Abstract
Since the dorsal root ganglia represent the first structure of pain modulation, they are the target of the newest therapies of neuropathic pain. Between these, pulsed radiofrequency (PRF) has been described among the promising non-invasive methods. Although the results encourage the clinical use of this procedure, their mechanism of action is still unclear. Aim of our study was to analyze acute effects of PRF on the rat lumbar ganglion and on nervous fibres running inside it. Clinical works describe PRF treatment as a techinque without any visible neurological deficit. The few disposable histological works are contractictory: some describe no signs of cellular damage and some demonstrate visible intracellular modifications. A total of 20 male Wistar rats were deeply anesthesized. Ten were positioned in a stereotactic system, and exposed to PRF at 2 Hz for 30 s after exposition of paravertebral muscles and positioning of a stimulation needle on left L4 ganglion. The other ten were used as controls. After 1 h, the left dorsal root ganglions L3, L4, L5 of the 20 animals were explanted, fixed in 2.5% Karnowsky solution and prepared for light and transmission electron microscopy. At light microscopy no differences between treated and control animals were observed; at transmission electron microscopy, instead, it was possible to observe that T gangliar cells contained an abnormal abundant smooth reticulum with enlarged cisternae and numerous vacuoles; myelinated axons presented pathological features and their myelin coverage was not adherent. Instead, unmyelinated axons appeared normal in shape and dimension and the Schwann cells surrounding it had intact plasmamembrane. Our results, obtained at acute stage, reveal that the PRF procedure should destroy the myelin envelope of nervous fibres. Further future studies, at chronic stage, should give other information on the prognosis of the myelinic damage.
Keywords: Pulsed Radiofrequency, Lumbar ganglion, Neuropathic pain
Introduction
Radiofrequency lesions have been used in clinical practice for the treatment of neuropathic chronic pain syndromes [8, 16, 17, 18,21] since 1965 [11]. As described in literature, the pain relief effect was essentially due to the high temperature (enclosed between 60 and 80°C) produced by the electrode tip in the target tissue resulting into a coagulative necrosis of both cells and fibres [2, 3, 13, 14].
Recently, pulsed radiofrequency (PRF) procedure was introduced as a valid treatment in comparison to continue radiofrequency (CRF) lesions and it has been rapidly accepted for its clinically demonstrated effectiveness and the lack of any permanent neurological deficits [5, 6, 7, 12, 13, 14, 19, 20].
To day, only few studies in literature [10, 4] described the morphological modifications occurred in the ganglion cells after the PRF treatment. Moreover the lack of information both on acute stages and on the cellular modification induced by the PRF procedure, convinced us to perform a detailed morphological study at both structural and ultrastructural level on the acute effects of PRF procedure on gangliar tissue to investigate the alterations occurred to its components: gangliar T cells, satellite cells, myelinated fibres and unmyelinated fibres, Schwann cells, intragangliar and perigangliar vessels.
Materials and methods
During the experiments principles of good laboratory animal care and animal experimentation of the specific national (DL 116 GU suppl 40, 18 febbraio 1992; Circolare n° 8 GU 14 luglio 1994) and international laws and regulations (EEC Council Directive 86/609, OJL 358,1, dec 12 1987) were followed.
Surgical procedure
Twenty male Wistar rats weighing 325 g were deeply anesthetised with an intraperitoneal injection of 1 ml ketamine. The abdominal and lumbar regions of the rats were then shaved and the indifferent electrode of the PRF device was attached to the abdominal skin of the rat. The animals were positioned prone in a stereotactic system. Their position was guarantee by rounded tip metallic sticks fixed at the iliac christa. A 1 cm long incision was performed on the skin along the spinous processes of L4–L5 and the sacrum. The paravertebral muscles were then exposed and the needle was inserted between the left L4 and L5 laminae till reaching the bone. The correct positioning of the stimulation needle was then checked observing the contraction of the lower limb muscles using 2 Hz electrical stimulation between 0.3 and 0.6 V.
Other ten animals were used as sham control: the surgical procedure was the same but the PRF lesion was not performed.
Pulsed radiofrequency procedure
PRF procedure was performed on the left L4 dorsal root ganglion using a 10-cm long electrode (Neurostick® 25 gauge, 5 mm active tip, Onkoline®) powered by an PRF generator (PMG-230 Baylis). The lesion generator was set to deliver RF at 5 bursts per second (2 Hz) of 20 millisecond duration each with the automatic temperature control activated and set to 42°C for 120 s.
