Abstract
The study design included a multidisciplinary examination of the mineral phase of ovine intervertebral disc calcifications. The objective of the study was to investigate the mineral phase and its mechanisms of formation/association with degeneration in a naturally occurring animal model of disc calcification. The aetiology of dystrophic disc calcification in adult humans is unknown, but occurs as a well-described clinical disorder with hydroxyapatite as the single mineral phase. Comparable but age-related pathology in the sheep could serve as a model for the human disorder. Lumbar intervertebral discs (n = 134) of adult sheep of age 6 years (n = 4), 8 years (n = 12) and 11 years (n = 2) were evaluated using radiography, morphology, scanning and transmission electron microscopy, energy dispersive X-ray spectroscopy, X-ray powder diffraction, histology, immunohistology and proteoglycan analysis. Half of the 6-year, 84% of the 8-year and 86% of the 11-year-old discs had calcific deposits. These were not well delineated by plain radiography. They were either: (a) punctate deposits in the outer annulus, (b) diffuse deposits in the transitional zone or inner annulus fibrosus with occasional deposits in the nucleus, or (c) large deposits in the transitional zone extending variably into the nucleus. Their maximal incidence was in the lower lumbar discs (L4/5–L6/7) with no calcification seen in the lumbosacral or lower thoracic discs. All deposits were hydroxyapatite with large crystallite sizes (800–1,300 Å) compared to cortical bone (300–600 Å). No type X-collagen, osteopontin or osteonectin were detected in calcific deposits, although positive staining for bone sialoprotein was evident. Calcified discs had less proteoglycan of smaller hydrodynamic size than non-calcified discs. Disc calcification in ageing sheep is due to hydroxyapatite deposition. The variable, but large, crystal size and lack of protein markers indicate that this does not occur by an endochondral ossification-like process. The decrease in disc proteoglycan content and size suggests that calcification may precede or predispose to disc degeneration in ageing sheep.
Keywords: Ageing, Animal model, Dystrophic calcification, Hydroxyapatite
Introduction
There are a number of diseases and disorders of diverse aetiology in which intervertebral disc calcification (IDC) may occur including calcium pyrophosphate dihydrate deposition, hyperparathyroidism, haemochromatosis, thalassemia, alkaptonuria, amyloidosis and acromegaly [2]. In addition, conditions that immobilise a spinal segment, such as ankylosing spondylitis and surgical posterior spinal fusion, may also result in IDC. However, the deposition of calcium salts per se has not been demonstrated to be of clinical significance in any of these conditions. Rather, they have been incidental radiographic findings. For instance, a radiographic study of 223 cadaveric spines revealed that IDC, particularly in the annulus fibrosus (AF), increases with age and the degree of reduction in disc height (a reliable index of degeneration), which are expected associations [7].
One type of IDC of unknown cause, which may have clinical significance, has been in the past non-specifically termed dystrophic [47]. The condition is not associated with any systemic disease or disorder (vide supra) and occurs without detectable disturbance of calcium/phosphorus metabolism. The peak incidence is in the fifth decade and it is more common in males than in females. It occurs almost exclusively in the thoracic and upper lumbar regions and most frequently in the T9–10 disc; multiple level deposits are not unusual [1, 7, 9, 47]. An entire nucleus pulposus (NP) may be calcified, but the outer AF is often unaffected, at least radiographically. The periphery of the NP deposit is in the transitional zone (TZ), the somewhat ill-defined region of variable width where the NP and AF merge.
A thoracic NP deposit may be an incidental radiographic finding [10, 52], but may still be responsible for a short-lived episode of acute thoracic pain [1]. Posterior prolapse of a calcific NP deposit, which initially has a toothpaste-like consistency, may not produce significant symptoms [47, 56], but root syndromes and spinal cord compression are well-documented complications. X-ray diffraction and electron microscopic examination of calcified prolapses show hydroxyapatite (HA), but no other mineral phases [46]. Giant crystallites are present in a markedly degraded matrix with a wide spectrum of crystallite size [45, 46]. It is regrettable and confusing that the above-described pathology has not been considered as a discrete entity [7, 9]. While IVD degeneration occurs as an age-related event in humans, it has also been detected as early as the third decade. Not surprisingly, it is commonly associated with small, often microscopic, foci of calcification. These minute deposits give HA diffraction patterns with broadened reflections, similar to those obtained from calcified cartilage, indicative of a small crystallite size; calcite and magnesium whitlockite are also frequently detected in small amounts of randomly selected postmortem disc tissue of subjects over 50 years of age [45]. An additional mineral phase found in older cadaver disc tissue is calcium pyrophosphate dihydrate [24]. The interrelationships between these mineral phases and the possible biological significances thereof are yet to be established. Hence, for the time being there is no alternative but to consider them all under the unsatisfactory collective label of dystrophic calcification(s). Nevertheless, a reasonable case can be made for using, as we have done, the term hydroxyapatite deposition disorder (HADD) for the unique discal lesions summarised above. This descriptive term has been also used for the common calcific lesions in human supraspinatus tendons [18]. It has some features in common with the thoracic discal pathology, and notably a peak age incidence in the fifth decade [11]. Further, supraspinatus HADD and comparable tendon calcification elsewhere in the body may produce intense, acute local pain or be asymptomatic [18].
The ovine intervertebral disc, which is also subject to calcification, has been widely used to investigate disc metabolism, biomechanical properties of the intact lumbar column and the discal response to injury [14, 20, 21, 26, 27, 29, 30, 37, 39, 48, 54, 55]. In the cited literature it is regarded as a suitable experimental model for its human counterpart, even though there is little evidence that IVD pathology or prolapse of calcific deposits is a clinical problem in this animal [4, 16]. The present paper reports on the mineral phases in ovine IDC, and attention is drawn to certain similarities and differences between the pathology in humans and sheep.
Materials and methods
All chemicals from Sigma-Aldrich were of AR grade or of the best quality available.
Animals and processing of spines
Purebred Merino sheep were used for this study (n = 18), which provided 132 IVDs for analysis. The sheep included: wethers, 6-year-olds, n = 4; and ewes, 8-year-olds, n = 12 and 11-year-olds, n = 2. Plain lateral radiographs were made of each sheep spine prior to killing by injection of an overdose of pentobarbitone sodium. The lumbar spines were removed within 30 min of death. Paraspinal muscle and ligaments were trimmed from the lumbar spinal segments and the dorsal spinal elements (facet joints, spinal processes, etc.) were removed. The IVDs were isolated by cutting midway through the cranial and caudal vertebral bodies using a band saw. Isolated IVDs were then bisected and the cut surfaces photographed.
