Abstract
The phenomenon of spinal deformity in the pinealectomized chicken has led researchers to postulate a disturbance of melatonin activity as a potential cause of adolescent idiopathic scoliosis (AIS). More recently, structural differences between curves seen in this model and those seen in scoliosis have been highlighted suggesting the deformities observed are not as similar as first thought. We examined melatonin levels, and the radiological and histological characteristics of scoliosis after pinealectomy in chickens. They underwent pinealectomy (P) at 2 days of age, sham surgery (S) or served as controls (C). Mean melatonin levels were 32.9 pmol/L (P), 175 pmol/L (S) and 227.3 pmol/L (C). Scoliosis developed in 75% of chickens after pinealectomy and 38% after a sham procedure. Nineteen percent of unoperated controls also developed scoliosis. A lower melatonin level was associated with the development of scoliosis (p ≤ 0.001), but exceptions were seen with levels up to 265 pmol/L observed in one case. Most of the curves occurring spontaneously and after sham surgery and almost half after pinealectomy were short angular curves: distinct from those resembling idiopathic scoliosis. These occur over one or two segments and are characterized by marked apical wedging, frequently associated with subluxation or dislocation. The intervertebral joint in the chicken is more like a synovial joint histologically than an intervertebral disc. This study highlights important differences between the chicken and the human, and between their respective spinal deformities. Caution is advised when drawing conclusions regarding the pathogenesis of AIS from this model.
Keywords: Scoliosis, Melatonin, Pineal gland, Histology, Chickens
Introduction
The phenomenon of spinal deformity after pinealectomy in the chicken [20] has led researchers to postulate the disturbance of melatonin activity as a potential cause of Adolescent Idiopathic Scoliosis (AIS) [14]. Early reports were of an incidence of scoliosis after pinealectomy that was as high as 100% [14]. Subsequent investigations have found the incidence to be as low as 50% [5, 6, 18, 21, 22, 24]. Factors proposed to account for this difference includes: the age at which the surgery is performed [10], the structures resected at pinealectomy [4], and the length of follow-up [13].
Melatonin was implicated in the aetiology of the deformity, as it is the most recognized product of the pineal. However, none of the early experimental evidence for this [15, 16] has been independently reproduced [1, 3, 8, 9]. Constant light treatment (which suppresses melatonin secretion) has not resulted in scoliosis in chickens [5], and so it remains unclear whether melatonin levels have a role in the pathogenesis of the deformity or not.
Initial reports commented on anatomical similarities between the deformity seen in the chicken and AIS, such as rotation of the apical vertebra [12, 14, 24]. More recently, anatomical differences have been highlighted. It has been noted that the chicken curves have a thoracolumbar apex and an oblique take off from the pelvis in association with iliac hypoplasia [6]. These features are not characteristic of AIS. A further anatomical difference that has been noted is that vertebral wedging is prominent early in the development of curves in the chicken [1] and late in the human.
We have conducted histologically confirmed pinealectomy in the chicken to further examine the relationship between the surgery, subsequent melatonin levels and the anatomical features of the resultant scoliosis.
Materials and methods
The experimental protocol was approved by our institutional Animal Ethics Committee. Pinealectomy or sham surgery was performed on 67 two-day-old Steggles chicks (46 pinealectomy, 21 sham operations). Thirty-one chicks were obtained concurrently and served as unoperated controls.
The pinealectomy was performed under inhalational anaesthesia (isoflurane 2–4% in Oxygen) through an endotracheal tube with positive pressure ventilation. The technique used has been described previously [14, 20, 25]. A midline longitudinal incision was made through the scalp centred on the confluence of the sinuses, visible through the cartilaginous skull. Operating with the aid of a dissecting microscope a distally based flap of cartilaginous skull with adherent dura was created with a scalpel. This was folded back to expose the pineal within the confluence of the sinuses adherent to the parietal dura. The pineal and attached stalk were divided from their attachment to the posterior aspect of the brainstem and examined under the dissecting microscope for completeness prior to being sent for histological confirmation by frozen section. The flap of skull was replaced and the skin was sutured.