The control and treated animals were kept in deep anesthesia for 1.5 h and then L3–L4, L4–L5 and L5–S1 dorsal root ganglions of both sides were surgically explanted, using bone fine rongeaurs.
The obtained specimens were fixed in 2,5% Karnowsky solution in 0.1 M cacodylate buffer and then prepared for light and transmission electron microscopy.
Light microscopy analysis
The specimens were maintained in the fixative solution for 6 h, washed in 0.1 M cacodylate buffer, dehydrated in graded ethanol and paraffin embedded in order to obtain longitudinal and transversal sections. About 3–5 μm thick sections were perfomed using a PFM Slide 2002 microtome (PFM, Köln, Germany) and collected on slide glasses. The obtained sections were then stained with eosin-haematoxylin according to the current protocol. The final observations were carried out with a Nikon Eclipse 600 (Nikon Corp., Tokio, Japan) microscope equipped by a Polaroid DMC photo camera (Polaroid Corp., Waltham, Massachusset, USA).
Transmission electron microscopy (TEM) analysis
The specimens were kept into the fixative solution for 6 h and then were washed in 0.1 M cacodylate buffer. The samples were postfixed in 1% OsO4 for 1 h, washed in 0.1 M cacodylate buffer, dehydrated in ascending ethanol and propylenoxyde, and then embedded in epon 812 mixture.
The embedded specimens were cut with a RMC MTX ultramicrotome (Boeckeler Instruments Inc., Tucson, Arizona, USA) and the obtained ultrathin sections were collected on 150 mesh grids and then were overstained with uranile acetate and lead citrate, and finally observed with a Philips Morgagni TEM (FEI, Eindhoven, The Netherlands).
Results
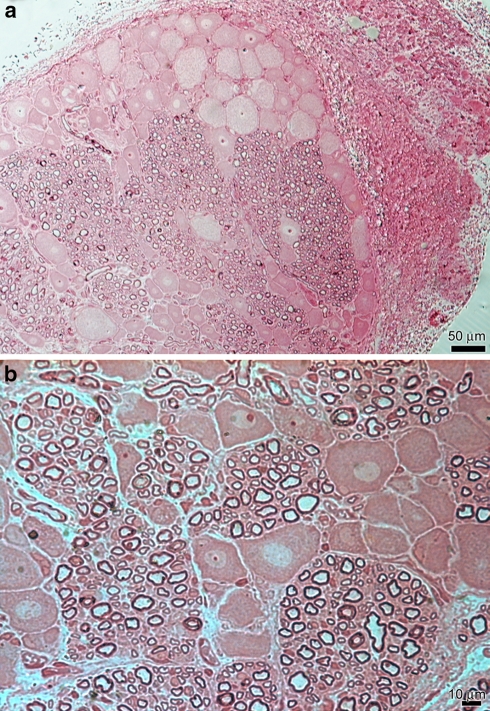
In control animals at light microscopy analysis, it was possible to observe the normal disposition of gangliar cells and nervous fibres in the rat lumbar ganglion (Fig. 1a). The rat lumbar ganglion revealed the coexistence of myelinated, unmyelinated fibres, Schwann cells and gangliar T cells (Fig. 1b). Among them it was also possible to see intragangliar capillaries and perigangliar medium sized blood vessels.
Fig. 1.
