Abstract
Postlaminectomy epidural adhesion is implicated as a main cause of “failed back surgery syndrome” and associated with increased risk of complications during revision surgery. Various materials acting as mechanical barriers to reduce fibroblasts infiltration into epidural space have met with limited success. In present research, amniotic membrane (AM) was studied to investigate its effects on reducing epidural scar adhesion after laminectomy in a canine model. Laminectomy sites were created at L-1, L-3, L-5, and L-7 levels in 24 adult mongrel dogs. Freeze dried AM (FAM), cross-linked AM (CAM), and autologous free fat (AFF) were implanted, respectively, at a randomly assigned site in each dog with the remaining untreated site serving as internal control. The animals were sacrificed at 1, 6, and 12 weeks postoperatively. Then, gross pathologic observation including scar amount and adhesion tenacity, qualitative histology evaluation, and quantitative histology analysis were compared. Gross observation demonstrated that scar amount and adhesion tenacity of CAM group were significantly lower in comparison with those of FAM and non-treatment groups. A white, slightly vascularized CAM layer covered the dura mater without tenacious scar adhesion. The histology analysis also indicated reduced fibroblasts infiltration and consequent epidural fibrosis, which were similar to the results of AFF group. In conclusion, the CAM is effective in reducing epidural fibrosis and scar adhesion after laminectomy in canine model. It is a promising biomaterial for future clinical applications.
Keywords: Laminectomy, Epidural fibrosis, Amniotic membrane, Dura mater
Introduction
Approximately 185,000 lumbar spine surgeries are performed every year in the US to treat various clinical conditions such as spondylolisthesis, spinal stenosis, and discogenic back pain [2]. Failed back surgery syndrome (FBSS) is characterized by the presence of intractable pain and varying degrees of functional incapacity after lumbar spine surgery. It occurs in 13–61% of patients who undergo back surgery [4, 34]. The reasons for FBSS include inadequate surgical decompression, recurrent disc herniation, lumbar instability, extensive epidural scar, and inadequate fusion [7]. The postoperative epidural scar can cause extradural compression or dural tethering, which results in recurrent radicular pain and physical impairment [27].
The intraspinal hemorrhage after laminectomy constitutes a scaffold for the migration of fibroblasts from the periosteum and paraspinal muscles. Then, a granulation tissue forms and deposits collagen fibers, which finally mature into a dense fibrotic scar recognized as the “postlaminectomy membrane” [17]. The epidural scar mainly originates from the erector spinae muscle mass and causes tractions on the dura mater or nerve roots, which result in low back pain. Furthermore, it makes re-exposure more time consuming and difficult in revision surgery with increased risk of complications [33]. Once the scar forms, there is no effective treatment. Although extensive epidural scar adhesions can be removed and the tethered nerve roots can be freed at the time of revision surgery, the adhesions will recur after secondary surgery [19]. Therefore, it is necessary to develop a therapy that reliably reduces epidural fibrosis and scar adhesions without causing side effects.
In addition to improvements of surgical techniques and administration of haemostatic agents, implantation of biomaterial serving as a mechanical barrier between dura mater and overlying tissue is of great importance. It can limit the migration of fibroblasts and reduce collagen deposition. Various materials including silastic, synthetic membranes or foams, and free or pedicle fat grafts have been applied as barriers to reduce scar formation [8, 14, 18]. However, none of them has been widely accepted with consistent results. Now, only some materials, such as Gelform, Adcon-L, and fat grafts are applied in humans with limited success [6, 8, 20]. The associated complications include cerebrospinal fluid leaks, seroma formation, scar dimpling, and migration of fat graft [10, 24, 37]. Hence, a material, which can effectively reduce epidural fibrosis is needed.
One particularly promising material is amniotic membrane (AM), which is the inner layer of fetal membrane. This translucent membrane is composed of an inner layer of epithelial cells planted on a basement membrane. AM can reduce inflammation, inhibit vascularization, combat infection, and limit postoperative adhesion [32]. It has been used to treat variable diseases such as non-healing skin ulcers, vaginal atresia, and severe ocular surface disease [9, 35]. Moreover, it can serve as adjunctive tissue to reduce surgical adhesions in abdominal surgery and otolaryngologic surgery [25, 29, 36]. Furthermore, AM is an immune-privileged tissue and rarely causes immunologic rejection [15]. So it is reasonable to hypothesize that AM is a suitable interposition membrane which can reduce postoperative epidural fibrosis and consequent scar adhesions in spine surgery.
To test this hypothesis, a total laminectomy canine model was established in current study. AM was implanted between the dura mater and overlying paraspinal muscles as a mechanical barrier to limit the formation of epidural fibrosis and consequent scar adhesions. The effects were assessed by gross observation and histology evaluation. The purpose of this study is to investigate the preventive effects of AM in vivo.
Materials and methods
Procurement of AM
Informed consents were received from pregnant donors in accordance with the Declaration of Helsinki. Normal human placentas were carefully obtained soon after cesarean sections and washed in saline solution. Intact AM was stripped from the chorion gently and cut into sheets of 20 mm × 30 mm in dimension. The sheets (n = 3) were washed with PBS and fixed in 10% neutral buffered formalin. Thereafter, they were dehydrated through a series of graded alcohols and embedded in paraffin. Sections of 5 μm thickness were cut and stained with hematoxylin and eosin (H&E) for histology observation. Then, AM sheets were treated differently to prepare implants. The freeze–dried AM (FAM) was prepared by freeze drying the AM under a vacuum of 0.2 Torr for 24 h. The cross-linked AM (CAM) was fabricated by immersing AM in 0.25% glutaraldehyde (GA) solution followed by incubation at room temperature for 1 h. Thereafter, the CAM was thoroughly washed with phosphate buffered saline solution (PBS) to remove the residual GA. Finally, the FAM and CAM were sterilized by ethylene oxide for implantation.
