Abstract
Here we investigated the biomechanical properties of spinal segments in patients with degenerative lumbar spondylolisthesis (DLS) using a novel intraoperative measurement system. The measurement system comprised spinous process holders, a motion generator, a load cell, an optical displacement transducer, and a computer. Cyclic displacement of the holders produced flexion-extension of the segment with all ligamentous structures intact. Stiffness, absorption energy (AE), and neutral zone (NZ) were determined from the load-deformation data. Forty-one patients with DLS (M/F = 15/26, mean age 68.6 years; Group D) were studied. Adjacent segments with normal discs in six patients (M/F = 3/3, mean age 35 years) were included as a control group (Group N). Flexion stiffness was significantly lower in Group D than in Group N. The NZ, however, was significantly greater in Group D than in Group N. Thus, compared to normal segments, spinal segments with DLS had a lower flexion stiffness and a higher NZ. NZs in Group D were, however, widely distributed compared to those in Group N that showed NZ <2 mm/N in all cases, suggesting that the segment with DLS is not always unstable and that the segments with NZ >2 mm/N can be considered as unstable.
Keywords: Intraoperative biomechanical analysis, Lumbar degenerative spondylolisthesis, Segmental instability
Introduction
Lumbar segmental instability is difficult to define, whether or not it involves olisthesis. Although radiographic evaluation of degenerative lumbar spines is extensively performed [3, 4, 7, 11, 16, 22, 29], its usefulness in the diagnosis of lumbar segmental instability remains controversial because the large range of normal motion significantly overlaps underlying pathologic conditions [4, 10]. Although biplanar, cineradiographic, and fluoroscopic measurements [13, 24, 28, 31] are more dynamic, a biomechanically-based conclusion about instability cannot be drawn, because the images do not provide information about the load-deformation behaviour.
Since 1997, we have been working to develop a new intraoperative measurement system to determine segmental properties with all ligamentous structures intact. In the preliminary basic and clinical studies, we confirmed that the measurement system safely and reliably provides multiple parameters based on continuous load-deformation data obtained during surgery [9].
The purpose of the present study was to investigate segmental biomechanical properties in patients with degenerative lumbar spondylolisthesis (DLS) using the new intraoperative measurement system.
Materials and methods
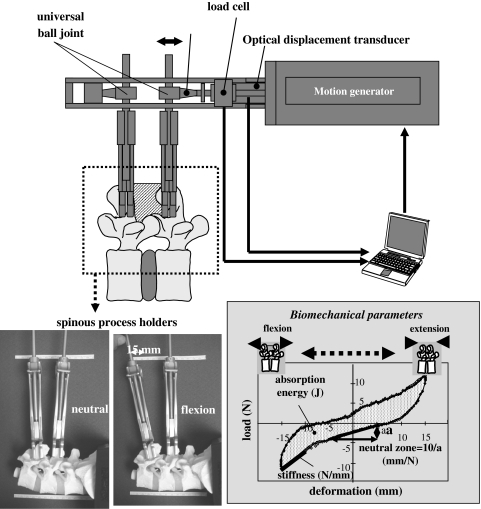
This intraoperative measurement system comprises spinous process holders (Gi-5, Mizuhoikakikai, Niigata, Japan), a motion generator (RC-RSW-L-50-S, IAI Corporation, Shimizu, Shizuoka), and a personal computer. The two holders firmly grip adjacent spinous processes. Cyclic displacement in a single direction at a speed of 2.0 mm/s is generated to the tips of the holders with a maximum displacement of 15.0 mm from the neutral position. Neutral position is defined as the position in which no load is recorded between the tips of the holders. Load at the tip of the caudal spinous process holders is measured with a load cell (LUR-A-200NSAI, Kyowadengyo Corporation, Chofu, Japan) and displacement is measured using an optical displacement transducer (LB-080, Keyence, Chofu, Japan). Real-time load-displacement data are obtained via a personal computer. The spinous process holder is connected to the motion generator through a multi-directional ball joint, producing flexion-extension of the segment (Fig. 1). The range of motion-induced by 15-mm translation of the spinous processes in the two directions is equivalent to approximately 9° of segmental flexion-extension. The induced motion has no adverse effects [9, 32].
Fig. 1.