Light microscopy (LM) control specimen. a Gangliar cells and nervous fibres had normal distribution. Scale bar 50 μm. b Nervous fibres of different diameters were grouped in bundles of 50–100 μm. Gangliar cells, ovalar or pyramidal in shape, had central roundish nucleus. Scale bar 10 μm
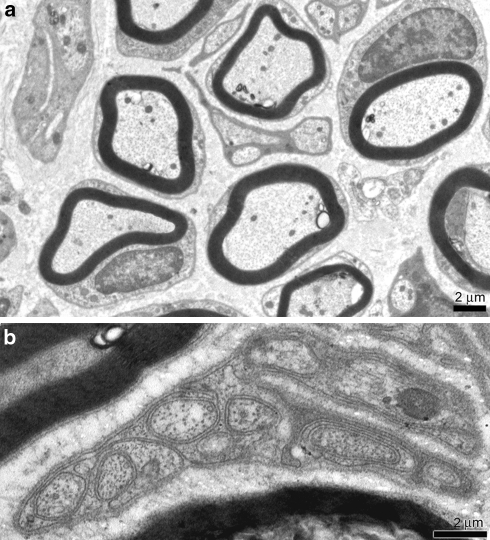
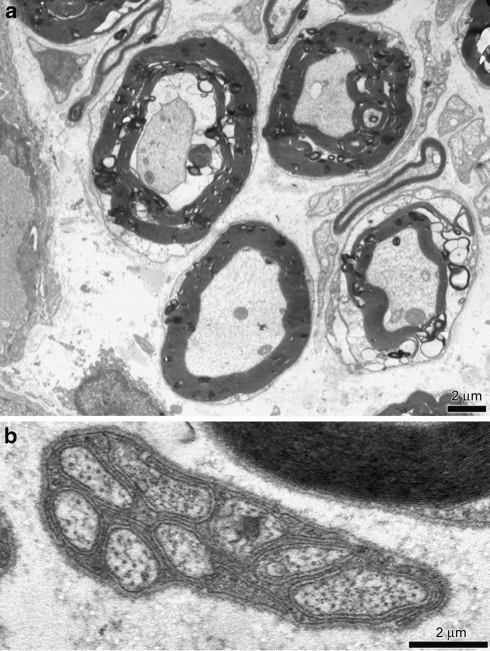
The TEM analysis revealed gangliar cells surrounded by satellite cells. The smooth and rough endoplasmic reticulum was regular in size and shape and their mitochondria had external membrane and cristae normally disposed. Myelin enveloped nervous fibres without defects and axons were well developed (Fig. 2a). Schwann cells surrounded unmyelinated well preserved axons (Fig. 2b).
Fig. 2.
Transmission electron microscopy (TEM) control specimen. a Myelin sheats of Schwann cells enveloped the axons. Nuclei and intracellular organelles were intact. Scale bar 2 μm. b Unmyelinated fibre. Subtle sheat of single Schwann cells membrane enveloped numerous bundles of axons. Scale bar 2 μm
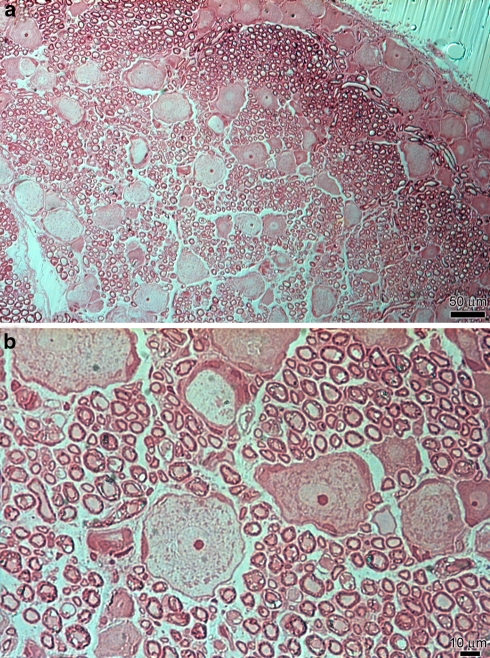
After 1 h the PRF procedure, LM analysis of the lumbar ganglions did not show any impairment of the gangliar architecture except for a slight interstitial oedema (Fig. 3a). The gangliar T cells were oval in shape and were surrounded by a single layer of well organised satellite cells that had normal morphology and distribution. Unmyelinated axon appeared to be normal and with no evident change in their shape and spatial distribution. Some myelinated axons seemed to have morphological alterations, because of their dark irregular staining, but not easily valuable due to the low magnification field (Fig. 3b).
Fig. 3.
