Abstract
Purpose
Afferent nerve firing has been linked to spontaneous bladder contractions in a number of lower urinary tract pathologies and it may lead to urgency and incontinence. Using optical mapping, single unit recording and tension measurements we investigated the correlation between afferent nerve firing and spontaneous bladder contractions in spinal cord transected mice.
Materials and Methods
Bladder-nerve preparations (bladder sheets and the associated L6-S2 pelvic nerves) were dissected from normal and spinal cord transected mice showing overactivity on cystometry and opened along the ventral aspect from base to dome. Bladder sheets were mounted horizontally in a temperature regulated chamber to simultaneously record Ca2+ transients across the mucosal surface, single unit afferent nerve firing and whole bladder tension.
Results
Single unit afferent fibers were identified by probing their receptive fields. Fibers showed a graded response to von Frey stimulation and a frequency of afferent firing that increased as a function of the degree of stretch. Optical maps of Ca2+ transients in control bladders demonstrated multiple initiation sites that resulted in high frequency, low amplitude spontaneous contractions. Alternatively in maps of the bladders of spinal cord transected mice Ca2+ transients arose from 1 or 2 focal sites, resulting in low frequency, high amplitude contractions and concomitant afferent firing.
Conclusions
Large amplitude, spontaneous bladder contractions evoke afferent nerve activity, which may contribute to incontinence.
Keywords: urinary bladder; spinal cord injuries; urinary incontinence; neurons, afferent; muscular contraction
Spontaneous bladder contractions have been shown to decrease in frequency but increase in magnitude in a number of lower urinary tract pathologies, including outlet obstruction1 and SCT.2 These contractions may underlie abnormal increases in intravesical pressure, resulting in urgency, frequency and urge incontinence.3 This activity may originate in the central nervous system4 or in detrusor smooth muscle.5 Alternatively it may originate in the urothelial/suburothelial region. It has been suggested that the urothelial release of acetylcholine and ATP interacts with lamina propria myofibroblasts to drive spontaneous activity.6,7 In the rat increases in the amplitude of spontaneous contractions have been noted after SCT.2 This activity was initiated at a focal site near the bladder dome and it spread over the detrusor in a coordinated fashion. In normal bladders spontaneous contractions originate at multiple changing sites in the detrusor and, therefore, they spread in an uncoordinated manner, resulting in low amplitude spontaneous contractions.2
Afferent nerves convey sensory information from the bladder to the central nervous system. These nerves comprise myelinated Aδ fibers that are primarily located in detrusor smooth muscle and unmyelinated C fibers that spread through all of the layers of the bladder wall.8 In the bladder 4 types of afferent nerves have been described, including serosal, muscular, muscular-mucosal and mucosal, of which 63% are muscular.9 Afferents respond to a number of different stimuli in the bladder wall, including stretch and tension as the bladder fills to convey sensations ranging from fullness to discomfort and pain. The sensitivity of afferents can also be modulated by chemical irritants and inflammatory mediators.10,11
The sensitization of afferent fibers and/or increases in the magnitude of spontaneous contractions have been implicated in a number of bladder pathologies.8 Accordingly we have developed an in vitro SCT bladder-nerve preparation and combined it with optical mapping, tension measurements and single unit recordings to determine the relationship between spontaneous contractions and afferent firing.
MATERIALS AND METHODS
Spinal Cord Transection
Five C57BL/10 female mice at ages 5 to 6 weeks were anesthetized with isoflurane (1% to 5%), and spinalized, as described previously.12 Briefly, laminectomy was performed to expose and transect the spinal cord between the T8–T9 vertebrae. After spinalization the bladders were expressed twice daily until somatospinal reflexes developed at 1 to 2 weeks.
Decerebration
Two weeks after SCT the mice were anesthetized with isoflurane (1% to 5%), and the carotids were exposed and tied with 7-zero silk suture. The trachea was exposed and a polyethylene-60 endotracheal tube was inserted. Craniotomy was performed, followed by precollicular decerebration.
Cystometry
After decerebration the bladder was exposed via a low midline abdominal incision. A flanged polyethylene-50 catheter was passed through a small opening at the apex of the bladder dome and secured with suture. The catheter was connected to an infusion pump and a pressure transducer. Saline was infused at room temperature at a rate of 0.01 ml per minute.
