Abstract
Objective
This study used a longitudinal design to investigate the buffering role of resilience on worsening HbA1c and self-care behaviours in the face of rising diabetes-related distress.
Method
A total of 111 patients with diabetes completed surveys and had their glycosylated hemoglobin (HbA1c) assessed at baseline and 1-year follow-up. Resilience was defined by a factor score of self-esteem, self-efficacy, self-mastery, and optimism. Diabetes-related distress and self-care behaviours were also assessed.
Results
Baseline resilience, diabetes-related distress, and their interaction predicted physical health (HbA1c) at 1-year. Patients with low, moderate, and high resilience were identified. Those with low or moderate resilience levels showed a strong association between rising distress and worsening HbA1c across time (r=.57, .56, respectively). However, those with high resilience scores did not show the same associations (r=.08). Low resilience was also associated with fewer self-care behaviours when faced with increasing distress (r= −.55). These correlation coefficients remained significant after controlling for starting points.
Conclusion
In patients with diabetes, resilience resources predicted future HbA1c and buffered worsening HbA1c and self-care behaviours in the face of rising distress levels.
Healthcare providers benefit from the knowledge and understanding of associations between psychosocial and physiological variables in patients with chronic illness. These associations can further inform treatment, prevention, and intervention protocols through a biopsychosocial perspective. In diabetes patients, a holistic view of disease can aid the prevention of a variety of debilitating physical complications that can result from consistently poor self-care behaviors and high glycosylated hemoglobin levels.
One well-known correlate of poor glycemic control and self-care is diabetes-related psychological distress (Polonsky, Anderson, Lohrer, Welch, & Jacobson, 1995, 2001; Weinger & Jacobson, 2001). Reducing diabetes-related distress has been successful through intensive, educational, or cognitive behavioral interventions (see Welch, Weinger, Anderson, & Polonsky, 2003, for review). Less is known about the personal factors that encourage successfully coping with rising levels of diabetes-related distress. Rising distress levels may accompany a diabetes patient at any stage of disease. For example, Shaban, Fosbury, Kerr, and Cavan (2006) report significant prevalence rates for moderate to severe depression and anxiety in type 1 patients living with diabetes for many years. Due to the extensive, daily nature of diabetes self-care, feeling “burned out” or overwhelmed is a risk for patients at any stage of their disease. However, psychosocial variables such as self-esteem, self-efficacy, self-mastery and optimism have been linked with less distress and lower glycosylated hemoglobin levels and may be good candidates for understanding the buffering the effects of distress on diabetes-related outcomes (Fournier, De Ridder, & Bensing, 2002; Johnston-Brooks, Lewis, & Garg, 2002; Rose, Fliege, Hildenbrandt, Schirop, & Klapp, 2002; Sousa, Zauszniewski, Musil, Price Lea, & Davis, 2005).
We chose to investigate the extent to which psychosocial resources protect, or buffer, individuals from the negative physical or behavioural effects of rising diabetes-related emotional distress. A buffering effect occurs when a personal or situational variable protects people from the potentially negative effects of stress (Cohen & Wills, 1985). Several variables have been investigated via the stress-buffering model in persons without diabetes, including hardiness, social support, finances, self-enhancement, and religiosity (Cohen & Wills, 1985; Kobasa, Maddi, & Kahn, 1982; Kornblith et al., 2001; Smith, Langa, Kabeto, & Ubel, 2005; Taylor, Lerner, Sherman, Sage, & McDowell, 2003; Wills, Yaeger, & Sandy, 2003). In diabetes patients, effective coping has been shown to buffer poor glycemic control in the face of chronic psychosocial stress (Peyrot & McMurry, 1992). Given the strong association of generalized stress with diabetes-related distress in the literature (e.g., Polonsky et al., 1995; Spencer et al., 2006) coupled with our primary interest in diabetes-specific outcomes (glycemic control and diabetes self-care behaviours), diabetes-related emotional distress was used as the theoretical “stress” variable in the buffering hypothesis for this study.
Positive psychosocial factors such as the ones mentioned above can be used to identify a diabetes patient who demonstrates “resilience” in the face of increasing diabetes-related distress. Resilience is a psychosocial construct referring to an individual’s capacity to maintain psychological and physical well-being in the face of adversity. Although much has been written about resilience in children and adolescents (e.g., Garmezy, Masten, & Tellegen, 1984; Masten et al., 1988; Werner & Smith, 1982), less is known about resilience in populations facing chronic illness or stress. The study of resilience in diabetes is virtually nonexistent, despite solid evidence that positive psychosocial resources influence glycemic control and quality of life (Rose et al., 2002; Sousa et al., 2005; Whittemore, Melkus, & Grey, 2005). In other areas of chronic illness, Becker and Newson (2005) found determination, perseverance, and tenacity were common among a qualitative study of elderly chronically ill African Americans, and psychological adjustment was found to be prevalent in families with HIV and cancer (Hodgkinson et al., 2006; New, Lee, & Elliott, 2006). Vedhara and Nott (1996) reported resilience predicted emotional distress in homosexual men with HIV. However, little more is known about the protective factors involved in resilience for many chronically ill populations.
One reason for the lack of research, perhaps, is that although studied in various contexts for many years, there is no universal agreement on what constitutes “resilience.” In fact, distinguishing factors that define resilience and ones that promote or reduce resilience can be difficult (Kinard, 1998). Theoretically, making the distinction between personal, definitional attributes of resilience and resilient correlates or outcomes is rarely practiced, yet understanding how these fundamentally different components change and affect each other over time may lead to advancement in the understanding of resilience.
