre C, Hecke A, Detrembleur C. Development of displacement of centre of mass during independent walking in children. Dev Med Child Neurol. 2004;46:533–539. doi: 10.1017/S0012162204000891. [DOI] [PubMed] [Google Scholar]
- 13.Dierick F, Penta M, Renaut D, Detrembleur C. A force measuring treadmill in clinical gait analysis. Gait Posture. 2004;20:299–303. doi: 10.1016/j.gaitpost.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Elftman H. The basic pattern of human locomotion. Ann N Y Acad Sci. 1951;51:1207–1212. doi: 10.1111/j.1749-6632.1951.tb27347.x. [DOI] [PubMed] [Google Scholar]
- 15.Gum JL, Asher MA, Burton DC, Lai SM, Lambart LM. Transverse plane pelvic rotation in adolescent idiopathic scoliosis: primary or compensatory? Eur Spine J. 2007;16:1579–1586. doi: 10.1007/s00586-007-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guth V, Abbink F, Gotze HG, Heinrichs W. Investigation of gait of patients with idiopathic scoliosis and the influence of the Milwaukee brace on gait (author’s transl) Z Orthop Ihre Grenzgeb. 1978;116:631–640. [PubMed] [Google Scholar]
- 17.Hopf C, Scheidecker M, Steffan K, Bodem F, Eysel P. Gait analysis in idiopathic scoliosis before and after surgery: a comparison of the pre and postoperative muscle activation pattern. Eur Spine J. 1998;7:6–11. doi: 10.1007/s005860050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kane WJ, Moe JH. A scoliosis-prevalence survey in Minnesota. Clin Orthop Relat Res. 1970;69:216–218. doi: 10.1097/00003086-197003000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Kramers-de Quervain IA, Muller R, Stacoff A, Grob D, Stussi E. Gait analysis in patients with idiopathic scoliosis. Eur Spine J. 2004;13:449–456. doi: 10.1007/s00586-003-0588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, Lowe TG, Blanke K. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83-A:1169–1181. [PubMed] [Google Scholar]
- 21.Lenke LG, Engsberg JR, Ross SA, Reitenbach A, Blanke K, Bridwell KH. Prospective dynamic functional evaluation of gait and spinal balance following spinal fusion in adolescent idiopathic scoliosis. Spine. 2001;26:E330–E337. doi: 10.1097/00007632-200107150-00020. [DOI] [PubMed] [Google Scholar]
- 22.Leong JC, Lu WW, Luk KD, Karlberg EM. Kinematics of the chest cage and spine during breathing in healthy individuals and in patients with adolescent idiopathic scoliosis. Spine. 1999;24:1310–1315. doi: 10.1097/00007632-199907010-00007. [DOI] [PubMed] [Google Scholar]
- 23.Lovejoy CO. The natural history of human gait and posture. Part 1. Spine and pelvis. Gait Posture. 2005;21:95–112. doi: 10.1016/j.gaitpost.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Lovejoy CO. The natural history of human gait and posture. Part 2. Hip and thigh. Gait Posture. 2005;21:113–124. doi: 10.1016/j.gaitpost.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Lovejoy CO. The natural history of human gait and posture. Part 3. The knee. Gait Posture. 2007;25:325–341. doi: 10.1016/j.gaitpost.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Lowe TG, Edgar M, Margulies JY, Miller NH, Raso VJ, Reinker KA, Rivard CH. Etiology of idiopathic scoliosis: current trends in research. J Bone Joint Surg Am. 2000;82-A:1157–1168. doi: 10.2106/00004623-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Mac-Thiong JM, Labelle H, Guise JA. Comparison of sacropelvic morphology between normal adolescents and subjects with adolescent idiopathic scoliosis. Stud Health Technol Inform. 2006;123:195–200. [PubMed] [Google Scholar]