The new intraoperative measurement system, comprising spinous process holders, a motion generator, an optical displacement transducer, and a personal computer
The patient was placed in the prone position on a Hall’s frame and the paraspinal muscles were detached from the spinous processes using standard procedures. Two spinous process holders were attached to the adjacent spinous processes. All ligamentous structures of the functional spinal unit, including the supra- and inter-spinous ligaments and facet joints, were preserved. The motion generator attached to the tips of the holders loaded the segment, producing five flexion-extension segmental motion cycles and real-time load-displacement data were obtained with a sampling rate of 5 Hz. Data from the third cycle were used for biomechanical analysis. We defined three motion parameters based on the load-displacement data, stiffness, neutral zone (NZ) [25], and absorption energy (AE). Stiffness (N/mm) was defined as the slope of the line fitting the load-displacement curve from −15 mm to −10 mm on flexion motion. NZ (mm/N) was defined as the reciprocal of the load necessary to displace the distance between the two tips of the holders from −5 mm (flexion) to 5 mm (extension) (Fig. 1). The least squares method was used for measuring stiffness and NZ. AE (J) was defined as the area of a hysteresis loop that was calculated using Simpson’s rule with 150 strips because sampling rate was 5 Hz for 30 s/1 cycle.
Forty-one patients (15 men and 26 women) with DLS (Group D) who presented neurogenic claudication were included in this study. The average age was 68.6 years old (range 47–80 years old). Ten patients had DLS in L3-4 and 31 had DLS in L4-5. None of the patients had a history of L5-S fusion or sacralized L5 vertebra. Biomechanical evaluation was performed at all levels using the original intraoperative measurement system. Decompression surgery in 15 patients, decompression with dynamic stabilization in 12 patients, and transforaminal interbody fusion in 14 were performed for the pathologic levels. Informed consent was obtained from all patients following the approval of the Committee of Medical Ethics of Niigata University (approval # 182, 2003).
Lateral X-rays were obtained under the following conditions; lines between the bilateral acromion processes and iliac crests were perpendicular to the X-ray film with a 2.5-m distance from the X-ray generator to the film, with 110 kV and 140 mA. Range of motion (ROM) was determined using Dupuis’ procedure [3]. Disc height was calculated as the mean value of the anterior and posterior disc height divided by the anteroposterior width of the upper vertebra. Magnetic resonance images were obtained in all patients with a 1.5 T magnetic resonance imager. Grading of disc degeneration was performed on T2-weighted midsagittal fast spin-echo images (repetition time 5,000 ms/echo time 130 ms) [30, 33]. The 41 discs with DLS were classified as grade III (inhomogeneous gray nucleus with unclear distinction of the nucleus and annulus): 5 discs; grade IV (inhomogeneous gray to black nucleus without distinction of the nucleus and annulus): 29 discs; and grade V (black nucleus with collapsed disc space): 7 discs. Regarding Modic changes [21], 5 discs showed type 1 and 4 discs type 2. For comparison, 6 segments classified as grade I and 2 segments classified as grade II in another series of patients (average age, 35 + 10.2 years, range 21–48 years, men/women = 3/3 cases, L3/4 = 3 cases, L4/5 = 3 cases) were used as a normal control group (Group N). These segments were adjacent to the operated symptomatic segments and verified to be asymptomatic by post-operative follow-up examination.
Shapiro–Wilks test elucidated that all the biomechanical data except AE in Group D were not normally distributed. Thus, the value of each biomechanical parameter was compared between Group N and D using a non-parametric Wilcoxon-signed ranks test. Linear regression analyses were performed to identify relationships among ROM, DH, and biomechanical parameters. JMP software package (ver.5.0.1a, SAS Institute, Cary, NC) was used for all statistical analyses. A P value of <0.05 was considered statistically significant.