Processing of disc specimens for electron microscopy
A total of 35 calcific deposits (2 mm3 blocks), identified by gross observation, were dissected out of the IVDs along with 12 morphologically normal adjacent non-calcified tissues. The calcific deposits were sampled from IVDs, which displayed punctate AF calcifications, such as those depicted in Fig. 1c, and from the more extensive TZ or NP calcifications demonstrated in Fig. 1d and e, where the punctate deposits were less prevalent. The punctate calcifications were more typical of the IVDs displaying an IVD calcification score of 1.0 or less, while the larger calcifications were found in IVDS with calcification scores >1.5. Of the 35 calcific deposits sampled for electron microscopy, these were sampled from the L1L2, L2L3, L3L4, L4L5 and L5L6 IVDs of two animals in the 6-yr-old sheep group; three animals from the 8-year-old and two animals from the 11-year-old sheep group. Thus a total of 35 IVDs were sampled.
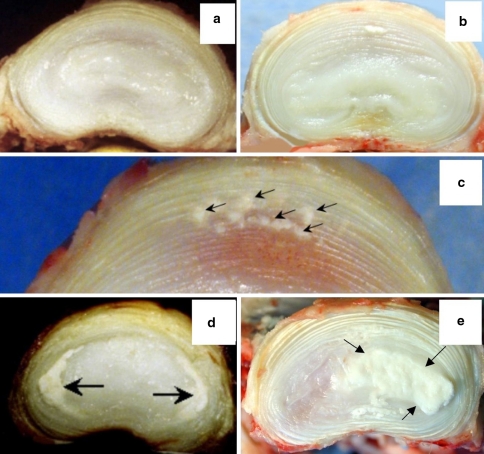
Fig. 1.
Morphologic appearance of horizontally bisected discs depicting no calcification in 2-year-old L3L4 and 4-year-old L5L6 IVDs, respectively (a, b). Small punctate annular calcific deposits in the anterior annulus of L4L5 of 4-year-old (c); moderate calcification predominantly in the transitional zone of 4-year-old L3L4 IVD (d); heavy deposition of calcium in the TZ/NP L5L6 11-year-old IVD (e). Arrows depict the deposits
The specimens were fixed in glutaraldehyde (2.5% in cacodylate buffer) for 2 h at room temperature. These specimens were selected from L2L3 to L5L6 spinal levels, as no cases of IDC were observed in the L7S1 IVD.
Processing of SEM specimens and energy dispersive spectroscopy
The disc specimens were rinsed in ultrapure water and dehydrated through graded ethanol (30–100%) followed by critical point drying. The dried specimens were mounted on stubs and gold-coated prior to examination in a Philips 505 scanning electron microscope using conventional imaging plus digital image capture. Energy dispersive X-ray spectroscopy (EDS) was conducted simultaneously on the specimens to undertake a microanalysis of the elemental compositions of macroscopically normal regions of the AF and calcific deposits, such as those depicted in Fig. 2a, b.
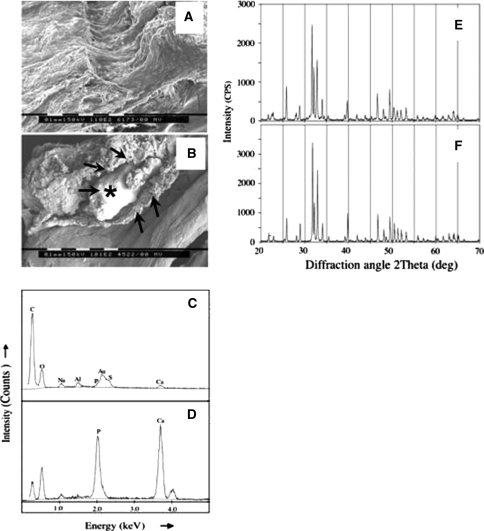
Fig. 2.
SEMs of: (a) normal AF with alternating lamellae evident and (b) a calcific deposit within an annular lamella (bar = 0.1 mm). Energy dispersive X-ray spectra of (c) normal non-calcified AF and (d) an annular calcific deposit showing the presence of Ca and P, which occurred in a molar ratio of 9:3 in (b) indicating the presence of hydroxyapatite as the mineral phase. XRD spectra of sintered calcific deposit (e) and ovine cortical bone (f). The arrow labeled with an asterisk in (b) indicates a calcific deposit examined by EDS in (d) surrounded by a shell-like material [unlabelled arrows in (b)]
Processing of TEM specimens
Fixed disc samples were rinsed in ultrapure water and cut into pieces of less than 1 mm3 size. The samples were post-fixed in 1% OsO4 at room temperature for 2 h, and then washed in ultra pure water for 10 min. The specimens were dehydrated in graded acetone (30–100%) and then infiltrated with acetone: resin (1:1), 100% resin (×3) and finally embedded in fresh resin, which was polymerised at 60°C overnight. Ultrathin tissue sections (50–100 nm) were cut using an Ultracut T microtome and were transferred to Formvar-coated copper grids (200 mesh) that had been carbon coated. The grids were examined in a Philips CM12 transmission electron microscope at 120 kV at various magnifications (2,300–260,000×).
Preparation of specimens for X-ray powder diffraction
Aliquots (0.5–1.0 g) of glutaraldehyde-fixed calcified IVD tissues from 35 IVDs representative of all calcific IVDs were rinsed in ultrapure water, freeze dried then pulverized at −195°C in a ball mill. Fresh normal ovine cortical bone (femoral shaft) was processed similarly for comparison. X-Ray diffraction (XRD) spectra were obtained from a Shimadzu XRD 6000 diffraction unit using a diffraction angle (2 θ) range from 20° to 70°. The samples were analysed before and after sintering the powders at 950°C for 1 h.
Preparation of crystal suspensions for determination of crystal size distributions
All organic material was removed from glutaraldehyde-fixed disc pieces (2 mm3) containing calcific deposits by immersion in 2.5% sodium hypochlorite solution for 15 min. Pooled calcific deposits from a representative mixture of the calcific IVDs, that is L1L2 to L6L7 IVDs (n = 35) from all of the 6–11-year-old animals were used for this analysis. Samples of normal pulverised ovine cortical bone were treated identically for comparison. The samples were washed with ultrapure water (5 × 10 min) and dehydrated through a series of 10 min washes in graded ethanol (50–100%). Specimens were recovered at each step using centrifugation (2 min, 14,000 rpm). The residual pellet was resuspended in 100% ethanol, briefly sonicated and a small drop of the crystal suspension was placed on Formvar-coated copper grids (200 mesh) that had been carbon coated. The grids were examined by TEM at 45,000× magnification, and crystal dimensions were measured as previously described [57].