- 28.MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech. 1993;26:633–644. doi: 10.1016/0021-9290(93)90027-C. [DOI] [PubMed] [Google Scholar]
- 29.Mahaudens P, Thonnard JL, Detrembleur C. Influence of structural pelvic disorders during standing and walking in adolescents with idiopathic scoliosis. Spine J. 2005;5:427–433. doi: 10.1016/j.spinee.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto T, Kitahara H, Minami S, Takahashi K, Yamagata M, Moriya H, Tamaki T. Flexibility in the scoliotic spine: three-dimensional analysis. J Spinal Disord. 1997;10:125–131. doi: 10.1097/00002517-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Miller NH. Genetics of familial idiopathic scoliosis. Clin Orthop Relat Res. 2007;462:6–10. doi: 10.1097/BLO.0b013e318126c062. [DOI] [PubMed] [Google Scholar]
- 32.Nicolopoulos KS, Burwell RG, Webb JK. Stature and its components in adolescent idiopathic scoliosis. Cephalo-caudal disproportion in the trunk of girls. J Bone Joint Surg Br. 1985;67:594–601. doi: 10.1302/0301-620X.67B4.4030857. [DOI] [PubMed] [Google Scholar]
- 33.Perdriolle R, Vidal J. Thoracic idiopathic scoliosis curve evolution and prognosis. Spine. 1985;10:785–791. doi: 10.1097/00007632-198511000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Riddle HF, Roaf R. Muscle imbalance in the causation of scoliosis. Lancet. 1955;268:1245–1247. doi: 10.1016/S0140-6736(55)91020-5. [DOI] [PubMed] [Google Scholar]
- 35.Roche AF, Eyman SL, Davila GH. Skeletal age prediction. J Pediatr. 1971;78:997–1003. doi: 10.1016/S0022-3476(71)80430-4. [DOI] [PubMed] [Google Scholar]
- 36.Rogala EJ, Drummond DS, Gurr J. Scoliosis: incidence and natural history. A prospective epidemiological study. J Bone Joint Surg Am. 1978;60:173–176. [PubMed] [Google Scholar]
- 37.Saji MJ, Upadhyay SS, Leong JC. Increased femoral neck-shaft angles in adolescent idiopathic scoliosis. Spine. 1995;20:303–311. doi: 10.1097/00007632-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Schwender JD, Denis F. Coronal plane imbalance in adolescent idiopathic scoliosis with left lumbar curves exceeding 40 degrees: the role of the lumbosacral hemicurve. Spine. 2000;25:2358–2363. doi: 10.1097/00007632-200009150-00015. [DOI] [PubMed] [Google Scholar]
- 39.Stoquart G, Detrembleur C, Lejeune T. Effect of speed on kinematic, kinetic, electromyographic and energetic reference values during treadmill walking. Neurophysiol Clin. 2008;38:105–116. doi: 10.1016/j.neucli.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Stoquart GG, Detrembleur C, Palumbo S, Deltombe T, Lejeune TM. Effect of botulinum toxin injection in the rectus femoris on stiff-knee gait in people with stroke: a prospective observational study. Arch Phys Med Rehabil. 2008;89:56–61. doi: 10.1016/j.apmr.2007.08.131. [DOI] [PubMed] [Google Scholar]
- 41.Thorstensson A, Nilsson J, Carlson H, Zomlefer MR. Trunk movements in human locomotion. Acta Physiol Scand. 1984;121:9–22. doi: 10.1111/j.1748-1716.1984.tb10452.x. [DOI] [PubMed] [Google Scholar]
- 42.Boxtel GJGL, Berg Lenssen MM, Brunia CH. Detection of EMG onset in ERP research. Psychophysiology. 1993;30:405–412. doi: 10.1111/j.1469-8986.1993.tb02062.x. [DOI] [PubMed] [Google Scholar]
- 43.Winter D. The biomechanics and motor control of human gait: normal, elderly and pathological. Canada: Waterloo Ontario; 1991. [Google Scholar]