Bladder-Nerve Preparation
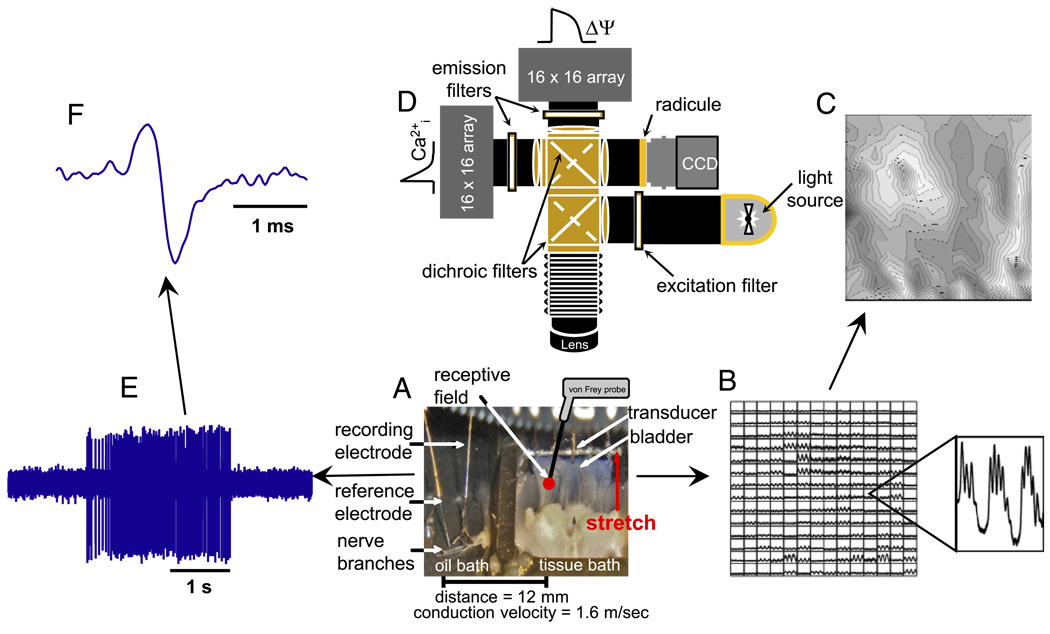
The bladder with the attached L6-S2 spinal nerves was dissected free and opened longitudinally along the ventral aspect to form a sheet. The preparation was mounted in a temperature regulated chamber with the urothelial surface up (fig. 1, A). The bladder sheet was attached to a tension transducer and the nerves were positioned on electrodes in adjacent oil filled recording chambers. The bladders were stained with Ca2+ sensitive dye (1 µM Rhod-2-AM) for 25 minutes. Tissue was perfused at 0.5 ml per minute with modified Krebs solution at 35C and the bladders were set to a tension of 1 gm.
Figure 1.
Experimental setup to record intracellular Ca2+ signals, tension and single unit afferent nerve activity. A, bladder-nerve sheet preparation. B, Ca2+ signals recorded in tissue using photodiode array. C, isochronal map derived from data in B. Initiation site corresponds to imaged tissue region with earliest Ca2+ transients (white areas). D, optical system arrangement of light source, filters, photodiode array and charge coupled device cameras. E, single unit afferent recordings. s, seconds. F, expanded single unit recording from E. ms, millisecond.
The nerve sheath was carefully peeled back and split into smaller bundles until 1 or 2 units could be discriminated. Receptive fields were identified by passing a soft brush along the length of the bladder and then probing with 0.4, 1.0 and 2.0 gm von Frey filaments. The bladder was stretched using a computer controlled stepper motor. Unit activity was differentially amplified, filtered, sampled at 20 kHz and stored on a computer hard drive. Figure 1, E shows 0.4 gm von Frey stimulation of a single afferent nerve acquired simultaneously along with optical data. Figure 1, F shows the trace expanded to reveal a single spike.
Optical Apparatus
Optical mapping was accomplished using an imaging system built at our institution (fig. 1, D).13 While the system has 2 photodiode array cameras for simultaneous measurement of voltage (action potentials) and Ca2+ (intracellular transients), only Ca2+ signals were recorded in this study. The conduction delays for Ca2+ transients between imaged regions of tissue were determined using cross-correlation analysis.13 Figure 1, B shows raw transient data for each pixel that imaged a region of tissue. These data were used to generate grayscale isochronal maps to visualize the spread of Ca2+ waves from their initiation sites (fig. 1, C). Figure 1, C shows white regions, which were the first areas to undergo transients, and black regions, which were the last areas.
Quantitative data are presented as the mean ± SD. The difference between 2 mean values was analyzed with the unpaired Student t test with nominal p <0.05 considered statistically significant. All experiments were reviewed and approved by the University of Pittsburgh institutional animal care use committee.