LM treated specimen. a The space between cells and fibres was enlarged by a slight oedema. Scale bar 50 μm. b Myelin sheat of myelinated fibres appeared delaminated and it was lost the organisation in bundles. Scale bar 10 μm
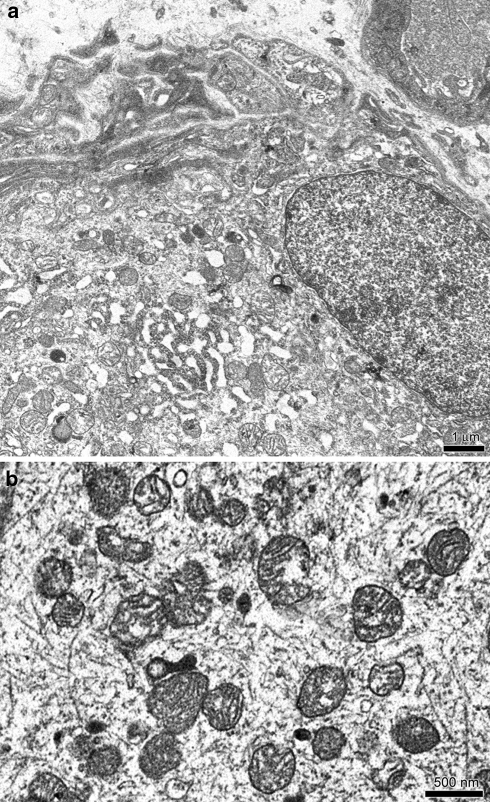
On the other hand TEM analysis showed no change in gangliar cells disposition while some alterations in their ultrastructural intracellular features were observed (Fig. 4a). Their cytoplasm enclosed numerous vacuoles and the intracellular organelles showed some pathological modifications: the smooth and rough endoplasmic reticulum cisternae were enlarged, mitochondria appeared to be damaged: their external membranes were often opened and their cristae diminished, enlarged or completely destroyed (Fig. 4b). In few gangliar cells it was possible to observe nuclear membrane disorders and loss of the integrity of the plasmalemma.
Fig. 4.
TEM treated specimens. a Intracellular compartment. Smooth reticulum was enlarged. Scale bar 1 μm. b Mitochondrial christae were diminished. Sometimes, mitochondrial external membrane were opened and the mitochondria were destroyed. Scale bar 500 nm
Some myelinated axons presented myelin sheats envelopment modifications: the myelin appeared delaminated and in some cases damaged clumping in dark ovoids (Fig. 5a). These changes could be enclosed into a thermal-like lesion affecting only the axon myelin bundles.
Fig. 5.
TEM treated specimens. a Myelin envelopes appeared delaminated and in some cases collapsed over the axons. The oedema was evident in the extracellular space. Scale bar 2 μm. b Unmyelinated fibres were normal in shape, dimension and organisation. Scale bar 2 μm
The Schwann cells enveloping unmyelinated fibres appeared to be normal and the unmyelinated axons enclosed did not show any pathological features (Fig. 5b). Satellite cells enveloping Schwann cells of myelinated fibres were enlarged while those enveloping unmyelinated fibres were normal both in number and shape.
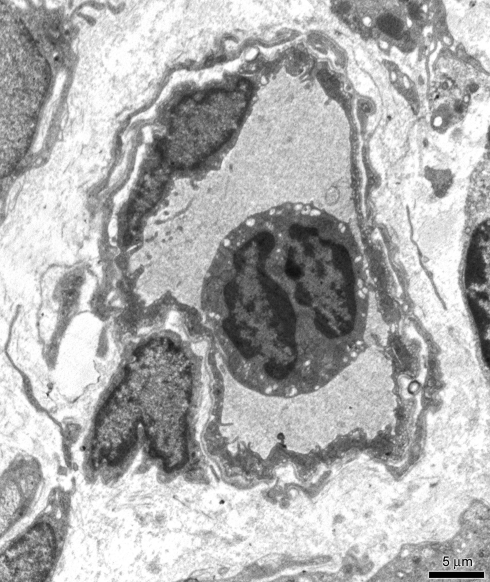
The extracellular environment was characterized by a slight oedema, represented by the enlargement of interstitial spaces between the nervous fibres even if the intragangliar and perigangliar vessels appeared to be normal and no thigh junction enlargements were observed in endothelial cells lining (Fig. 6).
Fig. 6.
TEM treated specimens. Vessels were surrounded by normal flat endothelial cells. Scale bar 5 μm
When observing the controlateral ganglions we did not find any impairment both in myelinated and unmyelinated fibres along with gangliar cells, satellite cells and vessels. Some slight modifications of myelin evelope was evidenced as in control animals probably due to technique-related artifacts. These morphological appearance resembled that observed in control animals and we could not find any differences between these two groups.
In all the specimens we evaluated the percentage of altered mielinated fibres counting for each specimen 5 fields at 3000× magnification. The alterations we took into account were any impairment of myelin disposition around fibers, intracytoplasmic vacuolizations or disruption of cellular membranes. We have to distinguish however the slight impairments found in fibres in control animals or contralateral ganglions, probably due to technical artefacts respect to those found in treated ganglions. We found the presence of altered mielinated axons in a median value of 79.81% of analyzed fibres in ganglions treated by PRF, while in controlateral ganglions the altered mielinated axons (with slighter alterations) had a median value of 23.21% and in control animals a median value of 24.35%.