Results
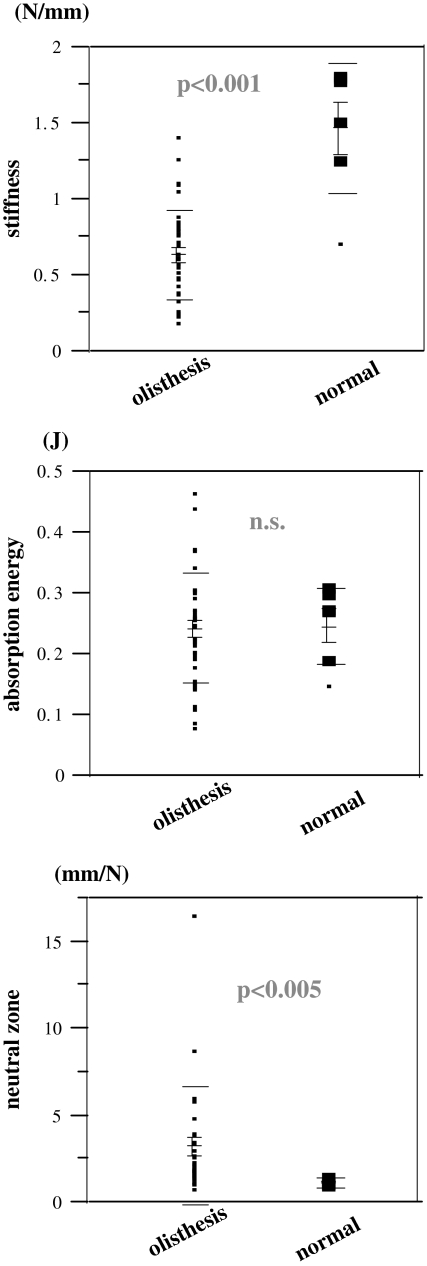
All the measurements were completed within 10 min without any complications related to the procedure. The spinous process holders were stable even after five loading cycles in all cases. The average stiffness value (average ± standard error) in Group D (0.63 ± 0.29 N/mm) was significantly lower than that in Group N (1.47 ± 0.43 N/mm; P < 0.001). There was no significant difference in the average AE value among Group D (0.24 ± 0.09 J) and Group N (0.25 ± 0.06 J), (P = 0.690). The NZ in Group D (3.25 + 3.43 mm/N) was significantly higher than that in Group N (1.12 + 0.32 mm/N; P < 0.005) (Fig. 2). AE negatively correlated with ROM (r2 = 0.240, P < 0.001) and flexion stiffness negatively correlated with disc height (r2 = 0.105, P < 0.05) (Table 1). 12.2% of the discs with olisthesis showed Modic type 1 change [21]. There was, however, no significant difference in any biomechanical parameter among the groups with different Modic change.
Fig. 2.
Comparison of biomechanical parameters between the segments with spondylolisthesis and those with normal discs. Olisthesis segments with spondylolisthesis, normal segments with normal discs
Table 1.
Relationships among biomechanical parameters and radiologic results
| Flexion stiffness (N/mm) | Extension stiffness (N/mm) | Absorption energy (J) | Neutral zone (mm/N) | |
|---|---|---|---|---|
| Range of motion | ||||
| r2 | 0.021 | 0.065 | 0.240 | 0.000 |
| P value | 0.336 | 0.087 | <0.001 | 0.988 |
| Disc heighta | ||||
| r2 | 0.105 | 0.021 | 0.003 | 0.000 |
| P value | <0.05 | 0.340 | 0.741 | 0.977 |
aDisc height was calculated as the mean value of anterior and posterior disc height divided by the anteroposterior width of upper vertebra
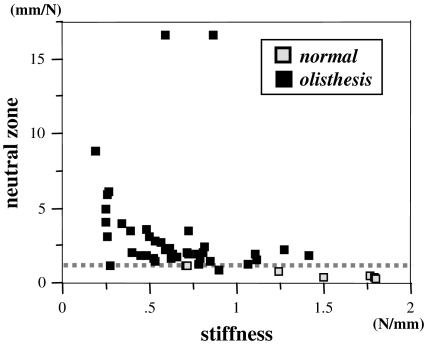
Regarding a relationship between stiffness and NZ, stiffness values are widely spread in both Group D and Group N. On the other hand, NZs of Group N are all <2 mm/N. In Group D, while NZ in most of the segments are >2 mm/N, some segments showed NZ ≤2 mm/N (Fig. 3).
Fig. 3.