Surgical procedure
Animal experiments were approved by Institutional Animal Care and Use Committee of the authors’ university. There were 24 adult mongrel dogs (weight: 14.3 ± 2.5 kg) used in this study. After the dogs were anesthetized and placed in prone position, a midline skin incision was made. The lumbosacral fascia was then incised and the paraspinal muscles were subperiosteally detached to expose laminae. Four separate total laminectomies (10 mm × 20 mm) were performed at L-1, L-3, L-5, and L-7 levels. The ligamentum flavum and epidural fat were removed and the dura mater was then exposed. The hemostasis was obtained by applying gentle pressure with cotton sheet. The laminectomy sites were treated differently with: (a) FAM implantation, (b) CAM implantation, (c) non-treatment (empty control), (d) autologous free fat (AFF) graft implantation (positive control). The AFF of 20 mm × 30 mm in dimension was harvested from subcutaneous tissue and trimmed to a thickness of ~5 mm. The FAM, CAM, and AFF were tucked under the laminar edges to cover the dura mater as a roof structure. Thereafter, they were fixed with 2–0 polyester suture (Ethibond, Johnson & Johnson, US) to the capsules of adjacent facet articulations. The control and implantation sites were randomly assigned in each animal to diminish the influence of level-specific variations. To prevent bias, the surgeon was blinded to the choice of implantation site and material type before operation. All treatments were followed by routine closure of muscle, fascia, and skin. Postoperatively, the dogs were housed in individual cages and allowed normal activity.
Gross anatomic examination
The dogs were sacrificed at 1, 6, and 12 weeks postoperatively. The sample size was eight for each group at each time point. The spine segments were removed en bloc. First, the examiners observed scar formation between the dura mater and paraspinal muscles. A previously described scale (0 = none, 1 = small, 2 = medium, 3 = large) was used to evaluate the amount of scar tissue [5]. Then, each sample was divided into two parts by cutting transversely through middle line. One part was for adhesion tenacity test and the other for histology analysis. The scar was peeled-off manually and the adhesion tenacity was evaluated using a reported scale. The scale was consisted of six grades: (1) Grade 0: no adhesion; (2) Grade 1: very slight adhesion and easily detached without applying manual force; (3) Grade 2: light adhesion and easily detached by weak traction; (4) Grade 3: moderate adhesion and detached by moderate traction; (5) Grade 4: tenacious adhesion and detached by strong traction; (6) Grade 5: highly tenacious adhesion and detached by sharp dissection [22].
Histology analysis
The other half of each sample was fixed in 10% formalin and then decalcified in 30% formic acid for 2 weeks before embedding. Thereafter, the sections of 5 μm thickness were cut horizontally and collected on slides. Slides were stained with H&E for histology evaluation. Each animal and each level had an equal number of typical sections for study. The results were evaluated by three individuals who were blinded to treatments. The cellular density of the scar tissue was measured as reported [28]. The number of fibroblasts per 40× magnification field was counted and the average was recorded. This was repeated for three fields (one from the middle of laminectomy area, two from the margins on each side). The average number of fibroblasts for these three fields was then recorded. The cell counting results for each group was expressed in cell number per millimeter square. Moreover, the cross-sectional area of epidural scar and spinal canal was calculated using a PC-Image analysis system (LEICA MTLA, Leica Ltd, Germany). The scar index was defined as the ratio of the area of epidural scar normalized to the area of spinal canal, which was used to indicate the severity of epidural fibrosis. In addition, the areas of newly formed bone originated from the vertebral lamina in laminectomy defect sites were compared among groups.
Statistical analyses
All data were analyzed using SPSS 13.0 software and statistically significant values were defined as P < 0.05. A Wilcoxon–Mann–Whitney test was used to determine significant difference in grades of gross scar formation and adhesion tenacity. The one-way analysis of variance (ANOVA) and Bonferroni test was used to check the significant difference in scar index, fibroblasts number, and new bone formation.
Results
Histology of AM
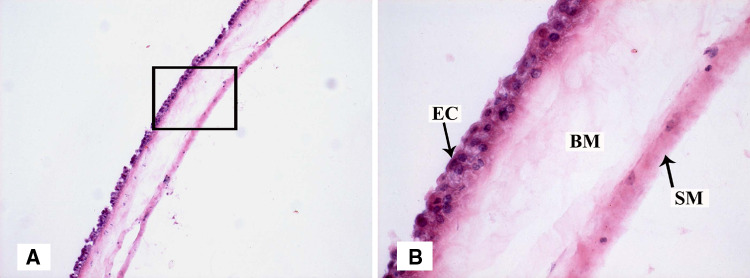
The AM comprised the innermost layer of the placenta. It consisted of a single layer of cuboidal epithelial cells, a thick basement membrane, and an avascular stromal matrix, which was loosely attached to the chorion (Fig. 1).
Fig. 1.
a Histology observation of AM by H&E staining (×100). b Magnified view of the black rectangle frame from a, demonstrating anatomic structure of AM (×400; EC epithelial cell, BM basement membrane, SM stromal matrix)
Clinical observation
All animals recovered from the surgical procedure and could walk within 1 day. There were no cases of infection and incisions healed within 1 week in all groups. The animals were ambulatory and healthy at the time of sacrifice. There was no obvious neurological deficit.