When we compared the number of alterated fibres observed in treated ganglions respect to those found in the controlateral ones or in control animals, using Student t test, we found a P value equal to 5.6 × 10−7 so demonstrating the statistical differences between the group of treated ganglions and control group when looking at alterated fibres.
Discussion
In previous clinical studies, [1, 13, 14, 15], the authors declared that PRF procedure is non-destructive and suggested that this method could be a valid alternative to continue radiofrequency (RF) lesion for the treatment of neuropathic pain. The reported clinical good results and evidence based observations along with the absence of permanent neurological deficits [19, 20], drove to an extensive application of this method in the field of neuropathic pain (e.g. trigeminal and great occipital neuralgia, postherpetic neuralgia) as well as lumbar back pain (e.g. neuralgia of Lutscka nerve), even if the pathophysiological reasons of its effectiveness has not been understood in detail yet.
Moreover, by an experimental point of view there are only few reports concerning the histological effects of PRF procedure on both ganglion and nerves: using light microscopic analysis at low power fields it has been demonstrated [10] that PRF treated ganglions with the exception of a slight interstitial oedema, showed no sign of cellular damage. These studies were always driven in comparison to continuous RF treatment and often highlighting only the differences observed in the effects on the both gangliar cells and myelinated fibres, seriously injured in continuous RF treatments compared to PRF procedures, neglecting the specific alterations occurred to the ganglion after PRF at acute stage. Furthermore, data of literature reported that two weeks after the procedure, at transmission electron microscopic analysis, the treated ganglions presented little intracellular modifications visible as the enlargement of the endoplasmic reticulum cisternae and a slight oedema [4].
Starting from these results, we focused our attention strictly on the acute effects of the PRF on the rat dorsal root ganglion.
The parameters we used to perform PRF procedure were equal to those experimentally used by other authors and comparable to those clinical used in humans.
The results we obtained at an acute stage showed some pathological effects on lumbar rat ganglion mostly affecting the myelinated fibres. In fact, these showed clear signs of a thermic-like effect consisting in the impairment of the architecture of myelin sheats bundles along the axons with the consequent swelling of pericytes. These alterations could be probably explained with the so called temperature burst that occasionally have been demonstrated to occur during PRF procedure.
The clinical observations on the patients treated with PRF report a documented pain relief during the first week following the procedure; that although gradually decrease during the 2–3 weeks later, finally is maintained [9].
In the light of our experimental results we can conclude that the morphological features we observed can well explain the evidence of the early post-procedure pain relief.
These results will be implemented by further functional studies in order to experimentally evaluate the morphological findings putting them into relation with the clinical course as to give a detailed timing of the modification occurred after PRF and to correctly identify the pathophysiological mechanism underlying this procedure.
In conclusion, trying to solve the debate whether this procedure can be considered as a neuromodulation technique or not, according to our results, those found in the literature and the clinical course we propose the term: neuro-remodelling technique.
Acknowledgments
The authors gratefully acknowledge the “Centro Grandi Attrezzature per la Ricerca Biomedica” of Insubria University for instruments availability.
Footnotes
Morphological PRF effects on ganglions.