Defining resilience solely in terms of salutary medical and psychosocial outcomes in the face of stress, while commonly practiced, provides little theoretical and empirical information about the psychosocial processes that moderate and mediate resistance to stressors. For example, although many use Kobasa, Maddi, and Kahn’s hardiness construct (1979) when referring to “resilience” (e.g., Baron, Eisman, Scuello, Veyzer, & Lieberman, 1996), in doing so, the distinction between predictor and outcome is often blurred. In Kobasa et al.’s study of business executives (1979), hardiness was operationalized as an outcome in terms of high life stress and low illness. However, the executives’ sense of commitment, challenge, and control, the three determinants of hardiness, were also reported as the factors that mediated positive adaptation in the face of stress. Similarly, in Baron et al.’s study on children of Holocaust victims (1996), the authors cite literature describing children of survivors as being surprisingly resilient (i.e., “no pattern of maladjustment or psychopathology,” p.514), while at the same time investigate stress-resilience (measured by hardiness) as a basic coping mechanism in this population. When the same term is used to refer to both an outcome and the processes that contribute to that outcome, definitional ambiguity can result.
Another potential reason for the reluctance to explore the construct of resilience in chronic illness may be that previous research has typically focused on only one or few variables thought to be protective (Rutter, 2000). This can lead to a lack of uniformity when using the term “resilience.” One possible solution is to attend to whether these commonly studied individual variables may actually be measures of the same construct (Judge, Erez, Bono, & Thoresen, 2002; Ozer, 1999). In a meta-analysis of popular traits such as self-esteem, neuroticism, locus of control, and generalized self-efficacy, Judge et al. (2002) reported that a single factor explained these relationships and each individual measure accounted for only a small percentage of unique variance. When applied to studies of resilience, closely related psychosocial resources have indeed been usefully analyzed and reported as a single factor (Hull, Lehn, & Tedlie, 1991; Major, Richards, Cooper, Cozzarelli, & Zubek, 1998; Vedhara & Nott, 1996).
Psychosocial resources such as self-esteem, optimism, self-efficacy and self-mastery are commonly used in other studies involving resilience and can avoid problems of circularity (Cederblad, Dahlin, Hagnell, & Hansson, 1994; Cicchetti, Rogosch, Lynch, & Holt, 1993; Rutter, 1985; Vedhara & Nott, 1996; Wagnild & Young, 1993). Further, they have been shown to be associated with mental and physical health (Fournier et al., 2002; Johnston-Brooks et al., 2002; Rose et al., 2002; Sousa et al., 2005). Therefore, optimism, self-esteem, self-efficacy and self-mastery were proposed as resilience resources that may be protective to those facing the stressors associated with living with diabetes.
The goal of our study was to explore the buffering effect of resilience on glycemia and self-care behaviors. We expected that resilience would differentiate levels of future glycemic control and self-care behaviours and that those with higher levels of resilience would be most protected from the adverse effects of increases in diabetes-related distress.
Method
Participants
Patients between the ages of 18 and 75 having diabetes were recruited either by mail or during a medical appointment at the Joslin Diabetes Center (JDC) in Boston, MA. The JDC Committee on Human Studies approved the protocol and voluntary written informed consent was obtained from each participant before the study. One hundred forty-five patients completed surveys and had their glycosylated hemoglobin percentage (HbA1c) assessed at baseline (BL) and 1-year later (1yr). Of the initial 145 patients, 34 were lost to follow-up at 1yr, having never returned for a clinic appointment within the study’s time frame. An additional three to five participants, depending on the survey instrument, failed to complete enough items on their surveys for scoring and were also dropped in subsequent analyses. Because the attrition rate was considerable, an attrition analysis comparing demographic and key variables between the attriters and completers was conducted, and the results are presented below.
Measures
Optimism was measured by the Life Orientation Test (LOT), an eight-item self-report measure (along with four filler items) assessing generalized expectancies for positive versus negative outcomes, scored on a 5-point Likert scale (Scheier & Carver, 1985). Cronbach’s alpha for the scale was .71, and test-retest reliability over the one-year period was .70. Adequate levels of convergent and discriminant validity have been reported (Scheier, Carver, & Bridges, 1994).
Self-esteem was measured using the Rosenberg Self-Esteem Scale (Rosenberg, 1965), a widely used scale assessing global self-esteem and feelings of personal self-worth. It includes 10 general statements assessing the degree to which respondents are satisfied with their lives and feel good about themselves. In our sample, Cronbach’s alpha was .91 and the 1yr test-retest reliability was .72.
Self-efficacy was measured with the Confidence in Diabetes Self-care scale (CIDS, Van Der Ven et al., 2003), a questionnaire assessing diabetes-specific self-efficacy with type 1 diabetes. A modified version was administered to type 2 patients. Each item is preceded by, “I believe I can…” with the strength of this belief rated on a 5-point Likert scale. Internal consistency was high (Cronbach’s alpha = .90) and 1yr test-retest reliability was .60. Examination of the factor structure indicated that the CIDS is best considered and used as a unidimensional scale (Van Der Ven et al., 2003).
Self-mastery was measured by Pearlin and Schooler’s (1978) Self-Mastery Scale (SMS). This 7-item questionnaire measures the extent to which a person generally feels as though he or she has personal mastery over life outcomes. Internal consistency (Cronbach’s alpha = .81) and 1yr test-retest reliability (r=.63) were adequate. Its psychometric properties have been well established (Pearlin & Schooler, 1978).
Diabetes-related emotional distress was our assessment of psychological adjustment, measured by the Problem Areas in Diabetes Scale (PAID), a 20-item measure assessing a broad range of feelings related to living with diabetes and its treatment, including guilt, anger, frustration, depressed mood, worry, and fear. Patients were asked to respond to a five point Likert scale ranging from “not a problem” to “serious problem” indicating the extent to which the statement was currently a problem to them. This scale has high internal consistency and validity (Polonsky et al., 1995; Welch, Jacobson, & Polonsky, 1997; Welch et al., 2003). Similar reliability was found in the current study, Cronbach’s alpha = 0.94. One-year test-retest reliability was high (r=.82). The responsiveness of the PAID (sensitivity to change over time) is also well established (Welch et al., 2003).