Sham surgery was identical, except that after raising the skull flap, it was replaced without removal of the pineal and its stalk.
Animals recovered promptly from the procedure and were kept in a controlled light environment (12 h on:12 h off) for the duration of the experiment. Each group (pinealectomy, sham and control) was divided into three for survival to 2, 4 and 6 weeks, respectively. The remainder where the group was not divisible into three were included in the group surviving to 6 weeks.
Euthanasia was by halothane overdose in the mid dark cycle, performed under infrared lighting to ensure that blood samples taken would reflect peak nocturnal melatonin secretion. Selected birds’ plasma was collected in heparinized syringes by intracardiac aspiration immediately post mortem (Table 1). These were sent for melatonin estimation by radioimmunoassay.
Table 1.
Cases undergoing melatonin estimation
| 2 weeks | 4 weeks | 6 weeks | |
|---|---|---|---|
| Pinealectomy | 4 | 4 | 3 |
| Sham | 1 | 2 | 2 |
| Control | 6 | 6 | 6 |
Anteroposterior X-rays of the spine were taken fortnightly and post mortem, after which the spines were removed and processed for histological examination. The brain was fixed in formalin prior to midsagittal section to permit examination of the pinealectomy site with the aid of a dissecting microscope. A mid sagittal section was also assessed histologically for residual pineal tissue.
Radioimmunoassay utilised reagents obtained from Buhlmann Laboratories (Allschwil, Switzerland), and was performed as previously described for human plasma [23]. Sensitivity of the assay was 4.3 pM. All samples were assayed in a single batch with intra-assay coefficients of variation <10% across the range of the standard curve.
X-rays were reviewed, recording the presence or absence of a scoliosis, the location of the apex and end vertebrae of each curve along with the side of the convexity, the number of curves present (single, double or triple) and the presence or absence of an oblique thoracolumbar take off. Each curve was classified as progressive, stable or regressive depending on whether it increased, remained the same or decreased with respect to the X-ray taken 2 weeks previously.
Statistical analysis (unpaired t test, ANOVA and chi square analysis) was performed using GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego, California, USA, http://www.graphpad.com.
Results
Ten birds died during the course of the experiment (six in the pinealectomy group and four in the control group). This left 40 in the pinealectomy group, 21 in the sham operation group and 27 control chickens.
Scoliosis
The overall incidence of scoliosis was 30 (75%) in the pinealectomy group, 8 (38%) in the sham operation group and 5 (19%) in the control group (Table 2).
Table 2.
Curve type following surgery (χ2, p < 0.001)
| Idiopathic scoliosis | Angular scoliosis | No scoliosis | |
|---|---|---|---|
| Pinealectomy | 17 | 13 | 10 |
| Sham | 1 | 7 | 13 |
| Control | 1 | 4 | 22 |
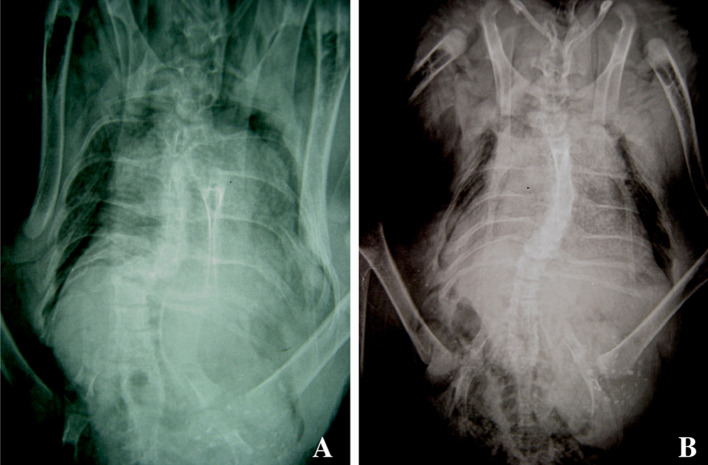
In this, 14 (33%) were single curves, 21 (49%) were double curves and 8 (19%) were triple curves. In 11 (79%) of the single curves, 13 (62%) of the double curves and 4 (50%) of the triple curves: the lowest curve was convex to the right. Twenty-nine (67%) birds with scoliosis had an oblique thoracolumbar take off. Many of the curves were unlike idiopathic curves. These curves had severe wedging of the apical vertebra (Fig. 3) which was often subluxed or dislocated from adjacent vertebrae (Fig. 1a). These were termed angular curves: occurring over a short segment (three or less vertebra). They were differentiated from the idiopathic curves (Fig. 1b) in all analyses.