References
- 1.Cohen SP, Foster A. Pulsed radiofrequency as a treatment for groin pain and orchialgia. Urology. 2003;61:645. doi: 10.1016/S0090-4295(02)02423-8. [DOI] [PubMed] [Google Scholar]
- 2.Cosman ER, Nashold BS, Ovelmann-Levitt J. Theoretical aspects of radiofrequency lesions in the dorsal root entry zone. Neurosurgery. 1984;15:945–950. doi: 10.1097/00006123-198412000-00032. [DOI] [PubMed] [Google Scholar]
- 3.Louw AJA, Vles HSH, Freling G, Herpers MJHM, Kleef M. The morphological effects of a radiofrequency lesion adjacent to the dorsal root ganglion (RF-DRG)—an experimental study in the goat. Eur J Pain. 2001;5:169–174. doi: 10.1053/eujp.2001.0228. [DOI] [PubMed] [Google Scholar]
- 4.Erdine S, Yucel A, Cimen A, Aydin S, Sav A, Bilir A. Effects of pulsed versus conventional radiofrequency current on rabbit dorsal root ganglion morphology. Eur J Pain. 2005;9:251–256. doi: 10.1016/j.ejpain.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Geurst JW, Wijk RM, Wynne HJ, Hammink E, Buskens E, Lousberg R, et al. Radiofrequency lesioning of dorsal root ganglia for chronic lumbosacral radicular pain: a tandomised, double-blinded, controlled trial. Lancet. 2003;361:21–26. doi: 10.1016/S0140-6736(03)12115-0. [DOI] [PubMed] [Google Scholar]
- 6.Mikeladze G, Espinal R, Finnegan R, Routon J, Martin D. Pulsed radiofrequency application in treatment of chronic zyapophyseal joint pain. Spine J. 2003;419:170–177. doi: 10.1016/s1529-9430(03)00065-2. [DOI] [PubMed] [Google Scholar]
- 7.Munglani R. The longer term effect of pulsed radiofrequency for neuropathic pain. Pain. 1999;80:437–439. doi: 10.1016/S0304-3959(98)00183-3. [DOI] [PubMed] [Google Scholar]
- 8.Nash TP. Percutaneous radiofrequency lesioning of dorsal root ganglia for intractable pain. Pain. 1986;24:67–73. doi: 10.1016/0304-3959(86)90027-8. [DOI] [PubMed] [Google Scholar]
- 9.Ochiai N, Tasto JP, Ohtori S, Takahashi N, Moriya H, Amiel D. Nerve regeneration after radiofrequency application. Am J Sports Med. 2007;35(11):1940–1944. doi: 10.1177/0363546507304175. [DOI] [PubMed] [Google Scholar]
- 10.Podajsky RJ, Sekiguchi Y, Kikuchi S, Myers RR. The histologic effects of pulsed and continuous radiofrequency lesions at 42°C to rat dorsal root ganglion and sciatic nerve. Spine. 2005;9:1008–1013. doi: 10.1097/01.brs.0000161005.31398.58. [DOI] [PubMed] [Google Scholar]
- 11.Rosomoff HL, Carrol F, Brown J, Sheptak P. Percutaneous radiofrequency cervical cordotomy technique. J Neurosurg. 1965;23:639–644. doi: 10.3171/jns.1965.23.6.0639. [DOI] [PubMed] [Google Scholar]
- 12.Shah RV, Racz GB. Long-term relief of posttraumatic headache by sphenopalatine ganglion pulsed radiofreuency lesioning: a case report. Arch Phys Med Rehabil. 2004;85:1013–1016. doi: 10.1016/j.apmr.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Sluijter ME, Kleef M. Characteristics and mode of action of radiofrequency lesions. Curr Rev Pain. 1998;2:143–150. [Google Scholar]
- 14.Sluijter ME, Cosman ER, Rittman WB, Kleef M. The effects of pulsed radiofrequency fields applied to the dosal root ganglion—a preliminary report. Pain Clin. 1998;11:109–117. [Google Scholar]
- 15.Sluijter ME. Radiofrequency. Switzerland: Flivo Press; 2001. [Google Scholar]
- 16.Stolker RJ, Vervest AC, Groen GJ. Percutaneous facet denervation in chronic thoracic spinal pain. Acta Neurochir (Wien) 1993;122:82–90. doi: 10.1007/BF01446991. [DOI] [PubMed] [Google Scholar]
- 17.Sweet WH, Wespic JG. Controlled thermocoagulation of trigeminal ganglion and rootlets for differential destruction of pain fibres. J Neurosurg. 1974;40:143–156. doi: 10.3171/jns.1974.40.2.0143. [DOI] [PubMed] [Google Scholar]
- 18.Kleef M, Leim L, Lousberg R, et al. Radiofrequency lesions adjacent to dorsal root ganglion for cervicobrachial pain: a prospective double blind randomized study. Neurosurgery. 1996;38:1127–1131. doi: 10.1097/00006123-199606000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Zundert J, Brabant S, Kelft E, Vercruyssen A, Buyten JP. Pulsed radiofrequency treatment of the Gasserian ganglion in patients with idiopathic trigeminal neuralgia. Pain. 2003;104:449–452. doi: 10.1016/S0304-3959(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 20.Zundert J, Lame IE, Louw A, et al. Percutaneous pulsed radiofrequency treatment of the cervical dorsal root ganglion in the treatment of chronic cervical pain syndromes: a clinical audit. Neuromodulation. 2003;6:6–14. doi: 10.1046/j.1525-1403.2003.03001.x. [DOI] [PubMed] [Google Scholar]
- 21.Vervest ACM, Stolker RJ. The treatment of cervical pain syndromes with radiofrequency procedures. Pain Clin. 1991;4:103–112. [Google Scholar]