Histology
Intact IVD-vertebral body segments or disc areas of specific interest were fixed in 10% neutral buffered formalin for 3 days and decalcified with several changes of 10% v/v formic acid in 5% neutral buffered formalin. The disc specimens were dehydrated in a graded methanol series and embedded in paraffin. Sections (4–7 μm) were cut using a Leica rotary microtome, adhered to positively charged microscope slides (Menzel-Glaser), deparaffinised in xylene and rehydrated in graded alcohols to water. Tissue sections were stained with H&E or toluidine blue/fast green [31]. Immunohistochemical localisation for type X collagen, osteopontin, bone sialoprotein and osteonectin was performed using specific polyclonal and monoclonal antibodies as outlined in Table 1.
Table 1.
Antibodies used for the localisation of IVD tissue components
| Antibody, clone (antigen) | Dilution used |
|---|---|
| Mouse polyclonal anti type X collagen [25] (deer antler cartilage) | 1/2,000 |
| Rabbit polyclonal anti-human osteopontin RGD domain [26] clone LF-86 (TVDTYDGRGDSVVYGLRSLS-[LPH]) | 1/4,000 |
| Rabbit polyclonal anti-human osteonectin [26] clone LF-37 (EALPDETEVVEETVAEVTEVP-[KLH]) | 1/4,000 |
| Rabbit polyclonal anti-human bone sialoprotein [26] clone LF-120 (amino acids 129-281, human sequence) | 1/2,000 |
The sections were pre-digested with chondroitinase ABC (0.25U/ml) for 1 h at 37°C in 50 mM Tris 30 mM sodium acetate buffer of pH 8.0, followed by bovine testicular hyaluronidase (1,000 U/ml) for 1 h at 37°C in phosphate buffer, pH 5.0
Disc proteoglycan analysis
Extraction of intervertebral disc tissues and determination of their sulphated GAG contents
Macroscopically normal and calcific regions of the AF and NP/TZ (~10 mg wet weight) were finely diced with scalpels and extracted with 4 M guanidinium hydrochloride (GuHCl) buffered in 50 mM sodium acetate, pH 5.8, containing proteinase inhibitors (1 mL) with constant end-over-end stirring for 48 h at 4°C. The tissue extract was then separated from the tissue residue by centrifugation and dialysed against distilled water. The tissue residue was washed with distilled water to remove GuHCl, then papain digested and an aliquot assayed for sulphated-GAG using the 1, 9-dimethylmethylene blue metachromatic dye binding assay of Farndale et al. [13] using bovine tracheal CS as a standard to construct a calibration curve for quantitation.
Sepharose CL2B gel permeation chromatography of the extracted disc proteoglycans
A column of Sepharose CL2B (92 cm × 1.6 cm) was calibrated using aggregated bovine nasal cartilage aggrecan to determine the void volume. Bovine tracheal cartilage CS was used to determine the total volume and to calculate the distribution coefficients (Kav) of the proteoglycan species. Normal and calcified AF and NP extracts (4.0 mL) were dialysed against 0.5 M sodium acetate (pH 6.8) and an excess of high molecular weight hyaluronan (Healon, 0.1 ml, 100 μg) was added and the samples chromatographed with the same buffer as eluent at a flow rate of 12 mL/h. Fractions (2.5 mL) were collected and aliquots were assayed for sulphated-GAG to determine the distribution of proteoglycan species [29, 48]. The proportion of disc proteoglycan present as aggregate (Kav −0.2 to 0.2) was determined relative to the total area of the chromatogram to calculate the percentage aggregate present in each sample [27, 28].
Quantitative scoring of IVD calcification
IVD calcifications were graded into several categories on the basis of the incidence and extent of the calcific deposits evident in non-fixed midline horizontal sections of the IVDs: 0 = no calcification; 1 = isolated small calcific deposits; 2 = multiple calcific deposits mainly in TZ but not affecting NP; 3 = extensive calcific deposits affecting over one-third of TZ and NP. The calcification scores were calculated by summing the grades of individual IVDs at each spinal level and dividing this by the number of IVDs examined.
Morphological assessment of intervertebral disc degeneration
A number of criteria were used to objectively determine the degeneracy level of the IVDs examined in the present study. (1) Toluidine blue stained IVD sections were assessed for depletion of anionic proteoglycan as an index of IVD degeneration. (2) Marked reduction in disc height relative to an age-matched normal control IVD was also indicative of disc degeneration. (3) Inversion of the normal anterior and posterior annular lamellae was also considered an indication of IVD degeneration as were the presence of focal defects such as (4) annular tears, and (5) cystic degeneration.
Results
Of the 28 IVDs examined from 6-year-old sheep, 14 contained calcific deposits with maximal deposition at the L4L5 and L5L6 spinal levels; no calcifications were evident in the L7S1 IVDs and only two of the four L1L2 had discernable isolated deposits (Table 2). Of the 12 8-year-old ovine spines (63% of IVDs), 5 had radiographic evidence of IVD calcification. On gross examination, however, 83% of these 8-year-old IVDs had calcific deposits, with maximum severity at L5L6 and L6L7 (Table 2). Of the 22 discs examined from the two 11-year-old sheep, 14 had calcifications with 12 out of the 14 lumbar IVDs (86% maximal calcification at L5L6 and L6L7) affected, while both lumbosacral IVDs were unaffected (Table 2). Three thoraco-lumbar IVDs (T12T13, T13T14, T14L1) were also available from each of the 11-year-old sheep with none containing any calcific deposits. As described in Table 2 and shown in Fig. 1, calcification in the ovine lumbar IVD fell into three patterns: (1) small punctate calcific deposits in the outer annular layers of the anterior AF often in the absence of other calcifications (Fig 1c); (2) diffuse calcification in the transitional or inner AF zone with occasional deposits evident in the NP (Fig. 1d), (3) heavy deposition in the transitional zone affecting large areas of the NP (Fig. 1e). Heavy deposition of calcific deposits in the AF was evident in three of the L5L6 (Fig. 1e) and L6L7 IVDs from the 11-year-old sheep. This was not observed in any of the younger age groups.
Table 2.