RESULTS
We developed a bladder nerve preparation and combined it with optical mapping and tension measurements to determine the influence of spontaneous activity on afferent nerve firing. We compared these parameters in bladder sheets from normal and SCT mice.
Afferent Fibers
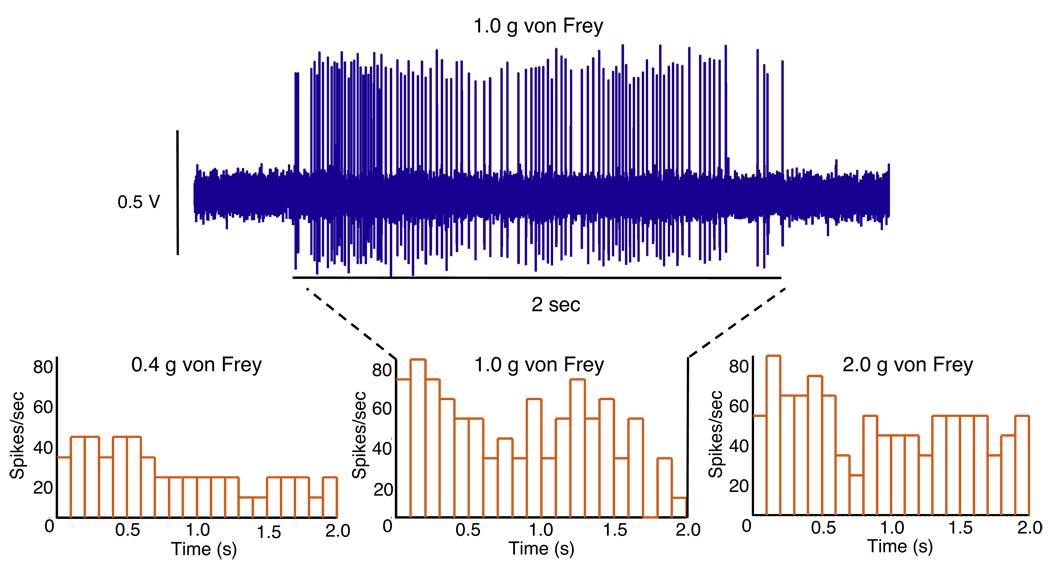
Afferent fibers were identified by softly stroking the mucosal surface with a fine brush, followed by von Frey stimulation to locate receptive fields. Single afferent fibers were recorded from the S1 branch of the bladder-nerve preparation. The afferent firing rate was enhanced by mechanical stimulation of the receptive field using a 0.4 gm von Frey probe (fig. 1, E and F). The receptive fields of a total of 14 fibers from 13 mice were located using this approach. Figure 2 shows histograms illustrating the fiber graded response to increasing von Frey probe stimulation of the corresponding receptive field. The rate of afferent firing using 0.4, 1.0 and 2.0 gm von Frey probes was 17.0 ± 8.29, 31.33 ± 15.63 and 33.33 ± 8.06 spikes per second, respectively. The afferent firing rate did not increase beyond 1.0 gm probing, indicating that the firing of the fibers had achieved saturation.
Figure 2.
Histograms show afferent fiber firing frequency in response to von Frey stimulation at 0.4, 1.0 and 2.0 gm (g) with 1 millisecond bin width. Afferent recordings correspond to 1.0 gm von Frey stimulation. s, seconds.
We determined conduction velocities to differentiate C fibers (less than 2 m per second) from Aδ fibers (2 to 20 m per second). This was accomplished by stimulating the receptive field using a small bipolar electrode and recording orthodromically propagating action potentials in the single afferent fiber being studied. Figure 1, F shows a single unit with a conduction velocity of 1.6 milliseconds, as measured over a distance of 12 mm (fig. 1, A), indicating a C fiber. However, one must be cautious because the peripheral portions of Aδ fibers are unmyelinated and their conduction velocity may appear to be within the range of that of C fibers. Therefore, conduction velocity was also determined by stimulating the axon bundle distal to the bladder sheet where Aδ fibers are myelinated.