Fig. 3.
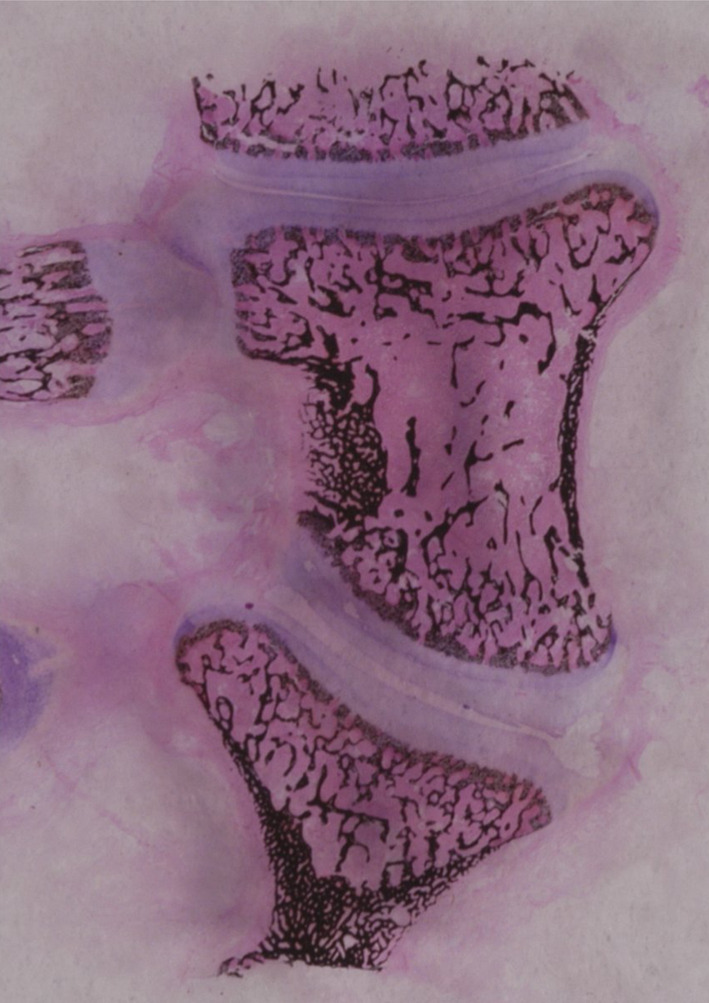
Vertebral wedging seen at the apex in angular curves suggests asymmetric growth
Fig. 1.
a X-ray of short angular curve showing severe wedging at the apex with subluxation of one vertebra on another. b X-ray of smooth curve, analogous to idiopathic scoliosis
The spontaneously occurring curves in the control group and those occurring in the sham group were predominantly angular in type (80 and 88%, respectively). The curves occurring in the pinealectomy group were more evenly distributed with a majority (57%) being idiopathic in type (Table 2). There was no significant difference in the frequency of each curve type in either age group.
Twenty-eight curves (65%) progressed throughout the observation period. Six curves (14%) decreased in size and nine curves (21%) remained stable.
A statistically significant increase was seen in the incidence of scoliosis after pinealectomy when compared with sham surgery and controls. This held true whether angular and idiopathic curves were considered together, or separately. The incidence after sham surgery appeared to be greater than the natural incidence, but this was not statistically significant (χ2, p = 0.30).
Melatonin levels
Similar results were found in all age groups and the results have therefore been pooled for presentation. Histological examination confirmed the removal of the pineal gland and its stalk in the surgical specimen, and the absence of residual pineal tissue in the brain stem post mortem.