Calcification scores in IVDs of sheep aged 6, 8 and 11 years old
| Disc level | 6-Year-old sheep (n = 4) | 8-Year-old sheep (n = 12) | 11-Year-old sheep (n = 2) |
|---|---|---|---|
| T12T13 | na | na | 0 |
| T13T14 | na | na | 0 |
| T14L1 | na | na | 0 |
| L1L2 | 0.5 | 0.4 | 0.5 |
| L2L3 | 1.0 | 1.5 | 0.5 |
| L3L4 | 1.5 | 2.2 | 1 |
| L4L5 | 2.25 | 2.3 | 2 |
| L5L6 | 2 | 2.5 | 3 |
| L6L7 | 1.75 | 2.5 | 3 |
| L7S1 | 0 | 0 | 0 |
IVD calcifications were graded as follows: 0 = no calcification; 1 = isolated small calcific deposits; 2 = multiple calcific deposits mainly in TZ but not affecting NP; 3 = extensive calcific deposits affecting over one-third of TZ and NP. The calcification scores were calculated by summing the grades of IVDs at each spinal level and dividing by the number of IVDs examined
na IVD not available for analysis
SEM demonstrated the alternating lamellar structure of the normal AF (Fig. 2a). The calcific deposits occurred between the annular lamellae and often appeared to contain an outer shell-like structure with amorphous calcific material deposited around a more compact central region (Fig. 2b). EDS demonstrated that calcium and phosphorus were the major elements detected in calcific deposits, while these were absent in the non-calcified AF (Fig. 2c, d). Minor quantities of aluminium and gold arising from sample preparation were also evident in all samples including the control tissues (Fig. 2c). The Ca and P occurred in a molar ratio of 5:3, indicative of hydroxyapatite as the predominant component of the mineral phase [6]. The composition of the calcific deposits was confirmed by XRD with spectra of poorly crystalline hydroxyapatite observed in the non-sintered samples (not shown) while sintering resulted in classical spectra of highly crystalline hydroxyapatite [5], identical to those obtained from sheep cortical bone (Fig. 2e, f).
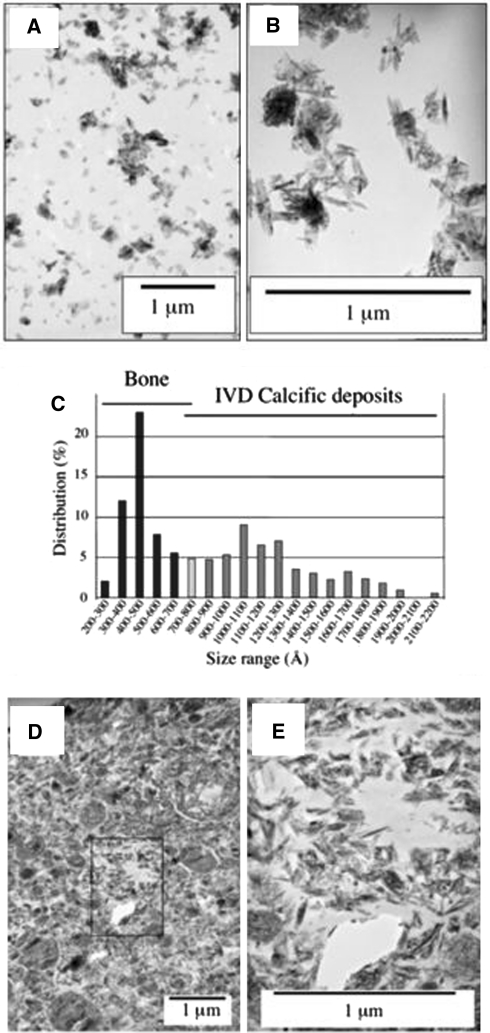
TEM of crystal suspensions prepared from normal ovine cortical bone and selected annular calcific deposits (Fig. 3a, b) demonstrated wide differences between the sizes of the crystals obtained (Fig. 3c). The size ranges of the hydroxyapatite crystals prepared from the IVD calcific deposits were from 500 to 2,200 Å, with 65% of the crystals falling in the 800–1,300 Å size range (Fig. 3c). In contrast, hydroxyapatite crystals from ovine cortical bone were more uniform in size and significantly smaller, ranging in size from 200 to 800 Å with the majority in the 300–600 Å size range (Fig. 3c). Similar crystal sizes were determined from TEM micrographs of ultrathin sections of IVD calcified deposits (Fig. 3d, e).
Fig. 3.
TEMs of crystal suspensions prepared from cortical bone (a) and calcific deposits from the ovine IVD (b). As much as 15 micrographs of bone and IVD crystal suspensions were examined at 45,000× magnification to determine the length of single crystals, as presented in (c). Crystals from the IVD deposits (n = 144) were measured in a size range of 500–2,200 Å, with 65% of the distribution within the 800–1,300 Å size range. The cortical bone crystals (n = 197) were significantly smaller (P < 0.001) and ranged in size from 200–800 Å with the majority in the 300–600 Å size range. d, e Appearance of the crystallites in an ultrathin section (80 nm) at 13,000× and 45,000× magnification demonstrating the heterogeneous nature of these deposits in the calcific mass. The boxed area in (d) is presented at higher magnifications in segment (e)
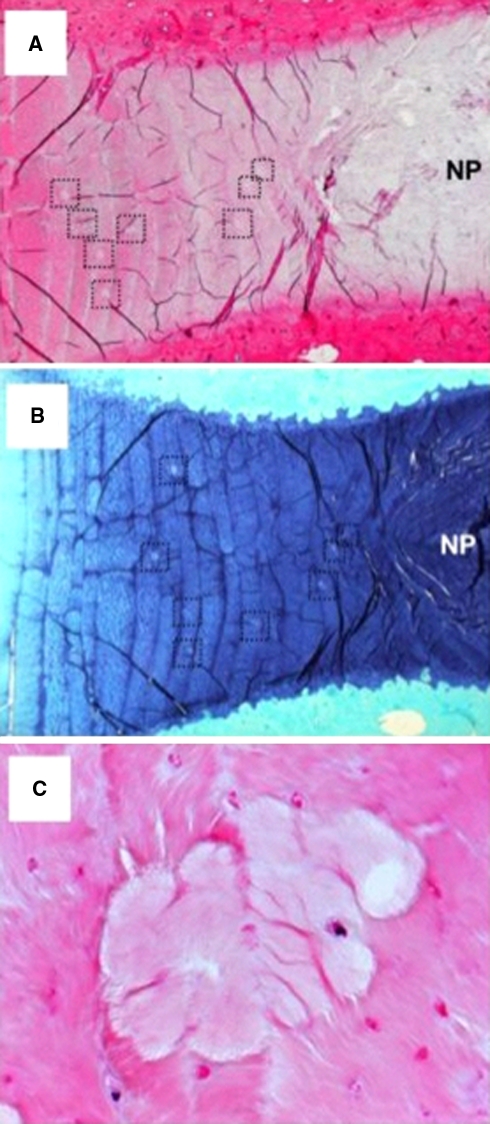
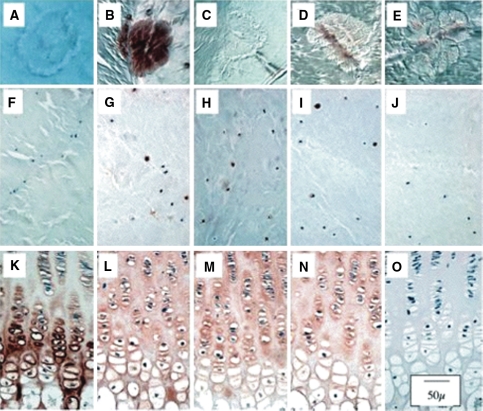