A relationship between stiffness and neutral zone. Open box normal (Group N), closed box olisthesis (Group D). Dotted line shows NZ = 2 mm/N
Representative cases
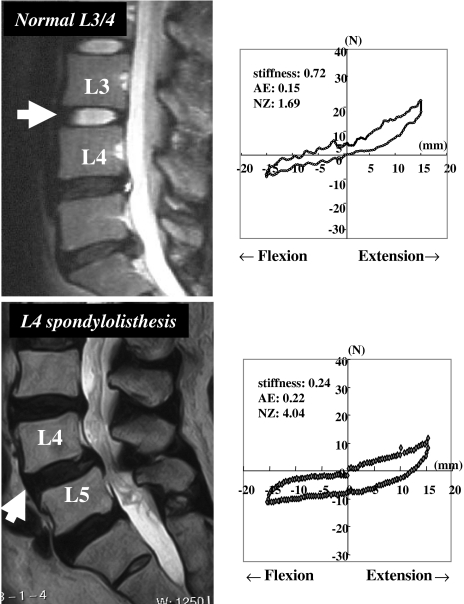
In one case of Group N, a 33-year-old woman, the measurements were: stiffness = 0.72 N/mm, AE = 0.15 J, and NZ = 1.69 mm/N (Fig. 4). In a representative case of Group D, a 73-year-old woman with L4 degenerative spondylolisthesis, the measurements were: stiffness = 0.24 N/mm, AE = 0.22 J, and NZ = 4.04 mm/N (Fig. 4). The NZ value in the case with degenerative spondylolisthesis was considerably higher than that in the case without disc degeneration. These typical cases had notably different hysteresis loops with a more flattened loop in Group D.
Fig. 4.
Biomechanical parameters and representative cases of normal segments (33-year-old woman) and of L4 degenerative spondylolisthesis (73-year-old female). Mid-sagittal view of T2-weighted MRIs (arrows represent the measured segment) and load-deformation data are illustrated. AE absorption energy, NZ neutral zone
Discussion
The segment with degenerative spondylolisthesis is generally considered “unstable” since the first report by Macnab [18]. Evidence to support this hypothesis, however, has not been presented. Moreover, the definition of clinical instability of the spine is yet to be clarified. One reason seems to be a gap between the facts elicited from ex vivo or animal studies [6, 8, 12, 20, 23, 25, 26] and clinical symptoms in patients with a suggestive segmental instability, mechanical low back pain, or motion-induced radiculopathy. Our approach is an attempt to bridge the gap between the basic biomechanical data and the clinical symptoms induced by instability.
Intraoperative measurements of a cervical or lumbar segment are occasionally performed to determine instability [1, 5, 14]. Common limitations of the previously used measurements include damage to the ligamentous or bone structures due to the fixation of pins, screws, or a spreader to the vertebrae and also the fact that data about the stiffness of only a single loading direction, flexion or extension can be obtained. From a biomechanical viewpoint, segmental properties of the spine cannot be determined by measuring stiffness alone. Measurements of multiple parameters, including the NZ, are necessary [27]. The present measurement system is the first clinically available method that can be used for measurement with all ligamentous structures intact, and provides multiple parameters based on continuous load-deformation data during surgery [9]. Among the biomechanical parameters, NZ is thought to be affected by degeneration, leading to painful motion [25]. An in vitro study of fresh human cadavers reported that the NZ is slightly increased with greater disc degeneration in lumbar flexion-extension motion [20]. Our measurement system demonstrated clear differences between the NZ of segments with DLS and the NZ of normal segments. Segments with DLS were characterised by lower flexion stiffness and a higher NZ compared to normal segments (Fig. 2). These results are compatible with Macnab’s hypothesis that osteoarthritis of the facet joints together with body-weight induces subluxation at these joints, and then the superior vertebra slips forward, producing spondylolisthesis [18].
According to Kirkaldy–Willis and Farfan’s concept, disc degeneration progresses from normal to dysfunctional, unstable, and restabilization phases [15]. On the other hand, segmental motion increases with an increasing severity of disc degeneration up to grade IV, but decreases when disc degeneration advances to grade V [33] in a cadaver study [6]. Most cases with disc degeneration in the present study were considered to be in the unstable to restabilization phases. The negative correlation between the range of motion and AE or between disc height and flexion stiffness (Table 1) can be explained by Kirkaldy–Willis’ concept [15] and the results of the cadaver study [6].