Plasma melatonin levels were diminished, but not abolished, by pinealectomy (32.9 pmol/L, SE 8.2). They were diminished to a lesser degree after the sham operation (175 pmol/L, SE 54.8). Control birds had a mean level of 227.3 pmol/L (SE 37.2). The difference between melatonin levels in the pinealectomy group and the control group was significant (t test, p = 0.01). But the difference between levels in the experimental and sham groups (t test, p = 0.3) and the sham and control groups (p = 0.2) was not significant.
Scoliosis was associated with diminished melatonin levels (Table 3). But not all birds with scoliosis had diminished levels. The highest level recorded in a bird with scoliosis was 265.6 pmol/L.
Table 3.
Melatonin level (pmol/L) according to curve pattern (Anova, p < 0.001)
| Number | Mean | SD | |
|---|---|---|---|
| Idiopathic curve | 7 | 102.7 | 83.6 |
| Angular curve | 7 | 107.8 | 157.1 |
| No curve | 20 | 192.7 | 162 |
The difference between melatonin levels in birds with scoliosis and controls was statistically significant only when angular and idiopathic curves were considered together (p = 0.048), but not when idiopathic (p = 0.088) or angular (p = 0.25) curves were considered individually.
There was no difference in the melatonin levels of birds with idiopathic curves and those with angular curves (p = 0.94).
Histology
Verification of completeness of the pinealectomy including removal of the stalk was best achieved by dissecting microscopy of the specimen post operatively, and of the operative site at post mortem. Histological examination of the operative specimen confirmed its pineal histology, and histology of the operative site confirmed the absence of the pineal and stalk after pinealectomy.
The stalk and pineal were present after sham surgery but no assessment of cytological detail was performed to enable us to comment on its viability. At operation, the sham technique was seen to stretch the stalk, which must partially damage it to a variable degree. Section of the stalk has been shown to reduce melatonin levels to a degree that is equivalent to pinealectomy [2]. It is also the critical step of the pinealectomy procedure that results in an increased incidence of scoliosis [4].
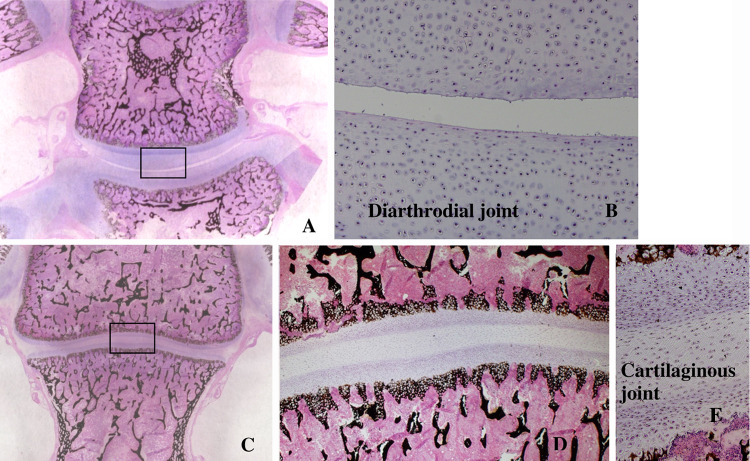
The chicken intervertebral joint is histologically quite different to the human disc. It lacks an annulus fibrosus and nucleus pulposus (Fig. 2).
Fig. 2.
Photomicrographs (Von Kossa, H&E) showing the two types of intervertebral joint in the chicken. a is a mobile diarthrodial type joint (×2). The central part of the joint (marked by a rectangle) is b (×10). c is a stiff cartilaginous joint without a cleft (×2). The central rectangle is shown at higher magnification at d (×5) and e (×10)
Two types of intervertebral joint were observed. The first was a mobile segment similar in appearance to a diarthrodial joint (Fig. 2a). The vertebral end plate was covered by hyaline cartilage with a well-formed intervening joint space and capsule, not unlike a synovial interphalangeal joint. In the second type, the endplates were similarly covered by hyaline cartilage but there was no intervening joint space. This is similar to what is seen at the costo-chondral junction (Fig. 2b). Both appearances were observed in cases from each of the age groups: 2, 4 and 6 weeks.