Self-care behaviours were assessed with the Self-Care Inventory-Revised (SCI-R) and served as our primary behavioural outcome (Greco et al., 1990; Polonsky et al., 1995; Weinger, Butler, Welch, & LaGreca, 2005). The 10 items most related to metabolic control were selected from the 15-item measure, assessing patients’ perceptions of self-care behaviours. Items were scored on a 5-point Likert scale from 1 (“never do it”) to 5 (“always do it without fail”). High scores indicate more frequent diabetes self-care behaviours. The SCI-R for our sample showed high internal consistency (α = .79), test-retest reliability (r=.76) and has been used in studies assessing self-care and its association with diabetes-related distress in type 1 and type 2 diabetes (Polonsky et al., 1995; Weinger, Butler et al., 2005; Wysocki, 1996).
Glycemic Control was our assessment of physical health in diabetes patients, measured by glycosylated hemoglobin (HbA1c). HbA1c is the definitive measure of glycemic control over the prior 2 to 3 months and is used in all major clinical trials of diabetes (Diabetes Control and Complications Trial Research Group, 1993). Higher HbA1c indicates poorer control. The JDC laboratory in Boston, MA analyzed all samples using high-performance liquid chromatography ion capture (Tosch Medics, San Francisco, CA; reference range: 4.0–6.0%). These methods conform to the Diabetes Control and Complications Trial Research Group (1993) standardized methods.
Statistical Analyses
Principal components analysis and structural equation modeling were used to derive the resilience factor. Paired t-tests and test-retest reliability were calculated for all variables to assess stability across the 1yr period. Comparisons between attriters and completers and differences in sex and type of diabetes were analyzed by use of independent t-tests, χ2 significance tests, and comparisons of correlation coefficients.
The main effects of the resilience factor, diabetes-related distress, and their interaction at baseline were tested for their prediction of 1yr HbA1c levels. Baseline HbA1c was entered in the first step, along with any other significant covariates of age, sex, education, type and duration of diabetes. The same procedures were used for the prediction of 1yr self-care. In post-hoc analyses, we used the upper, moderate, and lower tertiles of the resilience factor score distribution to designate low, moderate, and high groups. Repeated measures analyses of variances were used to estimate the effects of time, resilience group, and time by group interactions on HbA1c, PAID, and self-care scores.
Change scores were calculated for the key variables (1yr minus BL) in order to plot the effect of change in one variable (i.e., PAID) on change in another (i.e., HbA1c) for each individual. Pearson product-moment correlation coefficients were calculated to assess the association of change scores within each resilience group, and differences in these correlations were analyzed. Homogeneity of slopes tests explored potential differences in the slopes of the groups (Jackson et al., 2002; Pedhauzur, 1997). This test was run via factorial analysis of covariance, entering all covariates (including the baseline variable of interest) in conjunction with the interaction of resilience group status by the variable of interest. The interaction term was investigated for its significance and the null hypothesis of the homogeneity of slopes was rejected if the p-value was <.05. Change scores were also investigated using partial correlations coefficients controlling for the BL variable.
Results
Resilience Factor
Resilience resources were defined by a “Resilience Factor” (RF), derived from personal resources commonly associated with, or used to define, resilience (Cederblad et al., 1994; Cicchetti et al., 1993; Rutter, 1985; Vedhara & Nott, 1996; Wagnild & Young, 1993). These variables included optimism, self-esteem, self-efficacy and self-mastery. Development of the RF was two-fold.
First, total scores on the four scales were entered into a principal components analysis. A factor score was calculated for each participant by saving the scores as variables in the data reduction method using SPSS version 14.0 (SPSS, 2006). At baseline, 61.5% of the variance was explained by a single, unrotated component, which we designated as the “Resilience Factor” (RF). At the 1-year follow-up, 60.0% was explained by one component, demonstrating a relatively stable factor structure across time. Intercorrelation coefficients among scales are reported in Table 1 and 1yr test-retest reliability was high (r=.81). Table 1 also shows the loadings of each scale on the RF for both time points.
Table 1.
Intercorrelation Coefficients and Factor Loadings of Variables Comprising the Resilience Factor at BL and 1yr: Self-efficacy (CIDS), Self-esteem (SE), Self-mastery (SMS), and Optimism (LOT)
| Factor Loading | ||||||
|---|---|---|---|---|---|---|
| CIDS | SE | SMS | LOT | BL | 1yr | |
| CIDS | -- | .39 | .42 | .28 | .62 | .56 |
| SE | .30 | -- | .57 | .60 | .83 | .80 |
| SMS | .35 | .53 | -- | .61 | .85 | .86 |
| LOT | .27 | .58 | .69 | -- | .82 | .85 |
Note. Correlation coefficients at Baseline (BL) are non-italicized (upper diagonal) and correlation coefficients at 1-year follow-up (1yr) are italicized (lower diagonal). All correlation coefficients are significant (p<.01).
In addition, structural equation modeling was used to test the model fit of self-esteem, self-efficacy, self-mastery, and optimism as the components of the resilience factor. Resilience was entered as a single-factor latent variable and the four individual baseline measures were entered as observed variables. Two participant’s data were dropped from these analyses because they were missing data on one of the measures. The model was a reasonable fit with the data. The χ2 (2) = 5.42, p = NS. The fit indices were also acceptable (GFI = .98, Tucker-Lewis index = .94). The regression weights for the observed variables were as follows: self-efficacy = .38 (SE = .07), optimism = .92 (SE = .11), self-mastery = .97 (SE = .12), and self-esteem = 1.0, as one of the variables was constrained in order to obtain a model fit.
Demographic Characteristics
Demographic characteristics of the sample at baseline are presented in Table 2. There were no differences in demographic variables by sex, except that males tended to be older (t=2.09, p<.05). Patients with type 1 diabetes differed from those with type 2 diabetes in expected directions for the demographic variables: age (patients with type 2 older: t= −5.93, p<.001), duration of diabetes (patients with type 1 longer duration: t=5.40, p<.001), and HbA1c (patients with type 1 higher: t=2.25, p<.05). However, all resilience attributes and associations between key variables for type 1 and type 2 patients were similar; therefore, all patients were considered together.
Table 2.