Representative histological sections from a 6-year-old sheep demonstrating small focal calcific deposits mainly in the transitional zone are shown in Fig. 4. The calcified areas were evident as regions staining less strongly with H & E and toluidine blue-fast green (Fig. 4a, b). Based on morphology, live and dead cells were evident throughout the AF, and there was no distinct change in distribution around the calcific deposits (Fig. 4c). Furthermore, there was no evidence of cell hypertrophy surrounding or within the calcified areas. No type X collagen could be detected in the matrix around or in the calcific annular deposits, although occasional viable cells around such deposits were positive (Fig. 5a). The majority of cells in the normal AF showed little or no positive staining for type X collagen (Fig. 5a), while typical positive cellular and matrix staining for type X collagen was observed in the lower hypertrophic zone of the growth plate (Fig. 5k). Calcific deposits stained positively for bone sialoprotein (Fig. 6b) as did cells in the AF (Fig. 5g), NP, cartilage endplate and vertebral body (not shown). Strong positive matrix and cell staining for bone sialoprotein was seen in the lower proliferative and early hypertrophic zones of the growth plate (Fig. 5l). The IVD calcific deposits and surrounding matrix did not stain positive for antibodies to osteonectin or osteopontin (Fig. 5c, d). In contrast, positive immunostaining was observed in cells in the NP, cartilaginous endplates (not shown) and AF (Fig. 5h, i). In the growth plate the cells and matrix of the lower proliferative and upper hypertrophic zone stained positive for both osteopontin and osteonectin (Fig. 5m, n).
Fig. 4.
Low power (16×) H & E (a) and toluidine blue-fast green (b) stained vertical sections of two different 6-year-old sheep depicting multiple areas of small calcifications in the transitional zone, which are deficient in anionic proteoglycan (dotted box areas). c Higher power views of one of the annular calcific deposits and surrounding disc fibrochondrocytes (200×)
Fig. 5.
Immunolocalisation of type X collagen (a, f, k); bone sialoprotein (b, g, i), osteopontin (c, h, m), osteonectin (d, i, n) associated with calcific deposits (a–e), in non-calcified AF (f–j) and tibial growth plate (positive control, k–o); negative controls are depicted in the far right-hand lane (e, j, o). Nova Fast Red was used as chromogen and Nomarski differential interference contrast microscopy for visualisation. Of the proteins assessed, only bone sialoprotein localised positively to discrete calcific deposits in the AF (b). Isolated AF cells also stained positively for bone sialoprotein (g) osteopontin (h) and osteonectin (i)
Fig. 6.
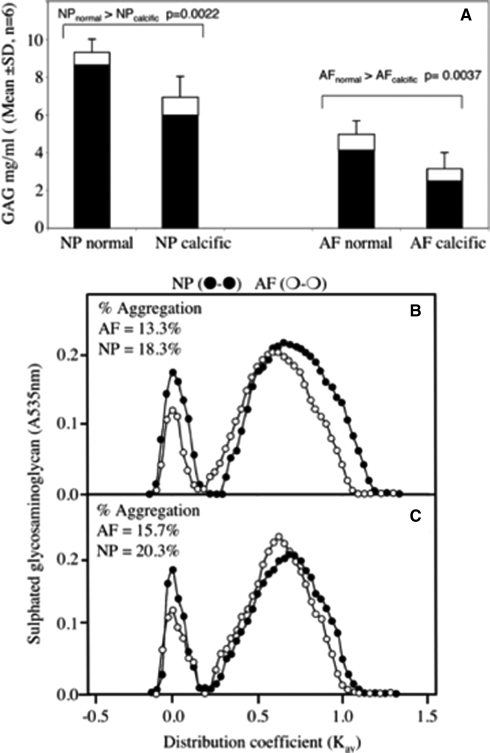
a Stacked histogram depicting the sulphated glycosaminoglycan content of 4 M GuHCl extracts (solid bars) and papain digested washed tissue residues (open bars) of normal and calcific AF and NP from two 11-year-old female sheep. The results shown are mean values calculated from six macroscopically normal and six calcific AF and NP tissue specimens dissected from the L1L2 to L6L7 IVDs of each spine, thus 12 tissue samples were examined per sample group. Sepharose CL2B Gel permeation chromatography of 4 M GuHCl extracts (4 mL) of pooled normal (b) and calcific (c) AF and NP from 11-year-old ovine IVDs. There was no significant difference in the aggregation levels in the normal and calcific AF (AFn = 13.3%; AFc = 15.7%) and NP (NPn = 18.3%; NPc = 20.3%). The non-aggregatable aggrecan monomers of the normal and calcific NP samples were of slightly smaller hydrodynamic size (Kav = 0.688) than the AF aggrecan monomers (Kav = 0.594)
In both calcified and normal discs, the NP contained significantly more GAG than the AF as expected (Fig. 6a). There was a significant reduction in the GAG content of both the AF and NP in discs containing calcific deposits compared to those with no evidence of calcification (Fig. 6a). Gel permeation chromatography demonstrated that the majority (80–87%) of proteoglycan in both the AF and NP did not aggregate with HA and eluted in the included volume of a CL2B column (Fig. 6b). There was no difference in aggregation percentage between calcified and non-calcified discs. The non-aggregating PG peak in the AF was eluted earlier than that of the NP, but at the same Kav in both calcified and non-calcified discs. In contrast, the non-aggregating PGs in the NP of calcified discs eluted 1–2 fractions later (i.e. slightly smaller hydrodynamic size) than those from the non-calcified discs (Fig. 6c).