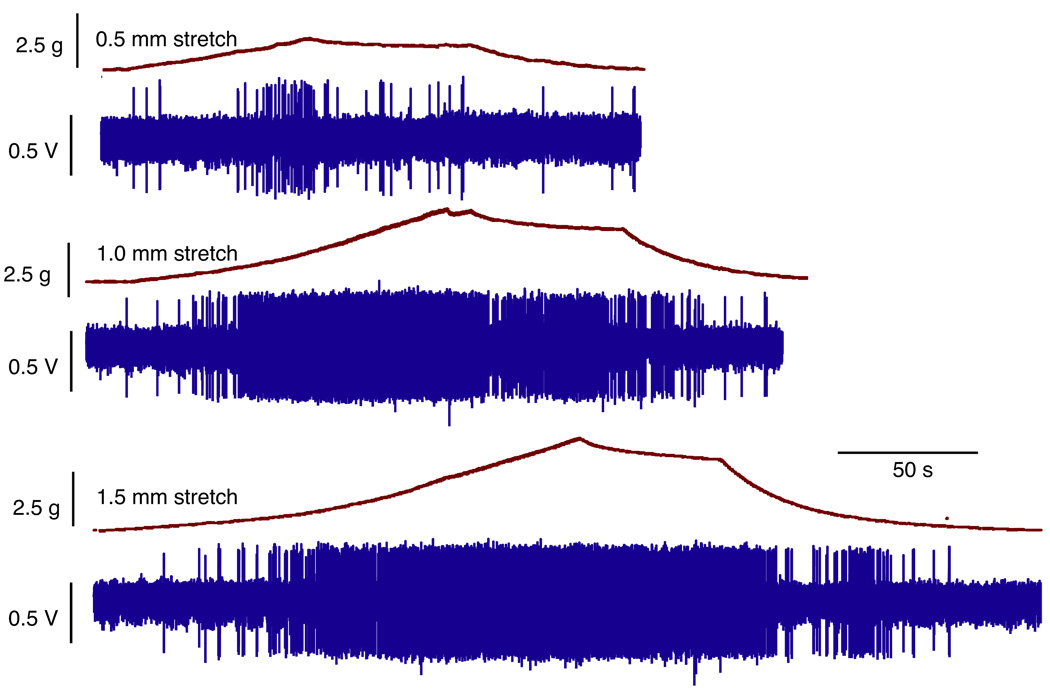
We examined the response of afferent fibers in normal adult mouse bladders after varying degrees of stretch. Bladder sheets were stretched along the base-to-dome axis at a rate of 10 µm per second, held for 60 seconds and then returned to resting tension. Four of the 8 fibers tested with stretch were mechanosensitive. Figure 3 shows changes in bladder tension and the firing of a mechanosensitive fiber in response to 0.5, 1.0 and 1.5 mm of stretch. Bladder tension increased with the degree of stretch, including 0.5 mm at 1.7 gm, 1.0 mm at 3.3 gm and 1.5 mm at 4.5 gm. The frequency of afferent firing also increased as a function of the level of stretch when held for 60 seconds. The mean firing rate at 0.5, 1.0 and 1.5 mm of stretch was 0.35 ± 0.60, 3.90 ± 2.92 and 5.45 ± 2.3 spikes per second, respectively. Firing ceased as stretch was reversed and bladder tension returned to