Means (M) or Percentages, Standard Deviations (SD), and Ranges of Demographic Variables at Baseline; n=145
| Variable | % or M | SD | Range |
|---|---|---|---|
| Age (yrs) | 49.4 | 15.1 | 18–77 |
| Education (yrs) | 15.3 | 2.9 | 8–20 |
| Baseline HbA1c (%) | 7.8 | 1.4 | 4.6–13.2 |
| Duration of DM (yrs) | 18.9 | 13.2 | 1–53 |
| Sex (% female) | 57 | ||
| Ethnicity (% Caucasian) | 88 | ||
| Type of DM (% Type 1) | 63 |
Attrition Analysis
Analyses were conducted to determine whether there were any differences between those who dropped out before completion of the study and those who completed both assessments. Independent t-tests, chi-squared significance tests, and comparisons of correlation coefficients revealed no differences between completers and attriters in any of the demographic characteristics or in any psychosocial, behavioural, or physiological variables (resilience, diabetes-related distress, self-care, or HbA1c) at T1.
Descriptive Statistics
Means and standard deviations for psychosocial and physiological variables are presented in Table 3. Paired t-tests were used to investigate stability in averages across time. Means for resilience variables and HbA1c did not change, while mean PAID scores dropped over time.
Table 3.
Means (M), Standard Deviations (SD), and Paired t-test of Physiological and Psychosocial Variables at Each Time Point
| Variable | Baseline | 1-year follow-up | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | n | t | |
| HbA1c | 7.83 | 1.40 | 7.72 | 1.36 | 111 | 1.65 |
| Resilience Factor | ||||||
| Self-esteem | 75.78 | 19.08 | 78.74 | 17.91 | 107 | −1.54 |
| Self-efficacy | 82.30 | 11.64 | 83.56 | 10.53 | 108 | −.25 |
| Optimism | 62.53 | 17.29 | 64.04 | 17.16 | 108 | −.26 |
| Self-mastery | 66.27 | 17.90 | 64.04 | 17.16 | 108 | 1.65 |
| Self-care | 37.33 | 6.27 | 37.43 | 7.64 | 108 | 1.17 |
| PAID | 32.80 | 20.82 | 26.04 | 20.09 | 106 | 3.61*** |
Note. PAID=Problem Areas in Diabetes Scale.
p<.001.
Testing the Buffering Hypothesis
The interaction effect between resilience and diabetes-related distress was examined using multiple regression analysis, with 1yr HbA1c as the dependent variable and baseline HbA1c, resilience factor, and diabetes-related distress entered stepwise into the model. Table 4 summarizes the effect of the resilience factor, diabetes-related distress, and their interaction on 1yr HbA1c. Covariates of age, sex, type of diabetes, education, and duration of diabetes were not significant, and not included in the final models. In predictions of 1yr HbA1c, there were significant main effects of BL HbA1c, resilience factor, and diabetes-related distress such that higher HbA1c was related to lower resilience and higher diabetes-related distress (Table 4). The interaction between resilience and diabetes-related distress was also related to 1yr HbA1c after accounting for BL HbA1c, resilience, and distress (β= .52, p<.001). This indicated that the interaction of higher distress with lower resilience associated with higher HbA1c levels. Self-care behaviours at 1yr were also investigated as a dependent variable using the same design. Future self-care behaviours were not predicted by the resilience factor, diabetes-related distress, or their interaction (Table 4).
Table 4.
Regressions of 1-Year HbA1c and Self-care Scores on the Resilience Factor (RF), Diabetes-related Distress (PAID), and their Interaction Effects at Baseline (BL)
| Step | Variable | 1-year HbA1c | 1-year Self-care | |||
|---|---|---|---|---|---|---|
| Unst. β | Stand. β | Variable | Unst. β | Stand. β | ||
| 1a | BL HbA1c | .67 | .69*** | BL Self-care | .88 | .80*** |
| 2 | BL RF | −.52 | −.39** | BL RF | .41 | .06 |
| 3 | BL PAID | .02 | .24** | BL PAID | .04 | .13 |
| 4 | RF × PAID | .01 | .52*** | RF × PAID | −.01 | −.08 |
Note. 1yr HbA1c: F (4,104) = 38.09, p<.001; 1yr self-care: F (4,100) = 36.05, p<.001.
Covariates of age, sex, education, duration and type of diabetes were not significant and not included in the final models.
p<.01,
p<.001.
Comparisons of Low, Moderate, and High Resilience Groups
Post-hoc analyses were conducted by identifying those with low, moderate, and high resilience resources as defined by the tertiles of the resilience factor (RF). Of the 111 participants completing both time points, 34 participants were labeled as having “low resilience” (LO; range of RF = −2.95 to −0.29), 37 participants were labeled as having “moderate resilience” (MOD; range of RF = −0.23 to 0.51), and 40 were labeled as having “high resilience” (HI; range of RF = 0.56 to 2.18).
First, repeated measures analyses of variances were used to examine the effects of time and resilience group status on HbA1c, distress, and self-care scores. Means and F-statistics are presented in Table 5. These analyses revealed a significant within-subjects main effect, indicating that diabetes-related distress decreased across time. Resilience group differences were found for diabetes-related distress (HI group lowest) and self-care scores (HI group highest) across time. A trend was found for the interaction effect of time by group status on HbA1c, such that the HI and MOD resilience groups improved their HbA1c more than the LO resilience group (p=.08).
Table 5.