Discussion
A natural incidence of scoliosis in chickens has not been previously reported in the literature on the pinealectomized chicken model. But references to both an inbred predisposition and a natural incidence of scoliosis exist in the veterinary literature [19]. It would be interesting to know if naturally occurring curves are associated with depressed melatonin levels. But unfortunately we only assayed melatonin levels in a selection of the chickens, and none of these happened to have naturally occurring curves.
In common with AIS, scoliosis in this model seems to have a multifactorial aetiology. This has been suggested previously [4]. In this study, scoliosis can occur without pinealectomy. But pinealectomy increases its incidence in a half to two-thirds of cases. A further factor that casts doubt on pinealectomy as a sole cause of scoliosis is the fact that scoliosis after pinealectomy has not been reliably reproduced in other species [7, 17].
In a previous series, sham surgery has been found to be ineffective in producing scoliosis [4]. Our findings are, strictly speaking, in agreement with this as the increase in scoliosis after sham surgery compared with controls was not statistically significant. However, our results suggest that sham surgery does introduce an insult that is sufficient to both suppress melatonin and produce scoliosis. We do not understand why a similar effect has not been reported by other groups after sham surgery unless their technique did not involve dural reflection to expose the pineal. We found it impossible to separate the cartilaginous skull from the parietal dura and so they were reflected together as a distally based flap. This would necessarily stretch the pineal stalk and result in a postoperative haematoma equivalent to that after a pinealectomy.
We concur with previous authors [15] that pinealectomy does not abolish melatonin levels. There is a wide scatter of melatonin level results in each of the groups in our study. This has also been true of other studies [15, 25]. These findings, together with the fact that melatonin suppression alone without pinealectomy is not associated with an increased incidence of scoliosis [5], and that some birds with scoliosis had quite high (normal) melatonin levels, weaken the case for melatonin deficiency as a sole cause of curve production. It is possible that melatonin deficiency is part of a multifactorial aetiology, or that both melatonin deficiency and scoliosis are effects of the surgery, and that the depressed melatonin levels play no role in the aetiology of the deformity.
Some of the features associated with the curves seen in this model are similar to what is observed in AIS. We observed a predominance of curves that were convex to the right, at rates similar to those previously reported for this model [11, 12]. The right sided predominance is of the same order of magnitude as those quoted for AIS [26]. The rate of spontaneous resolution observed in this series is similar to that previously reported [24]. This is rare in AIS and is mostly seen in the infantile age group (1–3 years) where there is significant remaining growth potential. A similar phenomenon is presumably at work in this model as chickens reach sexual maturity at 6 weeks.
There are, however, important differences between scoliosis in the chicken and the curves seen in AIS. One of the differences already referred to is that the deformity is predominantly at the thoraco–lumbar junction, associated with an oblique take off of the mobile spine from the pelvis [6]. We are able to confirm this finding. We did not, however, find it to be true of all curves: a third were thoracic in location with no thoracolumbar component.
A difference that has not been commented on before is the high proportion of short, angular curves. These comprised half of the curves seen after pinealectomy in this study. They are characterized by marked vertebral wedging at the apex. Frequently there is subluxation or dislocation of one vertebra on another.
We do not know whether the angular curves are an extreme end of the spectrum of scoliosis, or whether they have a separate dysplastic aetiology, analogous to congenital scoliosis. The vertebral wedging suggests a disorder of formation or asymmetrical growth arrest (Fig. 3).
Both angular and idiopathic curves are significantly more common after pinealectomy and are associated with lower melatonin levels, suggesting similar aetiologies. They were similarly distributed in each of the age groups in this study.
The distinction between smooth curves and shorter angular curves has not been made before in this model. The natural incidence is dominated by the angular curves. While idiopathic curves become equally prevalent after pinealectomy.
The most significant difference between curves in this model and those seen in humans is that the chicken spine lacks an intervertebral disc. In its place is a synovial joint, or in some cases a cartilage joint without a cleft. The absence of a joint has been noted previously [27] in 3-day-old chickens. We have observed this to persist to skeletal maturity in our series.