resting levels. The slight decrease in the firing rate for the 60 seconds during which stretch was held may have been due to an adaptive response of afferent fibers or to the small decrease in tension that was observed during this period.
Figure 3.
Changes in bladder tension and firing of single afferent fiber in response to 0.5, 1.0 and 1.5 mm stretch. s, seconds
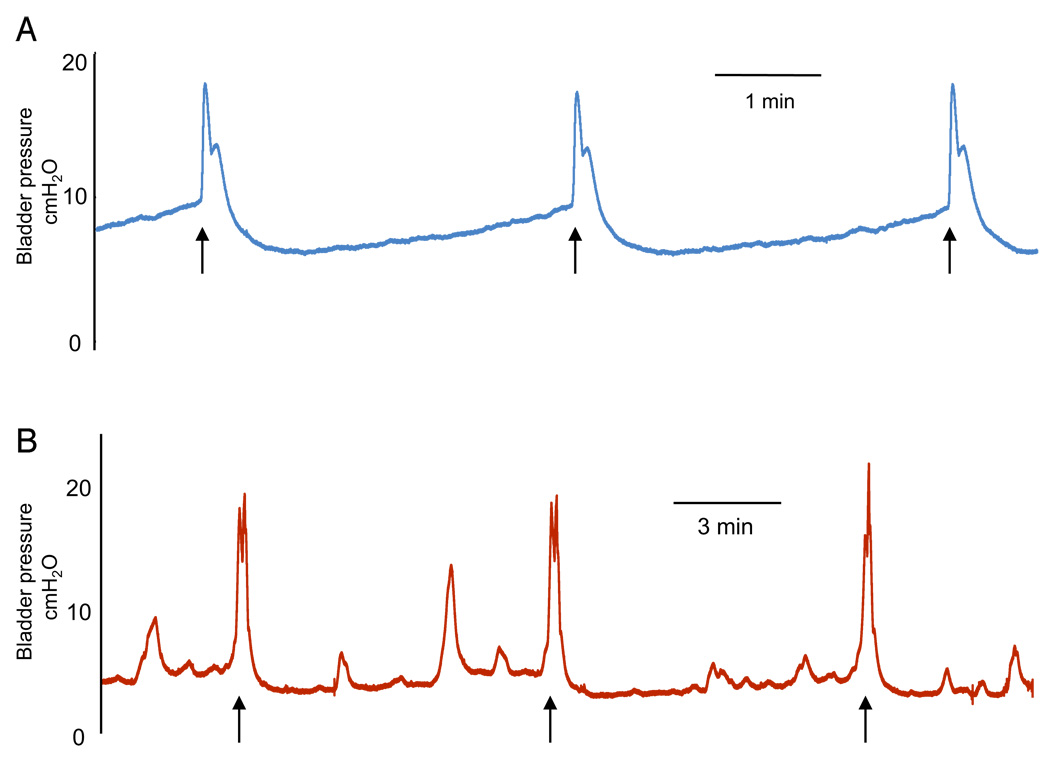
Cystometry
Cystometry was performed in SCT mice to screen for bladder overactivity. Mice with these symptoms were used immediately for the bladder-nerve preparation. Between 1 and 2 weeks after SCT the bladders typically demonstrated nonvoiding contractions, as shown on voiding cystometrograms (fig. 4, B). Pressure and contractile activities in SCT and control bladders were 4.74 ± 0.3 and 8.3 ± 0.2 mm H2O pressure threshold, 2.84 ± 0.19 and 6.3 ± 0.3 mm H2O baseline pressure, and 19.01 ± 1.46 and 19.3 ± 0.6 mm H2o