Repeated Measures Analysis of Variance (ANOVA) for HbA1c, Diabetes-related Distress (PAID), and Self-care
| Resilience Group | M ± SD | ANOVA | ||
|---|---|---|---|---|
| BL | 1yr | F (df) | ||
| HbA1c | LO | 8.15 ± 1.46 | 8.19 ± 1.52 | Time: (1,108) = 2.35 |
| MOD | 7.50 ± 1.18 | 7.48 ± 1.32 | Group: (2,108) = 2.56a | |
| HI | 7.99 ± 1.51 | 7.53 ± 1.19 | Interaction: (2,108) = 2.60a | |
| PAID | LO | 52.44 ± 19.83 | 43.04 ± 20.41 | Time: (1,103) = 13.50*** |
| MOD | 28.20 ± 14.90 | 25.26 ± 17.45 | Group: (2,103) = 36.25*** | |
| HI | 18.06 ± 13.31 | 12.57 ± 9.06 | Interaction: (2,103) = .61 | |
| Self-care | LO | 35.68 ± 6.32 | 37.04 ± 6.38 | Time: (1,104) = 1.00 |
| MOD | 39.05 ± 4.93 | 38.39 ± 7.04 | Group: (2,104) = 2.81b | |
| HI | 38.89 ± 7.02 | 38.03 ± 7.30 | Interaction: (2,104) = 1.94 | |
p=.08,
p=.07
p<.001
Second, we hypothesized that those with highest levels of resilience would be most protected from the adverse effects of increased distress. In order to test this hypothesis, we computed the correlation between change in distress and change in HbA1c. Any participant with missing data or with a change score outside of three standard deviations was considered an outlier and not considered in the analyses. This resulted in the exclusion of 9 HI, 3 MOD, and 6 LO participants. The correlation coefficients for change in distress with change in HbA1c were .57 and .56 for the LO and MOD resilience groups respectively (both p’s <.01). The correlation coefficient for the HI resilience group approached zero (r= .08, p=NS), and it differed from those for the LO and MOD groups (z’s = 2.07, 2.11 respectively, both p’s <.05).
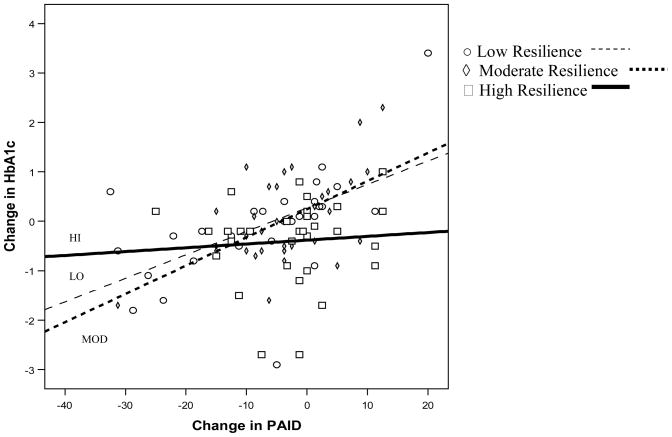
To represent the relation between change in distress and change in HbA1c visually, we plotted change scores for each participant in the LO, MOD, and HI resilience groups in order to understand trends on an individual level. Trend lines were fit separately for the three resilience groups. Figure 1 depicts the LO, MOD, and HI groups on change in diabetes-related distress and change in HbA1c. The slopes of the groups were different from each other as examined by testing for homogeneity of the group slopes (F(2,88) = 9.17, p<.001) (Jackson et al., 2002; Pedhauzur, 1997).
Figure 1.
Change in Diabetes-related Distress (PAID) and Change in HbA1c for Low (LO), Moderate (MOD), and High (HI) Resilience Groups
Although there was not a statistically significant interaction effect for the resilience factor and diabetes-related distress in predicting future self-care behaviours (Table 4), we explored the buffering effect of resilience on the deterioration of self-care behaviours in the face of increasing distress. Again, participants with missing data points or outlier data were excluded (total excluded: n=6 for HI group, n=4 for MOD group, n=6 for LO group). Results revealed that worsening diabetes-related distress scores were associated with worsening self-care scores more for the LO group (r= −.55, p<.01) than for the MOD or HI groups (MOD: r=−.13, HI: r= −.25; p’s=NS). This pattern of relations is consistent with a buffering effect on the relation between distress and self-care (differences in correlation coefficients for LO v. HI: z=−1.63, p=.05; LO v. MOD: z=−1.77, p<.05). However, the test for slope heterogeneity was not significant (F(2,89) = .27, p=NS; plot not shown).
To supplement these analyses and to ensure baseline data were not driving the correlations, we computed partial correlation coefficients, controlling for the baseline variable of interest. As shown in Table 6, the correlation coefficients were stronger for the LO and MOD groups than the HI group in both sets of associations. In fact, for both associations, the HI resilience group showed no significant associations and the associations of the HI group were statistically different from the LO and MOD groups (Table 6).
Table 6.
Partial Correlation Coefficients for Low (LO), Moderate (MOD), and High (HI) Resilience Groups
| Resilience Groups | z-statistic for: | |||||
|---|---|---|---|---|---|---|
| Association | Controlling for: | LO | MOD | HI | HI vs. LO | HI v. MOD |
| Δ PAID – Δ HbA1c | BL HbA1c | .50** | .62*** | −.24 | 2.76** | 3.60*** |
| Δ PAID – Δ Self-care | BL Self-care | −.49** | −.42* | −.09 | −1.67* | −1.42a |
Note. PAID = Problem Areas in Diabetes Scale; BL = Baseline
p=.08
p<.05,
p<.01,
p<.001
Discussion
Among patients with diabetes who experience worsening levels of diabetes-related distress, some are more resistant to deteriorating behavioural and physical effects than others. This study investigated resilience resources as a potential explanatory factor for these individual differences.
Although resilience is not commonly studied in patients with diabetes, a review of the resilience literature points to a few key variables that are commonly associated with, or used to define, stress-resilience. These include self-esteem (Fergusson & Lynskey, 1996; Parker, Cowen, Work, & Wyman, 1990; Werner & Smith, 1982), self-efficacy (Rutter, 1987; Sinclair & Wallston, 2004), optimism (Connor & Davidson, 2003; Segerstrom, Taylor, Kemeny, & Fahey, 1998; Taylor & Seeman, 1999), and self-mastery (Cederblad et al., 1994). These variables have consistently been associated with better health and reduced distress. Self-mastery has even been linked to lower mortality rates from all causes, including cardiovascular disease, and cancer (Surtees, Wainwright, Luben, Khaw, & Day, 2006). Individually, these positive psychosocial factors have shown promising results in predicting both mental and physical health in patients with diabetes (Fournier et al., 2002; Johnston-Brooks et al., 2002; Rose et al., 2002; Sousa et al., 2005). Thus, this study used these variables in a composite resilience factor to investigate how personal resources affect health and self-care in a diabetes sample.