Although photomicrographs of a chicken intervertebral joint showing all the features of a synovial joint have previously been published [21, 27] no comment was made on the dissimilarity between this and the human intervertebral disc. This is an important anatomical difference and should lead to caution in drawing parallels between the spine in this species and the human.
In conclusion, pinealectomy reliably produces scoliosis in chickens in approximately two-thirds of cases. There is however a natural incidence of scoliosis in chickens. Only a small increase that is not statistically, significant is observed after sham surgery. Curves after pinealectomy are associated with diminished melatonin levels, although exceptions to this are seen. The chicken spine does not have intervertebral discs like the human spine and the curves produced do not always have features characteristic of AIS. Caution should be exercised when drawing conclusions about the pathogenesis of idiopathic scoliosis based on observations from this animal model.
Acknowledgments
This study was funded by the Bone Growth Research Fund, Adelaide. Prof B Vernon Roberts assisted with histological interpretation. Ms Angela Poli assisted with typing the manuscript.
References
- 1.Bagnall K, Raso VJ, Moreau M, Mahood J, Wang X, Zhao J. The effects of melatonin therapy on the development of scoliosis after pinealectomy in the chicken. J Bone Joint Surg Am. 1999;81:191–199. doi: 10.2106/00004623-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bagnall KM, Beuerlein M, Johnson P, Wilson J, Raso VJ, Moreau M. Pineal transplantation after pinealectomy in young chickens has no effect on the development of scoliosis. Spine. 2001;26:1022–1027. doi: 10.1097/00007632-200105010-00007. [DOI] [PubMed] [Google Scholar]
- 3.Bagnall KM, Raso VJ, Hill DL, Moreau M, Mahood JK, Jiang H, Russell G, Bering M, Buzzell GR. Melatonin levels in idiopathic scoliosis. Diurnal and nocturnal serum melatonin levels in girls with adolescent idiopathic scoliosis. Spine. 1996;21:1974–1978. doi: 10.1097/00007632-199609010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Beuerlein M, Wilson J, Moreau M, Raso VJ, Mahood J, Wang X, Greenhill B, Bagnall KM. The critical stage of pinealectomy surgery after which scoliosis is produced in young chickens. Spine. 2001;26:237–240. doi: 10.1097/00007632-200102010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Cheung KM, Lu DS, Poon AM, Wang T, Luk KD, Leong JC. Effect of melatonin suppression on scoliosis development in chickens by either constant light or surgical pinealectomy. Spine. 2003;28:1941–1944. doi: 10.1097/01.BRS.0000083140.80750.93. [DOI] [PubMed] [Google Scholar]
- 6.Cheung KM, Wang T, Hu YG, Leong JC. Primary thoracolumbar scoliosis in pinealectomized chickens. Spine. 2003;28:2499–2504. doi: 10.1097/01.BRS.0000092366.30032.97. [DOI] [PubMed] [Google Scholar]
- 7.Cheung KM, Wang T, Poon AM, Carl A, Tranmer B, Hu Y, Luk KD, Leong JC. The effect of pinealectomy on scoliosis development in young nonhuman primates. Spine. 2005;30:2009–2013. doi: 10.1097/01.brs.0000179087.38730.5d. [DOI] [PubMed] [Google Scholar]
- 8.Fagan AB, Kennaway DJ, Sutherland AD. Total 24-hour melatonin secretion in adolescent idiopathic scoliosis. A case-control study. Spine. 1998;23:41–46. doi: 10.1097/00007632-199801010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Hilibrand AS, Blakemore LC, Loder RT, Greenfield ML, Farley FA, Hensinger RN, Hariharan M. The role of melatonin in the pathogenesis of adolescent idiopathic scoliosis. Spine. 1996;21:1140–1146. doi: 10.1097/00007632-199605150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Illes T, Horvath G, Bagnall KM, Raso VJ, Moreau M, Mahood J, Wang X, Zhao J. Pinealectomy and scoliosis: correspondence. J Bone Joint Surg Am. 2000;82:1197–1198. doi: 10.2106/00004623-200008000-00027. [DOI] [PubMed] [Google Scholar]