Gross observation
At 1 week postoperatively, grading scores of scar amount in all groups showed no significant difference (P > 0.05). After 6 weeks, abundant epidural scar was observed in non-treatment group. Because of severe adhesions between surrounding muscles and dura mater, dissection was difficult and time-consuming. Removal of scar tissue could easily lead to injuries to dura mater and nerve roots. Grading scores ranged from 2 to 3 and the mean score was 2.88 ± 0.35. In AFF group, less epidural scar was observed and re-exposure of dura mater and nerve roots was easy. The mean score was 0.63 ± 0.74, which was significantly lower than that of non-treatment group (P < 0.05). In FAM group, the dense scar was found between dura mater and overlying muscles with grade of 2.13 ± 1.13. There was no significant difference in scar amounts between FAM and non-treatment groups (P > 0.05). In CAM group, a much weaker or nearly absent epidural adhesion was recorded. The white, slightly vascularized membrane was found between dura mater and surrounding muscles to reduce scar intrusion. Furthermore, the CAM layer seldom adhered to the dura mater and was easily removed. Only a layer of fibrous tissue could be found between the CAM layer and dura mater in three samples. The grading score of CAM group was significantly lower than that of non-treatment group while showing no significant difference in comparison with that of AFF group. After 12 weeks, the results showed similar trends and there was still less scar formation in CAM group and AFF group (Table 1). The tenacity was recorded to indicate adhesion severity. At 1 week postoperatively, the tenacity showed no significant difference among groups due to less scar formation. The scores ranged from 0.25 ± 0.46 to 0.75 ± 0.71 in groups. After 6 weeks, the mean scores were 4.38 ± 0.74 and 0.50 ± 0.76 in non-treatment group and AFF group, respectively. The score of FAM group was 3.63 ± 1.51, which was not significantly different from that of non-treatment group (P > 0.05). In contrast, the scar was easily detached off in CAM group with rather lower score of 0.63 ± 0.74. The scar amounts and adhesion tenacity of CAM group were both significantly lower than that of non-treatment group. After 12 weeks, the score increased slowly with 0.88 ± 0.83 in CAM group, which was still significantly lower than those of FAM group and non-treatment group (Table 2).
Table 1.
Grading score of scar amount among groups (n = 8, mean ± SD)
| Group | 1 week | 6 weeks | 12 weeks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean score | P a | P b | Mean score | P a | P b | Mean score | P a | P b | |
| FAM | 0.38 ± 0.52 | 0.26 | 0.63 | 2.13 ± 1.13 | 0.02* | 0.10 | 2.50 ± 0.76 | 0.00* | 0.24 |
| CAM | 0.25 ± 0.46 | 0.54 | 0.32 | 0.5 ± 0.75 | 0.68 | 0.00* | 0.63 ± 0.92 | 0.71 | 0.00* |
| AFF | 0.13 ± 0.35 | 0.63 ± 0.74 | 0.38 ± 0.52 | ||||||