Discussion
There are some relevant similarities between HADD in humans and the described IDC in sheep [7, 9]. In both the single mineral phase is HA with a wide spectrum of crystallite size in contrast to the limited range of smaller crystallites seen in normal ovine cortical bone. Large HA crystallites suggestive of an unregulated calcification process have been reported in tuberculosis foci [25], in the degraded matrix of chondrosarcomata [23, 41, 53] and in calcific tendinitis [50]. The typical markers of normal endochondral ossification, type X collagen, osteopontin and osteonectin were not detected in the ovine deposits, although bone sialoprotein was present. The last molecule is one of the major non-collagenous proteins in normal bone where it plays a central role in mineralisation [3], although it does not induce calcification in all tissues with its effects being dependent on the local environment and cells [51]. Despite bone sialoprotein’s known roles in endochondral ossification, the absence of other key markers indicates that IDC does not occur through this ordered process. These results contrast with HA deposition in tendons and blood vessels where there is a positive staining for type X collagen, osteopontin and osteonectin [8, 17, 34, 35, 44, 49]. In these regards, it is probable that the avascular nature of the IVD plays a modulating role in the pathogenesis of calcification.
Hydroxyapatite deposition has been associated with IVD degeneration in the human lumbar spine [15], but the temporal discordance between the incidence of degeneration and calcification argues against a causal link [7, 9, 33]. Unequivocal evidence that disc degeneration is a predisposing factor for ovine IDC is wanting. However, in the present study, disc tissues from 11-year-old sheep containing calcific deposits had significantly less proteoglycan per tissue wet weight than age-matched non-calcified tissue from comparable disc regions, but no other overt morphological signs of IVD degeneration. The proteoglycans extracted from the calcific ovine disc tissues were of smaller hydrodynamic size compared to morphologically normal IVD tissues of comparable age and spinal level suggesting that the proteoglycans in the calcific mass may have undergone more extensive degradation than the age-matched tissue. Toluidine blue also demonstrated a marked decrease in anionic proteoglycan staining in the vicinity of calcific deposits suggesting that the calcification may be associated with proteoglycan degradation. It remains unclear as to whether such calcific deposits arise from, or result in, disc degeneration. However, it should be noted that Rees et al. [38] have shown that bone-derived small leucine rich repeat proteoglycans (SLRPs) inhibited HA crystal growth in vitro. Furthermore, some members of the SLRPs have been shown to be degraded in an ovine model of experimental disc degeneration [32] prior to deposition of such calcific deposits suggesting that they arise from degenerative changes in the IVD.
In man and sheep, the NP undergoes a chondroid metaplasia with skeletal maturation. This involves an increase in type II collagen with fibrocartilaginous cells concomitantly replacing notochordal cells. The same sequence of events occurs in certain breeds of dog and was the basis used by Hansen [20, 21] for dividing canines into two categories, chondrodystrophoid and non-chondrodystrophoid. The former are particularly prone to IDC and prolapse, whereas discal calcification is quite rare in the latter [20, 21]. The mineral phase in canine calcified prolapses is identical to that in human HADD [12] and that which we here describe in sheep. It is noteworthy that in the IVDs of humans, sheep and chondrodystrophoid dogs, the TZ is particularly susceptible to the process, which leads to HA deposition, and not infrequently in all three species this is the only discal region affected. The TZ is the site of maximal uptake of 35S (glycosaminoglycan synthesis) in the immature rabbit [42, 43] and in piglets [22] and the site of maximal uptake of 3H-glycine (protein synthesis) in the immature rabbit IVD [19]. Autoradiographic studies with 3H-thymidine in young rabbits show that these zones have the highest concentration of actively dividing cells and demonstrate that the TZ is a site of high metabolic activity and remodelling, which may predispose to HA deposition.
It is established that human, canine and ovine discal HADD occurs in fibrocartilaginous tissue. Also relevant is the observation that human supraspinatus tendon calcification occurs in that part of the tendon where fibrocartilaginous transformation takes place [50]. This is in the so called “critical zone”, which has a precarious blood supply [36, 40]. Supraspinatus tendon calcification, like human HADD, has a clear peak incidence in the fifth decade and so, strictly speaking, is not an ageing phenomenon [11]. Histological studies indicate that metaplasia is the event, which leads to this form of calcification and not degeneration or inflammation [50].
There are notable inexplicable dissimilarities between HADD in humans and sheep. No explanation can be offered for the different spinal distributions, thoracic and upper lumbar in the former [47] and lumbar in the latter (Table 2). HADD has occasionally been seen in the lumbar spines of 4-year-old sheep during the course of other studies, but not at an earlier age. Neither can an explanation be offered for the apparent immunity of the ovine lumbosacral disc to calcification. However, it is known that proteoglycan turnover is faster at this spinal level than at more proximal lumbar discs [48].
While we have been unable to elucidate the mechanism of HADD in sheep, clearly it is different from that in normal osteogenesis. This animal nevertheless provides a useful, naturally occurring model for investigation of the aetiology and pathogenesis of HADD, notwithstanding differences detailed above, which indicate that direct comparison with the human IVD is inappropriate.
Acknowledgments
Ms Susan Smith is thanked for undertaking the histology and immunolocalisations used in this study. Dr Larry Fisher, National Institutes of Health, National Institute of Dental Care and Research, Bethesda, USA is thanked for the generous gifts of antibodies to bone sialoprotein, osteopontin and osteonectin. This study was funded by: the Sir Charles Cutler Memorial Fund, Institute of Bone and Joint Research, University of Sydney, Royal North Shore Hospital; Medtronics Australia and NHMRC project Grant 352562.