The buffering model (Cohen & Wills, 1985) was used to investigate the potential for resilience to protect against negative effects of diabetes-related distress. Evidence for the buffering effect of resilience was first shown by the interaction effect of resilience and distress on the prediction of future HbA1c. This effect suggests that when faced with increasing distress, individuals with higher resilience are less likely than those with fewer resilience resources to have worsening HbA1c levels one year later.
When using tertile cutpoints of the resilience factor, strong associations were found for increases in diabetes-related distress with increases in glycemia in those with low and moderate level resilience scores; however, this was not found in those with high resilience. In fact, the slopes for the groups were statistically different. Though correlational in nature, the steeper slope for those in the LO and MOD resilience groups may indicate that an increase in diabetes-related distress affects HbA1c adversely in those with lower resilience, while the HI resilience group may be more resistant to this change.
Moderate buffering effects were also found for associations involving self-care. In these analyses, having low resilience resulted in fewer self-care behaviours when faced with rising distress. Partial correlation coefficients indicated that increases in PAID scores were related to changes in self-care behaviors in all but the high resilience group. Taken together, these results support previous findings that psychosocial resources affect self-care (Johnston-Brooks et al., 2002; Sousa et al., 2005; Syrjala, Ylostalo, Niskanen, & Knuuttila, 2004) while expanding on these results through application of buffering model.
This study was subject to limitations. The follow-up period was limited to approximately one year for all patients. In this time frame, 23% of the sample was lost to follow-up. Although attrition analyses comparing the completers to the attriters on means and percentages at baseline were not different, results still may have been impacted from this attrition rate. Moreover, previous work has shown that patients who fail to attend scheduled medical appointments may experience negative effects on their diabetes management (Griffin, 1998). They also have lower self-esteem, optimism, self-care, and higher diabetes-related distress (Weinger, McMurrich, Yi, Lin, & Rodriguez, 2005). Loss of these participants who did not return for a follow-up appointment within approximately one year may have resulted in a better-functioning sample than is typical in the diabetes population, and may provide some explanation for why mean levels of diabetes-related distress decreased and HbA1c was relatively stable in this observational study. Non-attendance may have suggested increases in diabetes-related distress over time and may have resulted in more patients with low resilience resources, and even greater differences between the low and high resilience groups versus our sample that was in consistent treatment over the course of the year.
Because the patients in our sample were varied in their years of diabetes duration (range 1–53 years) and it may be the case that those living with diabetes for a longer amount of time may have already adaptively or maladaptively adjusted to the disease, we cannot interpret these results as indicative of psychological adjustment to the illness itself. We can, however, be more confident that resilience buffers psychological adjustment over the course of living with the disease. This may be particularly important as recent data shows increased prevalence of clinically relevant anxiety and depression in patients with Type 1 diabetes (Shaban et al., 2006). In fact, this study by Shaban and colleagues (2006) reported mean duration of diabetes as 17.2 years (SD=12.0) which is comparable to our study sample.
Acknowledging these limitations, we feel that our results demonstrate that positive psychosocial resources can buffer the effects of rising distress levels on self-care behaviours and glycemic control in a diabetes population. Thus, assessment of resilience resources may be useful in cognitive behavioural interventions targeting coping with stress, improving self-care behaviours, or maintaining proper glycemic control. Future research of resilience in diabetes patients promises to advance our understanding of the psychological and biological processes that mediate stress-resistance while working toward the ultimate goal of improving mental and physical health in these patients.
Acknowledgments
This research was supported by grants from National Science Foundation (National Science Foundation Graduate Research Fellowship Grant to the first author), the National Institute of Mental Health (R01 MH57663), and the Harvard Medical School Priscilla White Fellowship, National Institute of Diabetes and Digestive and Kidney Diseases (R01 NIDDK24315, R01 NIDDK60115).
References
- Baron L, Eisman H, Scuello M, Veyzer A, Lieberman M. Stress resilience, locus of control, and religion in children of Holocaust victims. The Journal of Psychology. 1996;130:513–525. doi: 10.1080/00223980.1996.9915018. [DOI] [PubMed] [Google Scholar]
- Becker G, Newsom E. Resilience in the face of serious illness among chronically ill African Americans later in life. Journal of Gerontology. 2005;60B:S214–S223. doi: 10.1093/geronb/60.4.s214. [DOI] [PubMed] [Google Scholar]
- Cederblad M, Dahlin L, Hagnell O, Hansson K. Salutogenic childhood factors reported by middle-aged individuals. European Archives of Psychiatry and Clinical Neuroscience. 1994;244:1–11. doi: 10.1007/BF02279805. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Lynch M, Holt KD. Resilience in maltreated children: Processes leading to adaptive outcome. Development and Psychopathology. 1993;5:629–647. [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. [PubMed] [Google Scholar]