| Non-treatment | 0.50 ± 0.53 | 2.88 ± 0.35 | 2.88 ± 0.35 | ||||||
Scar amount was graded from 0 to 3 to denote none, small, medium, and large
a P value indicates treatment group versus AFF (positive control)
b P value indicates treatment group versus non-treatment group (empty control); * <0.05
Table 2.
Grading score of adhesion tenacity among groups (n = 8, mean ± SD)
| Group | 1 week | 6 weeks | 12 weeks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean score | P a | P b | Mean score | P a | P b | Mean score | P a | P b | |
| FAM | 0.50 ± 0.53 | 0.32 | 0.48 | 3.63 ± 1.51 | 0.00* | 0.34 | 4.13 ± 0.83 | 0.00* | 0.53 |
| CAM | 0.38 ± 0.52 | 0.60 | 0.26 | 0.63 ± 0.74 | 0.68 | 0.00* | 0.88 ± 0.83 | 0.36 | 0.00* |
| AFF | 0.25 ± 0.46 | 0.50 ± 0.76 | 0.63 ± 0.74 | ||||||
| Non-treatment | 0.75 ± 0.71 | 4.38 ± 0.74 | 4.50 ± 0.53 | ||||||
Tenacity was graded from 0 to 5 to denote none, very slight, light, moderate, tenacious, and highly tenacious adhesion
a P value indicates treatment group versus AFF (positive control)
b P value indicates treatment group versus non-treatment group (empty control); * <0.05
Qualitative histology evaluation
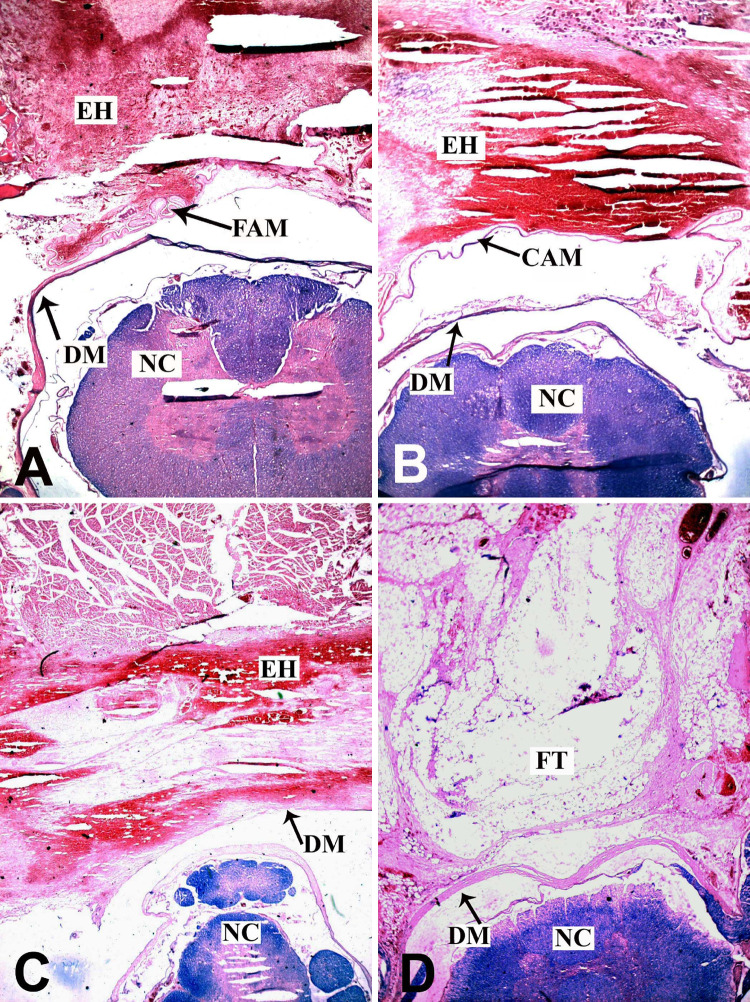
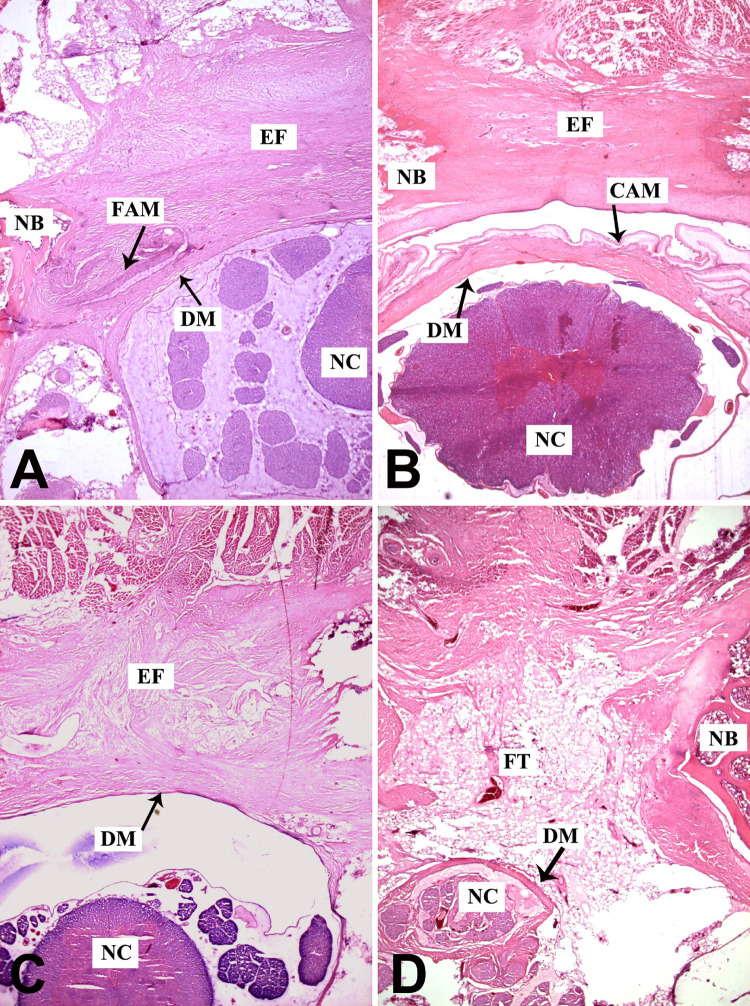
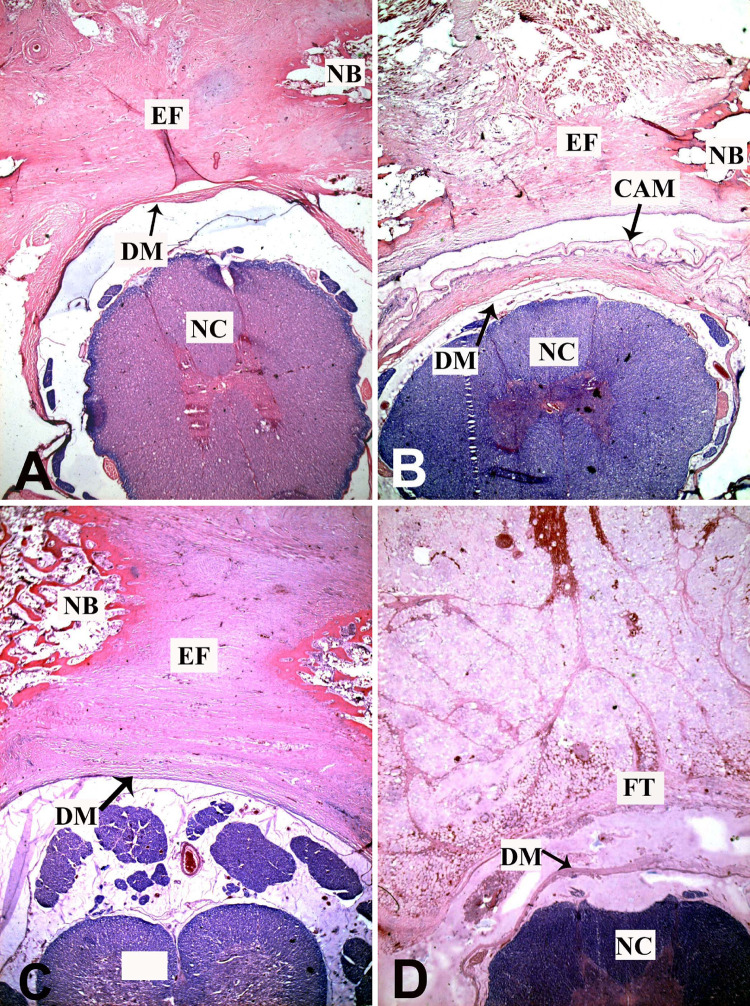
At 1 week postoperatively, epidural hemorrhage was clearly visible in FAM group, CAM group, and non-treatment group. The FAM and CAM acted as mechanical barriers to prevent epidural hemorrhage from intruding into spinal canal. In AFF group, the fat tissue filled the space between laminar edges and dura mater. There were no obvious inflammatory reactions at all laminectomy sites (Fig. 2). At 6 weeks postoperatively, the FAM degraded mostly and only small pieces around laminar edges could be found in FAM group. The epidural fibrosis was abundant and severe adhesion between the surrounding muscles and dura mater was observed. In CAM group, a fibrous membrane was developed underneath the laminar edge and dura mater was free of scar attachment. Only a thin layer of fibrous tissue around the dura was observed. A distinct layer between the scar tissue and dura mater could be discerned which facilitated the exposure in revision surgery. The epidural fibrosis appeared prominently in non-treatment group. The scar adhesion was severer and dense fibrous tissue with populated fibroblasts extended to the nerve roots. In contrast, there was less fibrotic tissue in AFF group. A layer of fat tissue was interposed between dura mater and paraspinal muscles to reduce adhesion (Fig. 3). After 12 weeks, the results showed similar trend. The FAM degraded completely and dense scar tissue thus formed in FAM group. In contrast, the CAM still maintained its integrity, which efficiently reduced the scar formation in spinal canal. With time on, the scar tissue became much denser in non-treatment group and there was still less scar formation in AFF group (Fig. 4).