maximal voiding pressure, respectively. The intercontraction interval was increased in SCT vs control mouse bladders (457 ± 38 vs 175 ± 9 seconds).
Figure 4.
Cystometrograms. A, controls. B, SCT decerebrated mice. Arrows indicate voiding
Spontaneous Contractile Activity
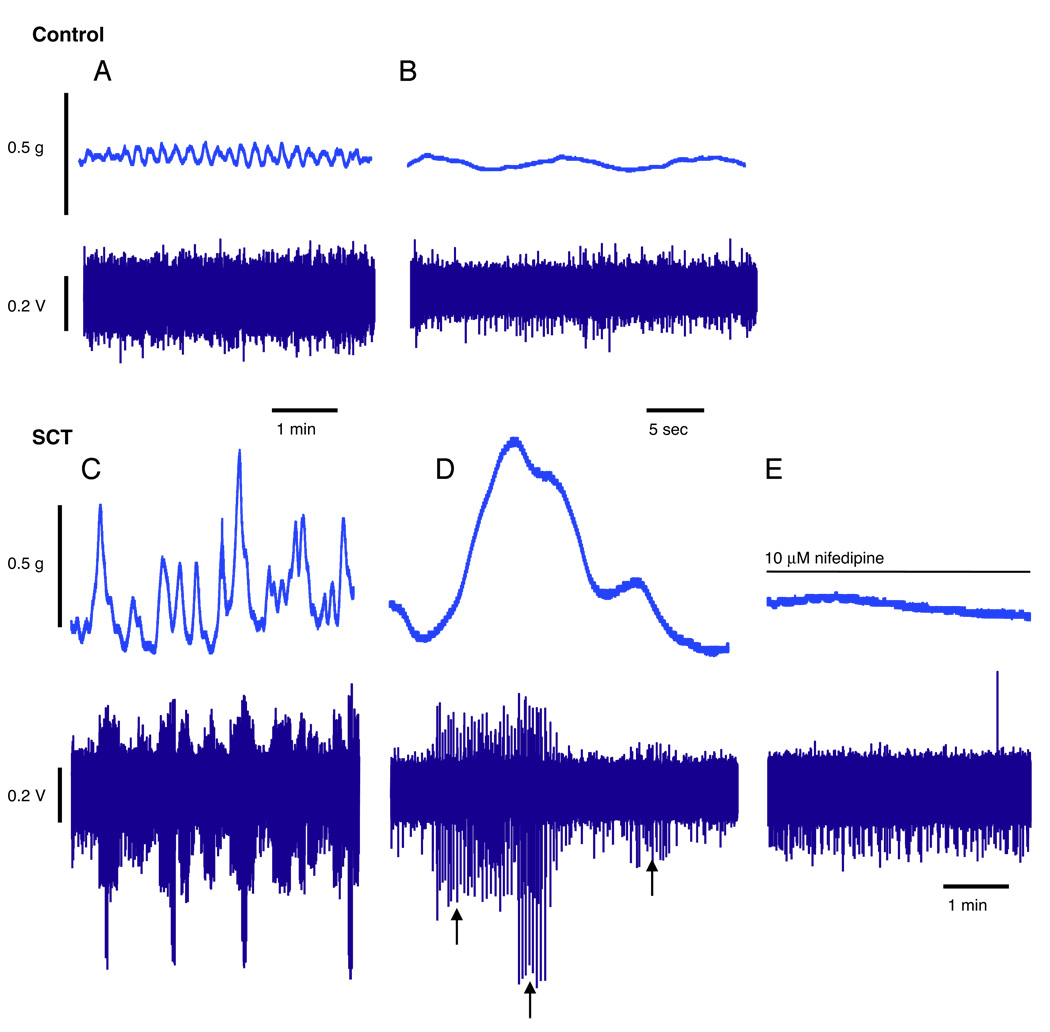
There were significant differences in spontaneous contractile activity in normal adult and SCT mouse bladder sheets (fig. 5, A and D). Normal adult mouse bladders demonstrated spontaneous contractions that were low in amplitude (0.15 ± 0.07 gm). In contrast, SCT mouse bladders demonstrated spontaneous contractions that were much higher in amplitude (1.0 ± 0.7 gm), which were also decreased in frequency compared with those in normal adults. Spontaneous contractions were insensitive to 1 µM tetrodotoxin (data not shown) but sensitive to 10 µM nifedipine (fig. 6, E).
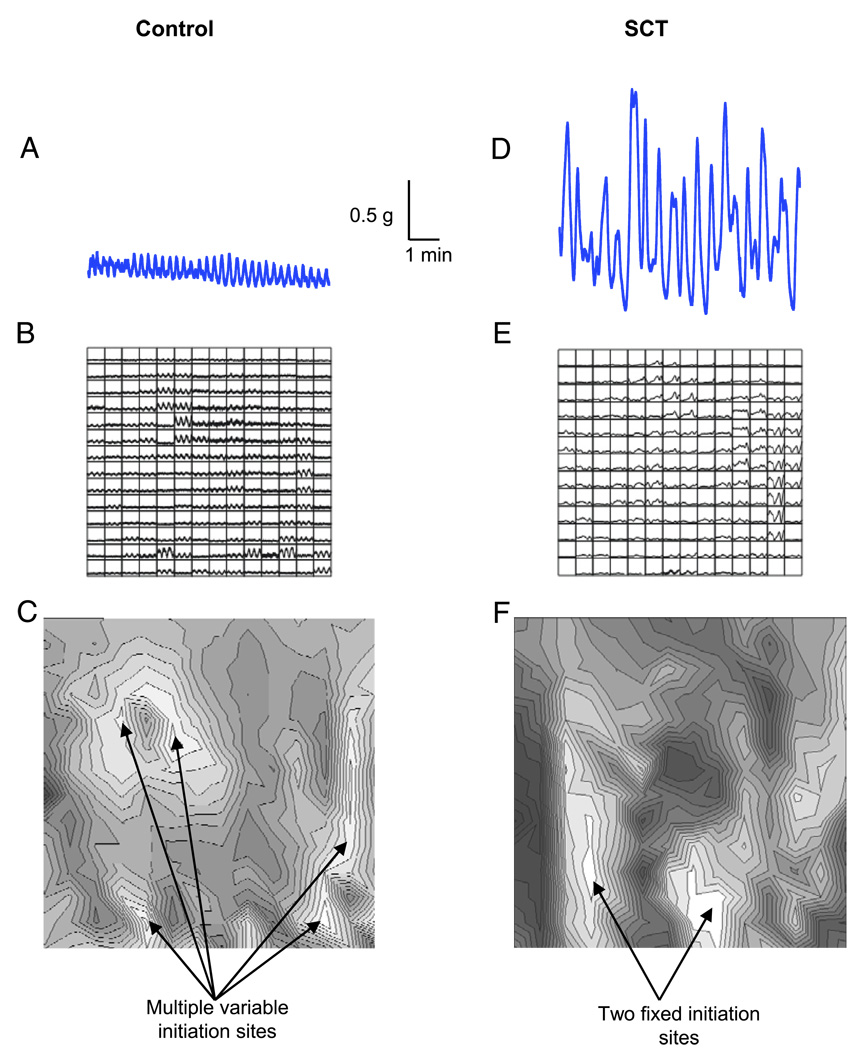
Figure 5.