References
- 1.Abbott KH, Leimbach WH, Retter RH. Further observations on thoracic disk protrusions. Bull Los Angel Neuro Soc. 1957;22:58–68. [PubMed] [Google Scholar]
- 2.Aessopos A, Tsironi M, Polonifi K, Baltopoulos P, Vaiopoulos G. Intervertebral disc calcification in thalassemia intermedia. Eur J Haematol. 2008;80:164–167. doi: 10.1111/j.1600-0609.2007.00987.x. [DOI] [PubMed] [Google Scholar]
- 3.Alford AI, Hankenson KD. Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–757. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Baker JR, Lyon DG. A case of intervertebral disc degeneration and prolapse with spondylosis in a sheep. Vet Rec. 1975;96:290. doi: 10.1136/vr.96.13.290. [DOI] [PubMed] [Google Scholar]
- 5.Bigi A, Cojazzi G, Panzavolta S, Ripamonti A, Roveri N, Romanello M, Noris Suarez K, Moro L. Chemical and structural characterization of the mineral phase from cortical and trabecular bone. J Inorg Biochem. 1997;68:45–51. doi: 10.1016/S0162-0134(97)00007-X. [DOI] [PubMed] [Google Scholar]
- 6.Bozzola J, Russell LD (1999) Electron microscopy, 2nd edn. In: Bozzola JJ, Russell LD (eds) Jone and Bartlett Pubs, Sudbury, pp 369–395
- 7.Chanchairujira K, Chung CB, Kim JY, Papakonstantinou O, Lee MH, Clopton P, Resnick D. Intervertebral disk calcification of the spine in an elderly population: radiographic prevalence, location, and distribution and correlation with spinal degeneration. Radiology. 2004;230:499–503. doi: 10.1148/radiol.2302011842. [DOI] [PubMed] [Google Scholar]
- 8.Chen NX, Moe SM. Uremic vascular calcification. J Investig Med. 2006;54:380–384. doi: 10.2310/6650.2006.06017. [DOI] [PubMed] [Google Scholar]
- 9.Cheng XG, Brys P, Nijs J, Nicholson P, Jiang Y, Baert AL, Dequeker J. Radiological prevalence of lumbar intervertebral disc calcification in the elderly: an autopsy study. Skeletal Radiol. 1996;25:231–235. doi: 10.1007/s002560050070. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JA, Abraham E. The calcified intervertebral disc: a non-specific roentgenologic sign. J Med Soc N J. 1973;70:459–460. [PubMed] [Google Scholar]
- 11.Depalma AF, Kruper JS. Long-term study of shoulder joints afflicted with and treated for calcific tendinitis. Clin Orthop Relat Res. 1961;20:61–72. [PubMed] [Google Scholar]
- 12.Durant DM, Riley LH, 3rd, Burger PC, McCarthy EF. Tumoral calcinosis of the spine: a study of 21 cases. Spine. 2001;26:1673–1679. doi: 10.1097/00007632-200108010-00009. [DOI] [PubMed] [Google Scholar]
- 13.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 14.Fazzalari NL, Costi JJ, Hearn TC, Fraser RD, Vernon-Roberts B, Hutchinson J, Manthey BA, Parkinson IH, Sinclair C. Mechanical and pathologic consequences of induced concentric annular tears in an ovine model. Spine. 2001;26:2575–2581. doi: 10.1097/00007632-200112010-00010. [DOI] [PubMed] [Google Scholar]
- 15.Feinberg J, Boachie-Adjei O, Bullough PG, Boskey AL. The distribution of calcific deposits in intervertebral discs of the lumbosacral spine. Clin Orthop Relat Res. 1990;254:303–310. [PubMed] [Google Scholar]
- 16.Fews D, Brown PJ, Alterio GL. A case of invertebral disc degeneration and prolapse with Schmorl’s node formation in a sheep. Vet Comp Orthop Traumatol. 2006;19:187–189. [PubMed] [Google Scholar]
- 17.Fitzpatrick LA, Turner RT, Ritman ER. Endochondral bone formation in the heart: a possible mechanism of coronary calcification. Endocrinology. 2003;144:2214–2219. doi: 10.1210/en.2002-0170. [DOI] [PubMed] [Google Scholar]
- 18.Garcia GM, McCord GC, Kumar R. Hydroxyapatite crystal deposition disease. Semin Musculoskelet Radiol. 2003;7:187–193. doi: 10.1055/s-2003-43229. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh P, Taylor TK, Horsburgh BA. The composition and protein metabolism in the immature rabbit intervertebral disc. Cell Tissue Res. 1975;163:223–238. doi: 10.1007/BF00221729. [DOI] [PubMed] [Google Scholar]
- 20.Hansen HJ. A pathologic–anatomical interpretation of disc degeneration in dogs. Acta Orthop Scand. 1951;20:280–293. doi: 10.3109/17453675108991175. [DOI] [PubMed] [Google Scholar]
- 21.Hansen HJ. A pathologic–anatomical study on disc degeneration in dog, with special reference to the so-called enchondrosis intervertebralis. Acta Orthop Scand Suppl. 1952;11:1–117. doi: 10.3109/ort.1952.23.suppl-11.01. [DOI] [PubMed] [Google Scholar]
- 22.Hansen HJ, Ullberg S. Uptake of S35 in the intervertebral discs after injection of S35-sulphate: an autoradiographic study. Acta Orthop Scand. 1960;30:84–90. doi: 10.3109/17453676109149527. [DOI] [PubMed] [Google Scholar]
- 23.Hermann G, Sacher M, Lanzieri CF, Anderson PJ, Rabinowitz JG. Chondrosarcoma of the spine: an unusual radiographic presentation. Skeletal Radiol. 1985;14:178–183. doi: 10.1007/BF00355558. [DOI] [PubMed] [Google Scholar]
- 24.Lee RS, Kayser MV, Ali SY. Calcium phosphate microcrystal deposition in the human intervertebral disc. J Anat. 2006;208:13–19. doi: 10.1111/j.1469-7580.2006.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindgren I. Anatomical and roentgenologic studies of tuberculous infections in BCG-vaccinated and non-vaccinated subjects with biophysical investigations of calcified foci. Acta Radiol Suppl. 1961;209:1–101. doi: 10.3109/00016926109173811. [DOI] [PubMed] [Google Scholar]
- 26.Melrose J, Ghosh P, Taylor TK, Hall A, Osti OL, Vernon-Roberts B, Fraser RD. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res. 1992;10:665–676. doi: 10.1002/jor.1100100509. [DOI] [PubMed] [Google Scholar]
- 27.Melrose J, Ghosh P, Taylor TK. Proteoglycan heterogeneity in the normal adult ovine intervertebral disc. Matrix Biol. 1994;14:61–75. doi: 10.1016/0945-053X(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 28.Melrose J, Ghosh P, Taylor TKF, McAuley L. Variation in the composition of the ovine intervertebral disc with spinal level and in its constituent proteoglycans. Vet Comp Orthop Traumatol. 1994;7:70–76. [Google Scholar]