Fig. 2.
Histology observation of laminectomy site in FAM (a), CAM (b), non-treatment (c), and AFF groups (d) by H&E staining at 1 week postoperatively (×100; CAM cross-linked amniotic membrane, FAM freeze–dried amniotic membrane, EH epidural hemorrhage, DM dura mater, NC neural cord, FT fat tissue)
Fig. 3.
Histology observation of laminectomy site in FAM (a), CAM (b), non-treatment (c), and AFF groups (d) by H&E staining at 6 weeks postoperatively (×100; CAM cross-linked amniotic membrane, FAM freeze–dried amniotic membrane, EF epidural fibrosis, DM dura mater, NC neural cord, FT fat tissue, NB newly formed bone)
Fig. 4.
Histology observation of laminectomy site in FAM (a), CAM (b), non-treatment (c), and AFF groups (d) by H&E staining at 12 weeks postoperatively (×100; CAM cross-linked amniotic membrane, FAM freeze–dried amniotic membrane, EF epidural fibrosis, DM dura mater, NC neural cord, FT fat tissue, NB newly formed bone)
Quantitative histology evaluation
Amount of fibroblasts
The number of fibroblasts showed no significant difference in FAM, CAM, and non-treatment groups at 1 week postoperatively. These groups all demonstrated higher amount of fibroblasts in comparison with AFF group. Then, the fibroblasts number of CAM group remained steady with 833 ± 42 and 879 ± 58 per mm2 at 6 and 12 weeks, respectively, which were significantly lower than those of non-treatment group (P < 0.05). In contrast, the fibroblasts number of FAM group increased steeply with 1,638 ± 46 and 1,720 ± 46 per mm2 at 6 and 12 weeks, respectively. There was no significant difference between FAM and non-treatment groups after 6 weeks (P > 0.05). The AFF group consistently showed a small number of fibroblasts during the whole experiment period (Table 3).
Table 3.
Fibroblasts number per square millimeter in scar tissue among groups (n = 8, mean ± SD)
| Group | 1 week | 6 weeks | 12 weeks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell number | P a | P b | Cell number | P a | P b | Cell number | P a | P b | |
| FAM | 860 ± 55 | 0.00* | 1.00 | 1638 ± 46 | 0.00* | 0.05 | 1720 ± 46 | 0.00* | 0.10 |
| CAM | 838 ± 22 | 0.00* | 0.20 | 833 ± 42 | 0.00* | 0.00* | 879 ± 58 | 0.00* | 0.00* |
| AFF | 113 ± 18 | 145 ± 13 | 150 ± 20 | ||||||
| Non-treatment | 893 ± 21 | 1762 ± 92 | 1781 ± 57 | ||||||
a P value indicates treatment group versus AFF (positive control)
b P value indicates treatment group versus non-treatment group (empty control); * <0.05
Extent of epidural scar
At 1 week postoperatively, the scar indexes showed no significant difference among FAM, CAM, and AFF groups, which were significantly lower than that of non-treatment group. Then, the scar indexes of CAM group increased with 11.23 ± 4.24 and 11.45 ± 3.83% at 6 and 12 weeks, respectively, which were significantly lower than those of non-treatment group (P < 0.05). However, CAM group showed no significant difference in comparison with AFF group (P > 0.05). The scar indexes of FAM group were 18.03 ± 7.55 and 19.35 ± 5.87% at 6 and 12 weeks, respectively. Although the scar indexes were mildly lower than those of non-treatment group (P > 0.05), they were significantly higher than those of AFF group (P < 0.05) (Table 4).
Table 4.
Scar indexes among groups (n = 8, mean ± SD)
| Group | 1 week | 6 weeks | 12 weeks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Scar index (%) | P a | P b | Scar index (%) | P a | P b | Scar index (%) | P a | P b | |
| FAM | 4.05 ± 0.31 | 1.00 | 0.00* | 18.03 ± 7.55 | 0.04* | 0.46 | 19.35 ± 5.87 | 0.01* | 0.22 |
| CAM | 3.90 ± 0.26 | 1.00 | 0.00* | 11.23 ± 4.24 | 0.67 | 0.03* | 11.45 ± 3.83 | 0.40 | 0.01* |
| AFF | 3.25 ± 0.26 | 3.48 ± 0.60 | 3.70 ± 0.73 | ||||||
| Non-treatment | 9.37 ± 1.55 | 26.78 ± 9.38 | 28.43 ± 8.28 | ||||||
a P value indicates treatment group versus AFF (positive control)
b P value indicates treatment group versus non-treatment group (empty control); * <0.05
Amount of newly formed bone
After 6 weeks, the newly formed bone originated from the vertebral lamina was observed in FAM, CAM, and non-treatment groups, which gradually decreased the size of laminectomy defects. In contrast, less newly formed bone was visible in AFF group. The areas of newly formed bone were 8.80 ± 3.58, 8.50 ± 2.73 and 9.78 ± 2.43, 10.07 ± 2.70 mm2 in FAM and CAM groups at 6 and 12 weeks, respectively. They were both significantly higher than that of AFF group (P < 0.05). However, there was no significant difference between theses two groups (P > 0.05) (Table 5).
Table 5.