Findings in normal adult and SCT mouse bladders. A and D, tension recordings. g, gm. B and E, Ca2+ transients. C and F, isochronal maps.
Figure 6.
Simultaneous recordings of spontaneous contractile activity and corresponding afferent firing in normal adult and SCT mouse bladders. A, control. g, gm. B, control expanded time base. C, SCT. D, SCT expanded time base. Arrows indicate action potential at different amplitude recorded in 3 separate fibers that responded to spontaneous contraction, as described. E, effect of 10 µM nifedipine on spontaneous contractions and afferent nerve firing.
Spontaneous Intracellular Ca2+ Transients and Isochronal Maps
Ca2+ activity across the mucosal surface of the bladder sheets was recorded during spontaneous contractions. Figure 5, B and E shows examples of the Ca2+ transients recorded in the bladder of a normal and an SCT mouse. The isochronal map of the normal bladder sheet demonstrated multiple initiation sites of Ca2+ transients (fig. 5, C, white areas). These initiation sites also varied in location among contractions. In contrast, in SCT bladders Ca2+ transients were initiated at 1 or 2 fixed sites (fig. 5, F).
Spontaneous Contractile and Afferent Activity
Spontaneous contractile activity and corresponding afferent firing were recorded in normal adult and SCT mouse bladders (fig. 6, A to D). Traces demonstrated that in SCT mouse bladders large amplitude spontaneous contractions induced the corresponding firing of afferent nerves (fig. 6, C and D). Figure 6 shows tension traces representing the overall tension generated by the bladder sheet. Figure 6, D shows the corresponding afferent recording with 3 separate fibers (arrows) that may have different receptive fields. As the tension changes, different fibers responded. In the presence of 10 µM nifedipine spontaneous contractile activity was abolished and the corresponding induction of afferent firing ceased (fig. 6, E). In contrast, small amplitude spontaneous contractions in the normal adult bladder did not induce afferent firing (fig. 6, A and B).
DISCUSSION
We report the usefulness of our mouse bladder sheetnerve preparation. The combination of optical mapping, tension measurements and afferent recordings allowed us to determine the initiation site(s) and magnitude of spontaneous contractions, and correlate them with concomitant afferent firing. Using whole bladder sheets with an intact mucosa permitted us to study the involvement of the urothelium/suburothelium in afferent activity. It also avoided the tissue damage associated with muscle strip preparations devoid of mucosa, which are frequently used in studies of detrusor activity.3
Afferent activity is important in micturition regulation. As the bladder fills and distends, stretch sensitive receptors on afferent nerves are activated and initiate micturition after an appropriate volume is attained. In this study we measured an increase in afferent firing as a function of stretch of the whole bladder sheet, as in previous studies in guinea pigs.14 Other studies have also shown increased afferent firing with stretch produced at a single site in the mouse bladder9 and with the distention of intact bladders in rats15 and cats.16 What these previous studies have not addressed is whether spontaneous bladder contractions can drive afferent firing.
A recent study used an in vitro whole rat bladder-pelvic nerve preparation and multiunit recordings to study the influence of spontaneous contractions on afferent excitability in normal and cyclophosphamide treated animals.10 In preparations from normal animals bladder distention resulted in phasic and tonic afferent activity. Phasic firing mirrored spontaneous contractions, while tonic activity increased as a function of stretch, which is a consequence of fiber recruitment in multi-unit recordings.