- 11.Inoh H, Kawakami N, Matsuyama Y, Aoki T, Kanemura T, Natsume N, Iwata H. Correlation between the age of pinealectomy and the development of scoliosis in chickens. Spine. 2001;26:1014–1021. doi: 10.1097/00007632-200105010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kanemura T, Kawakami N, Deguchi M, Mimatsu K, Iwata H. Natural course of experimental scoliosis in pinealectomized chickens. Spine. 1997;22:1563–1567. doi: 10.1097/00007632-199707150-00006. [DOI] [PubMed] [Google Scholar]
- 13.Machida M. Changes in serum melatonin levels in response to pinealectomy in the chicken and its correlation with development of scoliosis—point of view. Spine. 1998;23:2382. doi: 10.1097/00007632-199811150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J. An experimental study in chickens for the pathogenesis of idiopathic scoliosis. Spine. 1993;18:1609–1615. doi: 10.1097/00007632-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J. Role of melatonin deficiency in the development of scoliosis in pinealectomised chickens. J Bone Joint Surg Br. 1995;77:134–138. [PubMed] [Google Scholar]
- 16.Machida M, Dubousset J, Imamura Y, Miyashita Y, Yamada T, Kimura J. Melatonin. A possible role in pathogenesis of adolescent idiopathic scoliosis. Spine. 1996;21:1147–1152. doi: 10.1097/00007632-199605150-00005. [DOI] [PubMed] [Google Scholar]
- 17.Machida M, Murai I, Miyashita Y, Dubousset J, Yamada T, Kimura J. Pathogenesis of idiopathic scoliosis—experimental study in rats. Spine. 1999;24:1985–1989. doi: 10.1097/00007632-199910010-00004. [DOI] [PubMed] [Google Scholar]
- 18.O’Kelly C, Wang X, Raso J, Moreau M, Mahood J, Zhao J, Bagnall K. The production of scoliosis after pinealectomy in young chickens, rats, and hamsters. Spine. 1999;24:35–43. doi: 10.1097/00007632-199901010-00009. [DOI] [PubMed] [Google Scholar]
- 19.Rigdon RH, Mack J. Spontaneous occurrence of Scoliosis in the chicken. Avian Dis. 1968;12:530–543. doi: 10.2307/1588169. [DOI] [PubMed] [Google Scholar]
- 20.Thillard MJ. Vertebral column deformities following epiphysectomy in the chick. CR Hebd Seances Acad Sci. 1959;248:1238–1240. [PubMed] [Google Scholar]
- 21.Turgut M, Basaloglu HK, Yenisey C, Ozsunar Y. Surgical pinealectomy accelerates intervertebral disc degeneration process in chicken. Eur Spine J. 2006;15:605–612. doi: 10.1007/s00586-005-0972-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turgut M, Yenisey C, Uysal A, Bozkurt M, Yurtseven ME. The effects of pineal gland transplantation on the production of spinal deformity and serum melatonin level following pinealectomy in the chicken. Eur Spine J. 2003;12:487–494. doi: 10.1007/s00586-003-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 24.Wang XP, Jiang HX, Raso J, Moreau M, Mahood J, Zhao J, Bagnall K. Characterization of the scoliosis that develops after pinealectomy in the chicken and comparison with adolescent idiopathic scoliosis in humans. Spine. 1997;22:2626–2635. doi: 10.1097/00007632-199711150-00010. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Moreau M, Raso VJ, Zhao J, Jiang H, Mahood J, Bagnall K. Changes in serum melatonin levels in response to pinealectomy in the chicken and its correlation with development of scoliosis. Spine. 1998;23:2377–2381. doi: 10.1097/00007632-199811150-00002. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein SL. Natural history. Spine. 1999;24:2592–2600. doi: 10.1097/00007632-199912150-00006. [DOI] [PubMed] [Google Scholar]
- 27.Yoshihara H, Kawakami N, Matsuyama Y, Inoh H, Imagama S, Ishiguro N. A histomorphologic study of scoliosis in pinealectomized chickens. Spine. 2005;30:2244–2251. doi: 10.1097/01.brs.0000182095.00577.ee. [DOI] [PubMed] [Google Scholar]