Quantitative evaluation of newly formed bone among groups (n = 8, mean ± SD)
| Group | 6 weeks | 12 weeks | ||||
|---|---|---|---|---|---|---|
| Bone area (mm2) | P a | P b | Bone area (mm2) | P a | P b | |
| FAM | 8.80 ± 3.58 | 0.04* | 1.00 | 8.50 ± 2.73 | 0.04* | 1.00 |
| CAM | 9.78 ± 2.43 | 0.01* | 1.00 | 10.07 ± 2.70 | 0.01* | 1.00 |
| AFF | 3.28 ± 0.76 | 3.65 ± 0.83 | ||||
| Non-treatment | 8.00 ± 1.70 | 10.55 ± 1.46 | ||||
a P value indicates treatment group versus AFF (positive control)
b P value indicates treatment group versus non-treatment group (empty control); * <0.05
Discussion
The study demonstrates that CAM can be implanted as a physical barrier to reduce epidural fibrosis and scar adhesion without affecting wound healing. The results provide evidence that CAM is a promising biomaterial to reduce epidural fibrosis for future clinical application.
The formation of postoperative epidural fibrosis is an inevitable result of laminectomy. Although the relationship between epidural fibrosis and clinical symptoms of FBSS has not been unequivocally proven, as many as 24% of all FBSS cases may be attributed to epidural scar adhesion [27].The mechanical tethering of nerve roots, or the dura, by the excessive formation of epidural fibrosis may be a contributing factor for a significant subset of patients suffering from FBSS [3, 26, 27]. However, several authors have reported that there are no important differences between symptomatic and asymptomatic patients in fibrosis demonstrated by computed tomography (CT) and magnetic resonance imaging (MRI). They concluded that the degree of fibrosis was not related to recurrent symptoms following lumbar disc surgery [1, 23]. But at least epidural scarring can make a reoperation much more difficult, increasing the risks of dural tears, and nerve root injury. There is no way to predict the patients in whom symptomatic epidural fibrosis will develop, and once it occurs there is no effective treatment. The main reasons for scar formation include epidural fat destruction, hematoma, and paraspinal muscular fiber invasion. Regardless of the exact mechanisms of epidural scar adhesion, the crucial issue is to prevent the fibroblasts from migrating into the exposed dura in the early healing phase. The interposition of a physical barrier to limit cell migration is considered an effective strategy to reduce scar formation [30].
In this study, AM is selected as a barrier for its biocompatibility, absorbability, and easy manipulation. It is hypothesized to reduce inflammation, inhibit vascularization, and limit or prevent adhesion [31]. After implantation of FAM and CAM, there was no evidence of inflammatory reactions at 1, 6, and 12 weeks postoperatively. Few inflammatory cells (lymphocytes, neutrocytes, and histiocytes) could be observed adjacent to the dura mater and peripheral nerve roots. This was attributed to the immune-privileged properties of AM [15]. To prolong degradation time, we chose 0.25% GA as cross-linking reagent to prepare CAM instead of gamma ray, which resulted in the scission of collagen chains and decreased tensile properties. The AM is composed of a fibrous mesh structure from an assembly of collagen fibers. It is possible that cross-linking takes place in the interior of the fiber assembly without impairing the mesh structure [16]. The CAM degraded more slowly in comparison with FAM, which kept its morphology to prevent intrusion of fibrous tissue even after 12 weeks. In contrast, the FAM degraded faster and only some residuals could be found after 6 weeks (Fig. 2). At 12 weeks postoperatively, the FAM degraded completely and scar tissue filled the epidural cavity.
Nowadays, the AFF graft is one of the most commonly used methods in daily practice. Its main advantages include efficiency, availability, and compatibility. Various studies describe AFF graft to be superior to other interposition membranes and to have a long survival [13]. Although the fat graft remains the most commonly used material clinically, it has been associated with seroma formation, scar dimpling, limited laminectomy area coverage, and the migration of fat graft, which have been implicated as the causes of several cases of cauda equina syndrome [11, 21, 24]. The thickness and quality are important factors of the AFF graft. Till now, there is no guideline existed to determine the size of graft. It is known that the AFF graft will shrink to 30–50% of its original size by fibrotic and degenerative processes. Based on this, some authors suggest the thickness of graft should be 5 mm or thinner [11]. Others recommend the thickness of between 1 and 1.5 cm, which can protect the dura sufficiently and allow for some shrinkage without the formation of fibrous tissue [21]. The size of the AFF graft will depend on the size of the dural exposure. Graft which is smaller than the bony defect will not serve the purpose. But if it is too big, the graft may be infolded into the canal and might increase its thickness, causing dural compression [11]. Furthermore, the body mass indexes (BMI) of most Asian patients are usually lower than those of western patients. Some patients are even underweight. For thin patients who need revision spine surgery or multiple-level laminectomy, the harvested fat graft in operation may not big enough to meet the requirements for reducing epidural adhesions.
The AM has been used clinically to treat variable diseases such as non-healing skin ulcers, vaginal atresia, and severe ocular surface disease [9, 35]. These can confirm its biocompatibility and safety. For mass production of AM in tissue bank, the price is affordable. AM can be fabricated into different size and shape to meet the needs of operation. It can be used as an alternative of fat graft in patients who undergo spinal decompression, especially those with long-segment laminectomy or thin patients. In this study, AFF was used to reduce epidural fibrosis as positive control. The interposed graft significantly reduced epidural scar formation. In CAM group, few inflammatory cells and chronic reactions were observed due to excellent biocompatibility. The CAM acted as a roofing structure to separate the dura mater and dense scar tissue. The dura could remain relatively free from the overlying scar. A thin layer of fibrous tissue around dura mater and nerve roots were observed in three samples. It was attributed to the fibroblasts infiltration through the gap between CAM and lamina edges. Hence, the careful placement of CAM was of great importance. In comparison with CAM, the degraded FAM could not prevent the fibrosis tissue from intruding the epidural space after 6 weeks.
The fibroblasts may arise from the paraspinal musculature, ligamentum flavum, posterior longitudinal ligament, and the annulus fibrosis [17]. The free fat graft, by walling off the overlying muscles, may be more effective in limiting cellular trafficking and vascular in-growth into the epidural space. Therefore, the AFF group showed rather lower fibroblasts number in comparison with other groups. In CAM group, the number of fibroblasts was also at low level due to the intact mechanical barrier of implanted CAM. In contrast, the fibroblasts immigrated through the degraded FAM and proliferated robustly around dura in FAM group. The FAM showed rather weaker anti-adhesion capability. Although the fibroblasts number of CAM group was significantly higher than that of AFF group, the scar index showed no significant difference in these two groups. Both could efficiently reduce epidural scar formation (Tables 3, 4).