The criteria for spinal fusion as a treatment for DLS remain controversial [19]. In decision-making of fusion, clinical manifestations and the findings of imaging examinations (X-rays, MRI, CT, etc.) are obviously indispensable to determine a grade of instability [2–4, 7, 11, 14, 16, 17, 21, 22, 24, 28, 29, 31]. Spondylolisthesis is generally considered unstable [18], but the instability is thought to change continuously as a function of the progression of degeneration as proposed by Kirkaldy–Willis and Farfan [15]. If a patient has a certain grade of spondylolisthesis, the instability of the segment may be different from patient to patient. This is a point that makes surgeons distressed in determination of fusion. The final goal of the present research is to establish a reliable system to obtain the biomechanical data which can be referred for decision-making of treatment of choice. In the present study, while stiffness values are widely spread in both Group D and N, NZs of the segments with normal disc are all <2 mm/N. In Group D, even if NZs in most of the segments are >2 mm/N, some segments showed NZ <2 mm/N (Fig. 3). These results suggest that: (1) The segment with DLS is not always unstable. (2) Therefore, fusion to the segment is not at all times requisite. (3) A surgeon may be able to determine if fusion is necessary or not by referring NZ data obtained with our measurement system.
Conflict of interest statement
No grant or any other funding has been recieved.
Footnotes
A patent application for the intraoperative measurement system has been submitted.
References
- 1.Brown MD, Holms SH, Heiner AD. Intraoperative measurement of lumbar spine motion segment stiffness. Spine. 2002;27:954–958. doi: 10.1097/00007632-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 2.Chaput C, Padon D, Rush J, Lenehan E, Rahm M. The significance of increased fluid signal on magnetic resonance imaging in lumbar facets in relationship to degenerative spondylolisthesis. Spine. 2007;32:1883–1887. doi: 10.1097/BRS.0b013e318113271a. [DOI] [PubMed] [Google Scholar]
- 3.Dupuis PR, Yong-hing K, Cassidy JD, Kirkaldy-Willis WH. Radiographic diagnosis of degenerative lumbar spinal instability. Spine. 1985;10:262–276. doi: 10.1097/00007632-198504000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Dvoák J, Panjabi MM, Chang DG, Theiler R, Grob D. Functional radiographic diagnosis of degenerative lumbar spine: Flexion-extension and lateral bending. Spine. 1991;16:562–571. doi: 10.1097/00007632-199105000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebara S, Harada T, Hosono N. Intraoperative measurement of lumbar spinal instability. Spine. 1992;17:S44–S50. doi: 10.1097/00007632-199203001-00010. [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara A, Lim TH, An HS, Tanaka N, Jeon CH, Andersson GBJ, Haughton VM. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine. 2000;25:3036–3044. doi: 10.1097/00007632-200012010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Frymoyer JW, Selby D. Segmental instability: rationale for treatment. Spine. 1985;10:280–286. doi: 10.1097/00007632-198504000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Gertzbein SD, Seligman J, Holtby R, Chan KH, Kapasouri A, Tile M, Cruickshank B. Centrode patterns and segmental instability in degenerative disc disease. Spine. 1985;10:257–261. doi: 10.1097/00007632-198504000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa K, Kitahara K, Hara T, Takano K, Shimoda H, Homma T. Evaluation of lumbar segmental instability in degenerative diseases using a new intraoperative measurement system. J Neurosurg Spine. 2008;8:255–262. doi: 10.3171/SPI/2008/8/3/255. [DOI] [PubMed] [Google Scholar]
- 10.Hayes MA, Howard TC, Gruel CR, Kopta JA. Roentgenographic evaluation of lumbar spine flexion-extension in asymptomatic individuals. Spine. 1989;14:327–331. doi: 10.1097/00007632-198903000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Iguchi T, Kanemura A, Kasahara K, Kurihara A, Doita M, Yoshiya S. Age distribution of three radiologic factors for lumbar instability: probable aging process of the instability with disc degeneration. Spine. 2003;28:2628–2633. doi: 10.1097/01.BRS.0000097162.80495.66. [DOI] [PubMed] [Google Scholar]