In our normal preparations maintained at physiological temperature the observed low amplitude spontaneous contractions did not stimulate afferent firing. An explanation for this difference is that in the other study experiments were done at room temperature.10 As those investigators reported previously, spontaneous activity increases significantly in amplitude and decreases in frequency at lower temperatures.17 We hypothesize that decreased temperature inactivates some of the multiple Ca2+ initiation sites. Ca2+ waves initiated at multiple sites in the detrusor can travel only short distances before encountering other waves and stopping. This results in a large number of small spontaneous contractions, eg higher frequency and lower amplitude. A theory for the focal initiation of spontaneous activity in SCT and neonatal bladders, and multiple site initiation in normal bladders was recently proposed.2,13
Regarding the mentioned cyclophosphamide inflammatory model, those investigators observed no change in the amplitude of spontaneous contractions but they noted enhanced afferent firing (sensitization), which could be blocked by ATP antagonists.10 This suggests that there was increased ATP production following inflammation that excited P2X3/P2X2/3 receptors on afferent nerves. However, afferent sensitization cannot accurately be addressed or studied using multi-unit recording methods. In our SCT model of detrusor overactivity we measured an increase in the amplitude of spontaneous contractions and we are currently studying the contribution of afferent sensitization. A number of other studies have demonstrated enhanced, stretch induced urothelial ATP release in bladder pathologies.18–20 Thus, it is likely that the increased amplitude of spontaneous contractions and nerve sensitization contributed to the enhanced afferent firing seen in the bladders of mice with SCT.
CONCLUSIONS
SCT in the mouse resulted in larger amplitude, lower frequency spontaneous bladder contractions that could drive afferent nerve firing, which may contribute to lower urinary tract disorders. Similarly bladder afferent sensitization has been associated with a number of bladder pathologies. Therefore, we are currently determining the contribution of afferent sensitization to spontaneous, contractile evoked firing.
Acknowledgments
Study received University of Pittsburgh institutional animal care use committee approval.
Supported by National Institutes of Health Grants DK64280, DK71085 (AJK) and NS35790 (GFG).
Abbreviations and Acronyms
- ATP
adenosine triphosphate
- SCT
spinal cord transection
REFERENCES
- 1.Drake MJ, Hedlund P, Harvey IJ, Pandita RK, Andersson KE, Gillespie JI. Partial outlet obstruction enhances modular autonomous activity in the isolated rat bladder. J Urol. 2003;170:276. doi: 10.1097/01.ju.0000069722.35137.e0. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A. Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol. 2007;293:F1018. doi: 10.1152/ajprenal.00183.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng E, Young JS, Brading AF. Spontaneous activity of mouse detrusor smooth muscle and the effects of the urothelium. Neurourol Urodyn. 2008;27:79. doi: 10.1002/nau.20456. [DOI] [PubMed] [Google Scholar]
- 4.de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997;50:36. doi: 10.1016/s0090-4295(97)00587-6. [DOI] [PubMed] [Google Scholar]
- 5.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004;141:183. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillespie JI, Harvey IJ, Drake MJ. Agonist-and nerve-induced phasic activity in the isolated whole bladder of the guinea pig: evidence for two types of bladder activity. Exp Physiol. 2003;88:343. doi: 10.1113/eph8802536. [DOI] [PubMed] [Google Scholar]
- 7.Fry CH, Sui GP, Kanai AJ, Wu C. The function of suburothelial myofibroblasts in the bladder. Neurourol Urodyn. 2007;26:914. doi: 10.1002/nau.20483. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura N, Kaiho Y, Miyazato M, Yunoki T, Tai C, Chancellor MB, et al. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmiede Arch Pharmacol. 2007;377:437. doi: 10.1007/s00210-007-0209-z. [DOI] [PubMed] [Google Scholar]
- 9.Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99:244. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Y, de Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol. 2008;294:F1146. doi: 10.1152/ajprenal.00592.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong W, Spyer KM, Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541:591. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruse MN, Bray LA, de Groat WC. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Auton Nerv Syst. 1995;54:215. doi: 10.1016/0165-1838(95)00011-l. [DOI] [PubMed] [Google Scholar]
- 13.Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, et al. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol. 2007;292:F1065. doi: 10.1152/ajprenal.00229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zagorodnyuk VP, Costa M, Brookes SJ. Major classes of sensory neurons to the urinary bladder. Auton Neurosci. 2006;126–127:390. doi: 10.1016/j.autneu.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Shea VK, Cai R, Crepps B, Mason JL, Perl ER. Sensory fibers of the pelvic nerve innervating the rat’s urinary bladder. J Neurophysiol. 2000;84:1924. doi: 10.1152/jn.2000.84.4.1924. [DOI] [PubMed] [Google Scholar]
- 16.Habler HJ, Janig W, Koltzenburg M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J Physiol. 1993;463:449. doi: 10.1113/jphysiol.1993.sp019604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugaya K, de Groat WC. Influence of temperature on activity of the isolated whole bladder preparation of neonatal and adult rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R238. doi: 10.1152/ajpregu.2000.278.1.R238. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol. 1997;505:503. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- 20.Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Int. 2005;47:291. doi: 10.1016/j.neuint.2005.04.021. [DOI] [PubMed] [Google Scholar]