New bone formation from the margins of laminectomy defect was observed at 6 and 12 weeks postoperatively. There was no significant difference in the areas of newly formed bone among FAM, CAM, and non-treatment groups. These three groups demonstrated more newly formed bone tissue in comparison with AFF group (Table 5). The results were correlated with other reports [12, 29]. It is uncertain whether FAM or CAM can cause significant new bone formation in human spine because of species differences in osteogenicity. The role of the laminectomy defect healing in the pathological changes of spinal cord compression needs further study.
In conclusion, this study suggests that CAM is an effective anti-scar adhesion material, which can decrease adhesion tenacity and scar amount in epidural space. The findings also indicate the potentials of applying CAM in humans to minimize postoperative complications.
References
- 1.Annertz M, Jonsson B, Stromqvist B, Holtas S. No relationship between epidural fibrosis and sciatica in the lumbar postdiscectomy syndrome. Spine. 1995;20(4):449–453. doi: 10.1097/00007632-199502001-00007. [DOI] [PubMed] [Google Scholar]
- 2.Bertagnoli R, Yue JJ, Fenk-Mayer A, Eerulkar J, Emerson JW. Treatment of symptomatic adjacent-segment degeneration after lumbar fusion with total disc arthroplasty by using the prodisc prosthesis: a prospective study with 2-year minimum follow up. J Neurosurg Spine. 2006;4(2):91–97. doi: 10.3171/spi.2006.4.2.91. [DOI] [PubMed] [Google Scholar]
- 3.Coskun E, Süzer T, Topuz O, Zencir M, Pakdemirli E, Tahta K. Relationships between epidural fibrosis, pain, disability, and psychological factors after lumbar disc surgery. Eur Spine J. 2000;9(3):218–223. doi: 10.1007/s005860000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duggal N, Mendiondo I, Pares HR, Jhawar BS, Das K, Kenny KJ, Dickman CA. Anterior lumbar interbody fusion for treatment of failed back surgery syndrome: an outcome analysis. Neurosurgery. 2004;54(3):636–643. doi: 10.1227/01.NEU.0000108423.87889.9E. [DOI] [PubMed] [Google Scholar]
- 5.Einhaus SL, Robertson JT, Dohan FC, Jr, Wujek JR, Ahmad S. Reduction of peridural fibrosis after lumbar laminotomy and discectomy in dogs by a resorbable gel (ADCON-L) Spine. 1997;22(13):1440–1446. doi: 10.1097/00007632-199707010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Fischgrund JS. Perspectives on modern orthopaedics: use of Adcon-L for epidural scar prevention. J Am Acad Orthop Surg. 2000;8(6):339–343. doi: 10.5435/00124635-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Fritsch EW, Heisel J, Rupp S. The failed back surgery syndrome: reasons, intraoperative findings, and long-term results: a report of 182 operative treatments. Spine. 1996;21(5):626–633. doi: 10.1097/00007632-199603010-00017. [DOI] [PubMed] [Google Scholar]
- 8.Görgülü A, Simşek O, Cobanoğlu S, Imer M, Parsak T. The effect of epidural free fat graft on the outcome of lumbar disc surgery. Neurosurg Rev. 2004;27(3):181–184. doi: 10.1007/s10143-003-0310-9. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa T, Mizoguchi M, Haruna K, Mizuno Y, Muramatsu S, Suga Y, Ogawa H, Ikeda S. Amnia for intractable skin ulcers with recessive dystrophic epidermolysis bullosa: report of three cases. J Dermatol. 2007;34(5):328–332. doi: 10.1111/j.1346-8138.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 10.Hieb LD, Stevens DL. Spontaneous postoperative cerebrospinal fluid leaks following application of anti-adhesion barrier gel: case report and review of the literature. Spine. 2001;26(7):748–751. doi: 10.1097/00007632-200104010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Imran Y, Halim Y. Acute cauda equina syndrome secondary to free fat graft following spinal decompression. Singapore Med J. 2005;46(1):25–27. [PubMed] [Google Scholar]
- 12.Jacob A, Faddis BT, Chole RA. MeroGel hyaluronic acid sinonasal implants: osteogenic implications. Laryngoscope. 2002;112(1):37–42. doi: 10.1097/00005537-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Kanamori M, Kawaguchi Y, Ohmori K, Kimura T, Tsuji H, Matsui H. The fate of autogenous free-fat grafts after posterior lumbar surgery: Part 2. Magnetic resonance imaging and histologic studies in repeated surgery cases. Spine. 2001;26(20):2264–2270. doi: 10.1097/00007632-200110150-00019. [DOI] [PubMed] [Google Scholar]
- 14.Kato T, Haro H, Komori H, Shinomiya K. Evaluation of hyaluronic acid sheet for the prevention of postlaminectomy adhesions. Spine J. 2005;5(5):479–488. doi: 10.1016/j.spinee.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42(7):1539–1546. [PubMed] [Google Scholar]
- 16.Kumar TR, Shanmugasundaram N, Babu M. Biocompatible collagen scaffolds from a human amniotic membrane: physicochemical and in vitro culture characteristics. J Biomater Sci Polym Ed. 2003;14(7):689–706. doi: 10.1163/156856203322274941. [DOI] [PubMed] [Google Scholar]