- Connor KM, Davidson JR. Development of a new resilience scale: The Connor- Davidson resilience scale (CD-RISC) Depression and Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progressions of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT. Adolescent resiliency to family adversity. Journal Child Psychology Psychiatry. 1996;37:281–292. doi: 10.1111/j.1469-7610.1996.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Fournier M, De Ridder D, Bensing J. Optimism and adaptation to chronic disease: The role of optimism in relation to self-care options of type 1 diabetes mellitus, rheumatoid arthritis and multiple sclerosis. British Journal of Health Psychology. 2002;7:409–432. doi: 10.1348/135910702320645390. [DOI] [PubMed] [Google Scholar]
- Garmezy N, Masten AS, Tellegen A. The study of stress and competence in children: A building block for developmental psychopathology. Child Development. 1984;55:97–111. [PubMed] [Google Scholar]
- Greco P, LaGreca AM, Ireland S, Wick P, Freeman C, Agramonte R, Gutt M, Skyler JS. Assessing adherence in IDDM: A comparison of two methods (Abstract) Diabetes. 1990;39:165A. [Google Scholar]
- Griffin SJ. Lost to follow-up: The problem of defaulters from diabetes clinics. Diabetic Medicine. 1998;15(Supp 3):S14–S24. doi: 10.1002/(sici)1096-9136(1998110)15:3+<s14::aid-dia725>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Hodgkinson K, Butow P, Hunt GE, Wyse R, Hobbs KM, Wain G. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer. 2006 doi: 10.1007/s00520-00006-00148-00520. [DOI] [PubMed] [Google Scholar]
- Hull JC, Lehn DA, Tedlie JC. A general approach to testing multifaceted personality constructs. Journal of Personality and Social Psychology. 1991;61:932–945. doi: 10.1037//0022-3514.61.6.932. [DOI] [PubMed] [Google Scholar]
- Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, Skinner JS, Bouchard C, Wilmore JH. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. International Journal of Obesity. 2002;26:789–796. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- Johnston-Brooks CH, Lewis MA, Garg S. Self-efficacy impacts self-care and HbA1c in young adults with type 1 diabetes. Psychosomatic Medicine. 2002;64:43–51. doi: 10.1097/00006842-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Judge TA, Erez A, Bono JE, Thoresen CJ. Are measures of self-esteem, neuroticism, locus of control, and generalized self-efficacy indicators of a common core construct? Journal of Personality and Social Psychology. 2002;83:693–710. doi: 10.1037//0022-3514.83.3.693. [DOI] [PubMed] [Google Scholar]
- Kinard EM. Methodological issues in assessing resilience in maltreated children. Child Abuse and Neglect. 1998;22:669–680. doi: 10.1016/s0145-2134(98)00048-9. [DOI] [PubMed] [Google Scholar]
- Kobasa SC. Stressful life events, personality, and health: An inquiry into hardiness. The Journal of Personality and Social Psychology. 1979;37:1–11. doi: 10.1037//0022-3514.37.1.1. [DOI] [PubMed] [Google Scholar]
- Kobasa SC, Maddi SR, Kahn S. Hardiness and health: A prospective study. Journal of Personality and Social Psychology. 1982;42:186–177. doi: 10.1037//0022-3514.42.1.168. [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Herndon JE, Zuckerman E, Viscoli CM, Horowitz RI, Cooper MR, Harris L, Tkaczuk KH, Perry MC, Budman D, Norton LC, Holland J Cancer and Leukemia Group B. Social support as a buffer to the psychological impact of stressful life events in women with breast cancer. Cancer. 2001;91:443–454. doi: 10.1002/1097-0142(20010115)91:2<443::aid-cncr1020>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Major B, Richards C, Cooper ML, Cozzarelli C, Zubek J. Personal resilience, cognitive appraisals, and coping: An integrative model of adjustment to abortion. Journal of Personality and Social Psychology. 1998;74:735–752. doi: 10.1037//0022-3514.74.3.735. [DOI] [PubMed] [Google Scholar]
- Masten AS, Garmezy N, Tellegen A, Pellegrini DS, Larkin K, Larsen A. Competence and stress in school children: The moderating effects of individual and family qualities. Journal Child Psychology Psychiatry. 1988;29:745–764. doi: 10.1111/j.1469-7610.1988.tb00751.x. [DOI] [PubMed] [Google Scholar]
- New MJ, Lee SS, Elliott BM. Psychological adjustment in children and families living with HIV. Journal of Pediatric Psychology. 2006 doi: 10.1093/jpepsy/jsj1121. [DOI] [PubMed] [Google Scholar]
- Ozer DJ. Four principles for personality assessment. In: Pervin LA, John OP, editors. Handbook of personality: Thoery and Research. 2. New York: Guilford Press; 1999. pp. 671–686. [Google Scholar]
- Parker GR, Cowen EL, Work WC, Wyman PA. Test correlates of stress resilience among urban school children. Journal of Primary Prevention. 1990;11:19–35. doi: 10.1007/BF01324859. [DOI] [PubMed] [Google Scholar]
- Pearlin LI, Schooler C. The structure of coping. Journal of Health and Social Behavior. 1978;19:2–21. [PubMed] [Google Scholar]
- Pedhauzur EJ. Multiple regression in behavioral research: explanation and prediction. 3. New York: Harcourt Brace; 1997. [Google Scholar]
- Peyrot MF, McMurry JF. Stress buffering and glycemic control. Diabetes Care. 1992;15:842–846. doi: 10.2337/diacare.15.7.842. [DOI] [PubMed] [Google Scholar]
- Polonsky WH, Anderson BA, Lohrer PA, Welch GW, Jacobson AM. Assessment of diabetes-related emotional distress. Diabetes Care. 1995;18:754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- Rose M, Fliege H, Hildenbrandt M, Schirop T, Klapp BF. The network of psychological variables in patients wth diabetes and their importance for quality of life and metabolic control. Diabetes Care. 2002;25:35–42. doi: 10.2337/diacare.25.1.35. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Adolescent self-image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- Rutter M. Resilience in the face of adversity: Prospective factors and resistance to psychiatric disorder. British Journal of Psychiatry. 1985;147:598–611. doi: 10.1192/bjp.147.6.598. [DOI] [PubMed] [Google Scholar]
- Rutter M. Psychosocial resilience and protective mechanisms. American Orthopsychiatric Association. 1987;57:316–331. doi: 10.1111/j.1939-0025.1987.tb03541.x. [DOI] [PubMed] [Google Scholar]