- 29.Melrose J, Ghosh P, Taylor TK, Latham J, Moore R. Topographical variation in the catabolism of aggrecan in an ovine annular lesion model of experimental disc degeneration. J Spinal Disord. 1997;10:55–67. doi: 10.1097/00002517-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Melrose J, Ghosh P, Taylor TK, Vernon-Roberts B, Latham J, Moore R. Elevated synthesis of biglycan and decorin in an ovine annular lesion model of experimental disc degeneration. Eur Spine J. 1997;6:376–384. doi: 10.1007/BF01834063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melrose J, Smith S, Ghosh P. Cartilage and osteoarthritis. Vol 2. Structure and in-vivo analysis. Chapter 3 Histological and immunohistological studies on cartilage. Totawa: Humana Press; 2004. [Google Scholar]
- 32.Melrose J, Smith SM, Fuller ES, Young AA, Roughley PJ, Dart A, Little CB. Biglycan and fibromodulin fragmentation correlates with temporal and spatial annular remodelling in experimentally injured ovine intervertebral discs. Eur Spine J. 2007;16(12):2193–2205. doi: 10.1007/s00586-007-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13:173–178. doi: 10.1097/00007632-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 35.Moe SM, Chen NX. Inflammation and vascular calcification. Blood Purif. 2005;23:64–71. doi: 10.1159/000082013. [DOI] [PubMed] [Google Scholar]
- 36.Moseley HF. The vascular supply of the rotator cuff. Surg Clin North Am. 1963;43:1521–1522. doi: 10.1016/s0039-6109(16)37138-9. [DOI] [PubMed] [Google Scholar]
- 37.Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration: an experimental study using an animal model. Spine. 1990;15:762–767. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- 38.Rees SG, Shellis RP, Embery G. Inhibition of hydroxyapatite crystal growth by bone proteoglycans and proteoglycan components. Biochem Biophys Res Commun. 2002;292:727–733. doi: 10.1006/bbrc.2002.6699. [DOI] [PubMed] [Google Scholar]
- 39.Reid JE, Meakin JR, Robins SP, Skakle JM, Hukins DW. Sheep lumbar intervertebral discs as models for human discs. Clin Biomech (Bristol, Avon) 2002;17:312–314. doi: 10.1016/S0268-0033(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 40.Rothman RH, Parke WW. The vascular anatomy of the rotator cuff. Clin Orthop Relat Res. 1965;41:176–186. doi: 10.1097/00003086-196500410-00020. [DOI] [PubMed] [Google Scholar]
- 41.Sanerkin NG, Watt I. Enchondromata with annular calcification in association with fibrous dysplasia. Br J Radiol. 1981;54:1027–1033. doi: 10.1259/0007-1285-54-648-1027. [DOI] [PubMed] [Google Scholar]
- 42.Souter WA, Taylor TK. Acid mucopolysaccharide metabolism in the rabbit intervertebral disc. J Bone Joint Surg Br. 1969;51:385–386. [PubMed] [Google Scholar]
- 43.Souter WA, Taylor TK. Sulphated acid mucopolysaccharide metabolism in the rabbit intervertebral disc. J Bone Joint Surg Br. 1970;52:371–384. [PubMed] [Google Scholar]
- 44.Takeuchi E, Sugamoto K, Nakase T, Miyamoto T, Kaneko M, Tomita T, Myoui A, Ochi T, Yoshikawa H. Localization and expression of osteopontin in the rotator cuff tendons in patients with calcifying tendinitis. Virchows Arch. 2001;438:612–617. doi: 10.1007/s004280000367. [DOI] [PubMed] [Google Scholar]
- 45.Taylor TK, Little K. Calcification in the intervertebral disk. Nature. 1963;199:612–613. doi: 10.1038/199612b0. [DOI] [PubMed] [Google Scholar]
- 46.Taylor TK, Little K. Prolapsed calcified thoracic intervertebral disc. J Pathol Bacteriol. 1964;88:153–157. doi: 10.1002/path.1700880120. [DOI] [PubMed] [Google Scholar]
- 47.Taylor TKF, Ghosh P, Bushell GR, Stephens RW. Scientific basis of the treatment of intervertebral disc disorders. London: Heinemann Mediacl Books; 1981. [Google Scholar]
- 48.Taylor TK, Melrose J, Burkhardt D, Ghosh P, Claes LE, Kettler A, Wilke HJ. Spinal biomechanics and aging are major determinants of the proteoglycan metabolism of intervertebral disc cells. Spine. 2000;25:3014–3020. doi: 10.1097/00007632-200012010-00008. [DOI] [PubMed] [Google Scholar]
- 49.Uhthoff HK. Calcifying tendinitis, an active cell-mediated calcification. Virchows Arch A Pathol Anat Histol. 1975;366:51–58. doi: 10.1007/BF00438677. [DOI] [PubMed] [Google Scholar]
- 50.Uhthoff HK, Sarkar K, Maynard JA (1976) Calcifying tendinitis: a new concept of its pathogenesis. Clin Orthop Relat Res (118):164–168 [PubMed]
- 51.Wang J, Zhou HY, Salih E, Xu L, Wunderlich L, Gu X, Hofstaetter JG, Torres M, Glimcher MJ. Site-specific in vivo calcification and osteogenesis stimulated by bone sialoprotein. Calcif Tissue Int. 2006;79:179–189. doi: 10.1007/s00223-006-0018-2. [DOI] [PubMed] [Google Scholar]
- 52.Weinberger A, Myers AR. Intervertebral disc calcification in adults: a review. Semin Arthritis Rheum. 1978;8:69–75. doi: 10.1016/0049-0172(78)90035-5. [DOI] [PubMed] [Google Scholar]
- 53.Wicks IP, Fleming A. Chondrosarcoma of the calcaneum and massive soft tissue calcification in a patient with hereditary and acquired connective tissue diseases. Ann Rheum Dis. 1987;46:346–348. doi: 10.1136/ard.46.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22:2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 55.Wilke HJ, Kettler A, Wenger KH, Claes LE. Anatomy of the sheep spine and its comparison to the human spine. Anat Rec. 1997;247:542–555. doi: 10.1002/(SICI)1097-0185(199704)247:4<542::AID-AR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 56.Williams R. Complete protrusion of a calcified nucleus pulposus in the thoracic spine: report of a case. J Bone Joint Surg Br. 1954;36-B:597–600. doi: 10.1302/0301-620X.36B4.597. [DOI] [PubMed] [Google Scholar]
- 57.Ziv V, Weiner S. Bone crystal sizes: a comparison of transmission electron microscopic and X-ray diffraction line width broadening techniques. Connect Tissue Res. 1994;30:165–175. doi: 10.3109/03008209409061969. [DOI] [PubMed] [Google Scholar]