- 17.LaRocca H, MacNab I. The laminectomy membrane: studies in its evolution, characteristics, effects and prophylaxis in dogs. J Bone Joint Surg Br. 1974;56B(3):545–550. [PubMed] [Google Scholar]
- 18.Lee CH, Cho DS, Jin SC, Kim SH, Park DB. Usefulness of silicone elastomer sheet as another option of adhesion preventive material during craniectomies. Clin Neurol Neurosurg. 2007;109(8):667–671. doi: 10.1016/j.clineuro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Boutrand JP, Bittoun J, Tadie M (2002) A collagen-based sealant to prevent in vivo reformation of epidural scar adhesions in an adult rat laminectomy model. J Neurosurg 97(1)(Suppl):69–74 [DOI] [PubMed]
- 20.MacKay MA, Fischgrund JS, Herkowitz HN, Kurz LT, Hecht B, Schwartz M (1995) The effect of interposition membrane on the outcome of lumbar laminectomy and discectomy. Spine 20(16):1793–1796 [DOI] [PubMed]
- 21.Mayer PJ, Jacobsen FS. Cauda equina syndrome after surgical treatment of lumbar spinal stenosis with application of free autogenous fat graft. J Bone Joint Surg Am. 1989;71(7):1090–1093. [PubMed] [Google Scholar]
- 22.Miyamoto K, Masuda K, Inoue N, Okuma M, Muehleman C, An HS. Anti-adhesion properties of a thrombin-based hemostatic gelatin in a canine laminectomy model: a biomechanical, biochemical, and histologic study. Spine. 2006;31(4):E91–E97. doi: 10.1097/01.brs.0000199902.80607.ce. [DOI] [PubMed] [Google Scholar]
- 23.Nygaard QP, Jacobsen EA, Solberg T, Kloster R, Dullerud R. Nerve root signs on postoperative lumbar MR imaging. A prospective cohort study with contrast enhanced MRI in symptomatic and asymptomatic patients one year after microdiscectomy. Acta Neurochir (Wien) 1999;141(6):619–622. doi: 10.1007/s007010050351. [DOI] [PubMed] [Google Scholar]
- 24.Prusick VR, Lint DS, Bruder WJ. Cauda equina syndrome as a complication of free epidural fat-grafting: a report of two cases and a review of the literature. J Bone Joint Surg Am. 1988;70(8):1256–1258. [PubMed] [Google Scholar]
- 25.Rennekampff HO, Dohrmann P, Fory R, Fändrich F. Evaluation of amniotic membrane as adhesions prophylaxis in a novel surgical gastroschisis mode. J Invest Surg. 1994;7(3):187–193. doi: 10.3109/08941939409018285. [DOI] [PubMed] [Google Scholar]
- 26.Robertson JT. Role of peridural fibrosis in the failed back: a review. Eur Spine J. 1996;5(Suppl 1):S2–S6. doi: 10.1007/BF00298565. [DOI] [PubMed] [Google Scholar]
- 27.Ross JS, Robertson JT, Frederickson RC, Petrie JL, Obuchowski N, Modic MT, de Tribolet N. Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. ADCON-L European Study Group. Neurosurgery. 1996;38(4):855–861. doi: 10.1097/00006123-199604000-00053. [DOI] [PubMed] [Google Scholar]
- 28.Sabuncuoğlu H, Bavbek M, Sabuncuoğlu B, Gadelha E, Köse K, Preul M. Attenuation of postlaminectomy epidural fibrosis with monoclonal antibodies against intercellular adhesion molecule-1 and CD-18. Spine J. 2007;7(4):459–465. doi: 10.1016/j.spinee.2006.06.398. [DOI] [PubMed] [Google Scholar]
- 29.Shih HN, Fang JF, Chen JH, Yang CL, Chen YH, Sung TH, Shih LY. Reduction in experimental peridural adhesion with the use of a crosslinked hyaluronate/collagen membrane. J Biomed Mater Res B Appl Biomater. 2004;71(2):421–428. doi: 10.1002/jbm.b.30106. [DOI] [PubMed] [Google Scholar]
- 30.Tatsui CE, Martinez G, Li X, Pattany P, Levi AD. Evaluation of DuraGen in preventing peridural fibrosis in rabbits. J Neurosurg Spine. 2006;4(1):51–59. doi: 10.3171/spi.2006.4.1.51. [DOI] [PubMed] [Google Scholar]
- 31.Tosi GM, Massaro-Giordano M, Caporossi A, Toti P. Amniotic membrane transplantation in ocular surface disorders. J Cell Physiol. 2005;202(3):849–851. doi: 10.1002/jcp.20181. [DOI] [PubMed] [Google Scholar]
- 32.Von Versen-Höynck F, Syring C, Bachmann S, Möller DE. The influence of different preservation and sterilisation steps on the histological properties of amnion allografts-light and scanning electron microscopic studies. Cell Tissue Bank. 2004;5(1):45–56. doi: 10.1023/B:CATB.0000022276.47180.96. [DOI] [PubMed] [Google Scholar]
- 33.Welch WC, Thomas KA, Cornwall GB, Gerszten PC, Toth JM, Nemoto EM, Turner AS (2002) Use of polylactide resorbable film as an adhesion barrier. J Neurosurg 97(4)(Suppl):413–422 [DOI] [PubMed]
- 34.Winkelmüller M, Winkelmüller W. Long-term effects of continuous intrathecal opioid treatment in chronic pain of nonmalignant etiology. J Neurosurg. 1996;85(3):458–467. doi: 10.3171/jns.1996.85.3.0458. [DOI] [PubMed] [Google Scholar]
- 35.Zafar M, Saeed S, Kant B, Murtaza B, Dar MF, Khan NA. Use of amnion in vaginoplasty for vaginal atresia. J Coll Physicians Surg Pak. 2007;17(2):107–109. [PubMed] [Google Scholar]
- 36.Zohar Y, Talmi YP, Finkelstein Y, Shvili Y, Sadov R, Laurian N. Use of human amniotic membrane in otolaryngologic practice. Laryngoscope. 1987;97(8Pt1):978–980. [PubMed] [Google Scholar]
- 37.Zou X, Li H, Egund N, Lind M, Bünger C. Inhibition of spinal fusion by use of a tissue ingrowth inhibitor. Eur Spine J. 2004;13(2):157–163. doi: 10.1007/s00586-003-0645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]