- Rutter M. Resilience reconsidered: Conceptual considerations, empirical findings, and policy implications. In: Shonkoff JP, Meisels SJ, editors. Handbook of Early Childhood Intervention. 2. Cambridge: Cambridge University Press; 2000. pp. 651–682. [Google Scholar]
- Scheier MF, Carver CS. Optimism, coping, and health: Assessment and implications of generalized outcomes expectancies. Health Psychology. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Taylor SE, Kemeny ME, Fahey JL. Optimism is associated with mood, coping and immune change in response to stress. Journal of Personality and Social Psychology. 1998;74:1646–1655. doi: 10.1037//0022-3514.74.6.1646. [DOI] [PubMed] [Google Scholar]
- Shaban MC, Fosbury J, Kerr D, Cavan DA. The prevalence of depression and anxiety in adults with Type 1 diabetes. Diabetic Medicine. 2006;23:1381–1384. doi: 10.1111/j.1464-5491.2006.02012.x. [DOI] [PubMed] [Google Scholar]
- Sinclair VG, Wallston KA. The development and psychometric evaluation of the brief resilient coping scale. Assessment. 2004;11:94–101. doi: 10.1177/1073191103258144. [DOI] [PubMed] [Google Scholar]
- Smith DM, Langa KM, Kabeto MU, Ubel PA. Health, wealth and happiness: Financial resources buffer subjective well-being after the onset of a disability. Psychological Science. 2005;16:663–666. doi: 10.1111/j.1467-9280.2005.01592.x. [DOI] [PubMed] [Google Scholar]
- Sousa VD, Zauszniewski JA, Musil CM, Price Lea PJ, Davis SA. Relationships among self-care agency, self-efficacy, self-care, and glycemic control. Research and Theory for Nursing Practice. 2005;19:217–230. doi: 10.1891/rtnp.2005.19.3.217. [DOI] [PubMed] [Google Scholar]
- Spencer MS, Kieffer EC, Sinco BR, Palmisano G, Guzman JR, James SA, Graddy-Dansby G, Feathers JT, Heisler M. Diabetes-specific emotional distress among African Americans and Hispancis with Type 2 diabetes. Journal of Health Care for the Poor and Underserved. 2006;17:88–105. doi: 10.1353/hpu.2006.0095. [DOI] [PubMed] [Google Scholar]
- SPSS. SPSS for Windows (Version 14.0) Chicago, IL: 2006. [Google Scholar]
- Sultan S, Heurtier-Hartemann A. Coping and distress as predictors of glycemic control in diabetes. Journal of Health Psychology. 2001;6:731–739. doi: 10.1177/135910530100600616. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NWJ, Luben R, Khaw K, Day NE. Mastery, sense of coherence, and mortality: evidence of independent associations from the EPIC-Norfolk prospective cohort study. Health Psychology. 2006;25:102–110. doi: 10.1037/0278-6133.25.1.102. [DOI] [PubMed] [Google Scholar]
- Syrjala AMH, Ylostalo P, Niskanen MC, Knuuttila MLE. Relation of different measures of psychological characteristics to oral health habits, diabetes adherence and related clinical variables among diabetic patients. European Journal of Oral Sciences. 2004;112:109–114. doi: 10.1111/j.1600-0722.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sherman DK, Sage RM, McDowell NK. Are self-enhancing cognitions associated with health or unhealthy biological profiles? Journal of Personality and Social Psychology. 2003;85:605–615. doi: 10.1037/0022-3514.85.4.605. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Seeman TE. Psychosocial resources and the SES-health relationship. Annals of the New York Academy of Sciences. 1999;896:210–225. doi: 10.1111/j.1749-6632.1999.tb08117.x. [DOI] [PubMed] [Google Scholar]
- Van Der Ven N, Weinger K, Yi J, Pouwer F, Ader H, Van Der Ploeg HM, Snoek FJ. The confidence in diabetes self-care scale: Psychometric properties of a new measure of diabetes-specific self-efficacy in Dutch and US patients with type 1 diabetes. Diabetes Care. 2003;26:713–718. doi: 10.2337/diacare.26.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedhara K, Nott KH. Psychosocial vulnerability to stress: A study of HIV-positive homosexual men. Journal of Psychosomatic Research. 1996;41(3):255–267. doi: 10.1016/0022-3999(96)00096-7. [DOI] [PubMed] [Google Scholar]
- Wagnild GM, Young HM. Development and psychometric evaluation of the resilience scale. Journal of Nursing Measurement. 1993;1(2):165–178. [PubMed] [Google Scholar]
- Weinger K, Butler HA, Welch GW, LaGreca AM. Measuring diabetes self-care: A psychometric analysis of the Self-Care Inventory-R with adults. Diabetes Care. 2005;28:1346–1352. doi: 10.2337/diacare.28.6.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Education and Counseling. 2001;42:123–131. doi: 10.1016/s0738-3991(00)00098-7. [DOI] [PubMed] [Google Scholar]
- Weinger K, McMurrich SJ, Yi JP, Lin S, Rodriguez M. Psychological characteristics of frequent short-notice cancellers of diabetes medical and education appointments. Diabetes Care. 2005;28:1791–1793. doi: 10.2337/diacare.28.7.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch GW, Jacobson AM, Polonsky WH. The problem areas in diabetes scale: An evaluation of its clinical utility. Diabetes Care. 1997;20:760–766. doi: 10.2337/diacare.20.5.760. [DOI] [PubMed] [Google Scholar]
- Welch GW, Weinger K, Anderson BA, Polonsky WH. Responsiveness of the problem areas in diabetes (PAID) questionnaire. Diabetic Medicine. 2003;20:69–72. doi: 10.1046/j.1464-5491.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- Werner EE, Smith RE. Vulnerable but invincible: A study of resilient children. New York: McGraw-Hill; 1982. [Google Scholar]
- Whittemore R, Melkus GD, Grey M. Metabolic control, self-management and psychosocial adjustment in women with type 2 diabetes. Journal of Clinical Nursing. 2005;14:195–203. doi: 10.1111/j.1365-2702.2004.00937.x. [DOI] [PubMed] [Google Scholar]
- Wills TA, Yaeger AM, Sandy JM. Buffering effect of religiosity for adolescent substance use. Psychology of Addictive Behaviors. 2003;17:24–31. doi: 10.1037/0893-164x.17.1.24. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Taylor A, Hough BS, Linscheid TR, Yeates KO, Naglieri JA. Deviation from developmentally appropriate self-care autonomy association with diabetes outcomes. Diabetes Care. 1996;19:119–125. doi: 10.2337/diacare.19.2.119. [DOI] [PubMed] [Google Scholar]