- 12.Kaigle AM, Holm SH, Hansson TH. Experimental instability in the lumbar spine. Spine. 1995;20:421–430. doi: 10.1097/00007632-199502001-00004. [DOI] [PubMed] [Google Scholar]
- 13.Kanayama M, Abumi K, Kaneda K. Phase lag of the intersegmental motion in flexion-extension of the lumbar and lumbosacral spine: an in vivo study. Spine. 1996;21:1416–1422. doi: 10.1097/00007632-199606150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Kanayama M, Hashimoto T, Shigenobu K, Oha F, Ishida T, Yamane S. Intraoperative biomechanical assessment of lumbar spinal instability: validation of radiographic parameters indicating anterior column support in lumbar spinal fusion. Spine. 2003;28:2368–2372. doi: 10.1097/01.BRS.0000085357.24025.27. [DOI] [PubMed] [Google Scholar]
- 15.Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res. 1982;165:110–123. [PubMed] [Google Scholar]
- 16.Knutsson F. The instability associated with disc degeneration in the lumbar spine. Acta Radiol. 1944;25:593–609. doi: 10.3109/00016924409136488. [DOI] [Google Scholar]
- 17.Luk KD, Chow DH, Holmes A. Vertical instability in spondylolisthesis: a traction radiographic assessment technique and the principle of management. Spine. 2003;28:819–827. doi: 10.1097/00007632-200304150-00016. [DOI] [PubMed] [Google Scholar]
- 18.Macnab I. Spondylolisthesis with an intact neural arch—the so-called pseudo-spondylolisthesis. J Bone Joint Surg Br. 1950;32:325–333. doi: 10.1302/0301-620X.32B3.325. [DOI] [PubMed] [Google Scholar]
- 19.Martin CR, Gruszczynski AT, Braunsfurth HA, Fallatah SM, O’Neil J, Wai EK. The surgical management of degenerative lumbar spondylolisthesis. A systematic review. Spine. 2007;32:1791–1798. doi: 10.1097/BRS.0b013e3180bc219e. [DOI] [PubMed] [Google Scholar]
- 20.Mimura M, Panjabi MM, Oxland TR. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine. 1994;19:1371–1380. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 22.Morgan FP, King T. Primary instability of lumbar vertebrae as a cause of low back pain. J Bone Joint Surg Br. 1957;39:6–22. doi: 10.1302/0301-620X.39B1.6. [DOI] [PubMed] [Google Scholar]
- 23.Ogon M, Bender BR, Hooper DM, Spratt KF, Goel VK, Wilder DG, Pope MH. A dynamic approach to spinal instability. Part I: sensitization of intersegmental motion profiles to motion direction and load condition by instability. Spine. 1997;22:2841–2858. doi: 10.1097/00007632-199712150-00007. [DOI] [PubMed] [Google Scholar]
- 24.Okawa A, Shinomiya K, Komori H, Muneta T, Arai Y, Nakai O. Dynamic motion study of the whole lumbar spine by videofluoroscopy. Spine. 1998;23:1743–1749. doi: 10.1097/00007632-199808150-00007. [DOI] [PubMed] [Google Scholar]
- 25.Panjabi MM, Goel VK, Takata K. Physiologic strains in the lumbar spinal ligaments. Spine. 1982;7:192–203. doi: 10.1097/00007632-198205000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Panjabi MM, Krag M, Chung T. Effects of disc injury on mechanical behaviour of the human spine. Spine. 1984;9:707–713. doi: 10.1097/00007632-198410000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Panjabi MM. The stabilizing system of the spine. PartII. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5:390–397. doi: 10.1097/00002517-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Pearcy M, Portek I, Shepherd J. The effect of low back pain on lumbar spinal movements measured by three-dimensional x-ray analysis. Spine. 1985;10:150–153. doi: 10.1097/00007632-198503000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Pennal GF, Conn GS, McDonald G, Dale G, Garside H. Motion studies of the lumbar spine. A preliminary report. J Bone Joint Surg Br. 1972;54:442–452. [PubMed] [Google Scholar]
- 30.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 31.Stokes IA, Wilder DG, Frymoyer JW, Pope MH. Assessment of patients with low back pain by biplanar radiographic measurement of intervertebral motion. Spine. 1981;6:233–240. doi: 10.1097/00007632-198105000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Takano K, Hasegawa K, Kitahara K, Hara T, Sato S, Endo N. Lumbar segmental motion properties in vivo determined by a new intraoperative measurement system. Acta Med Biol (Niigata) 2006;54:1–8. [Google Scholar]
- 33.Thompson JP, Pearce RH, Schechter MT. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]