Abstract
The existence and importance of an accelerated adjacent segment disc degeneration (ASD) after lumbar fusion have previously not been demonstrated by RCTs. The objectives of this study were, to determine whether lumbar fusion in the long term accelerates degenerative changes in the adjacent disc and whether this affects the outcome, by using a prospective randomised design. A total of 111 patients, aged 18–55, with isthmic spondylolisthesis were randomised to exercise (EX, n = 34) or posterolateral fusion (PLF, n = 77), with (n = 37) or without pedicle screw instrumentation (n = 40). The minimum 10 years FU rate was 72%, with a mean FU time of 12.6 years (range 10–17 years). Three radiographic methods of ASD quantification were used, i.e. two digital radiographic measurement methods and the semi quantitative UCLA grading scale. One digital measurement method showed a mean disc height reduction by 2% in the EX group and by 15% in the PLF group (p = 0.0016), and the other showed 0.5 mm more disc height reduction in the PLF compared to the Ex group (ns). The UCLA grading scale showed normal discs in 100% of patients in the EX group, compared to 62% in the PLF group (p = 0.026). There were no significant differences between instrumented and non-instrumented patients. In patients with laminectomy we found a significantly higher incidence of ASD compared to non laminectomised patients (22/47 vs. 2/16 respectively, p = 0.015). In the longitudinal analysis, the posterior and anterior disc heights were significantly reduced in the PLF group, whereas in the EX group only the posterior disc height was significantly reduced. Except for global outcome, which was significantly better for patients without ASD, the clinical outcome was not statistically different in patients with and without ASD. In conclusion, the long-term RCT shows that fusion accelerates degenerative changes at the adjacent level compared with natural history. The study suggests that not only fusion, but also laminectomy may be of pathogenetic importance. The clinical importance of ASD seems limited, with only the more severe forms affecting the outcome.
Keywords: Adjacent segment degeneration, Outcome, Isthmic spondylolisthesis, Degenerative disc disease, Chronic low back pain, Laminectomy
Introduction
There is major controversy over whether fusion can result in a so-called adjacent segment disorder (ASD) caused by accelerated disc degeneration, and whether this affects the clinical outcome. ASD has been reported in 5–100% patients after lumbar fusion [29, 37]. It is, however, not known whether this is an expression of increased mechanical stress at the level adjacent to a fusion or simply an effect of ageing. No randomised trial has compared the effect of fusion with the natural history of disc degeneration at the “adjacent” level.
All previous studies are retrospective without a valid control group not operated on, representing only level 3 or 4 evidence. Whereas many authors have observed ASD [7, 20, 29, 37, 39, 53], several have not [4, 19, 28, 40, 45, 48, 49], resulting in conflicting conclusions. Furthermore, the clinical implication of a radiologically demonstrated ASD is uncertain. Many authors have found no correlation between radiological ASD and clinical symptoms [15, 21, 22, 26, 30, 31, 35, 44, 46].
A wide variety of spinal procedures, most notably disc prosthesis, are being offered because of the theoretical advantage of reducing the risk of ASD in the long-term perspective. The rationale for this is that biomechanical studies have shown that a fused part of the spine imposes increased stress on adjacent levels in animal [6, 9, 18, 36, 41] as well as in human models [3, 8, 10, 17, 25, 50].
The purpose of this study was to determine the long-term prevalence of ASD in non-operated and fused patients with adult isthmic spondylolisthesis, with the ultimate aim to determine whether fusion compared with natural history results in accelerated degenerative changes at the adjacent segment. Further aims were to study the effect of instrumentation, and whether adjacent level degeneration is of clinical significance.
Materials and methods
This prospective randomised study included 111 patients aged 18–55 years with isthmic spondylolisthesis. From 1990 to 1995, patients were randomised to one of three different treatments: posterolateral fusion (PLF) without instrumentation (n = 40), PLF with pedicle screw instrumentation (CDI) (n = 37) and a one year exercise program (EX) (n = 34). The study was originally designed to investigate the outcome and effect of conservative treatment compared with fusion, and also to detect differences in outcome for PLF with and without instrumentation. Thus these three equally large groups were initially included in the study.
The inclusion criteria were: Isthmic spondylolisthesis of all grades and levels, with low back pain with or without sciatica, severely restricted functional ability for more than 1 year and age 18–55 years. The exclusion criteria were previous spine surgery, psychiatric disorders and drug or alcohol abuse.
Patients with sciatica were investigated with MRI or CT-myelography for documentation of nerve root compression. No patient showed any radiological sign of disc prolapse or central spinal stenosis.
Initially 116 consecutive patients fulfilled the criteria for participating in the study, two patients refused randomisation, one patient had surgery arranged at another hospital due to long waiting time and two patients improved during the waiting time rendering surgery unnecessary, leaving 111 patients in the study.
The randomisation was done without stratification. After the patient had given consent, a nurse at the outpatient ward blindly chose one of three different notes indicating one of the three different treatment modalities. The randomisation resulted in similar pre-treatment age, symptoms, grade and level of slip and life style factors in the three groups (Table 1).
Table 1.
Demographics, symptoms, level and grade of slip and life style factors in per-cent (except age and sick-leave period) before treatment according to type of randomised treatment (observed numbers within brackets)
| All (n = 111) | Non-instrumented (N = 40) | Instrumented (n = 37) | Exercise (n = 34) | |
|---|---|---|---|---|
| Mean age (years) | 39 | 39 | 39 | 37 |
| Mean age at onset of symptoms (years) | 26 | 25 | 29 | 25 |
| Women | 49 (54) | 45 (18) | 57 (21) | 44 (15) |
| Men | 51 (57) | 55 (22) | 43 (16) | 56 (19) |
| Low back pain only | 31 (33) | 25 (10) | 30 (11) | 39 (12) |
| Low back pain + sciatica | 62 (67) | 68 (27) | 62 (23) | 55 (17) |
| Sciatica only | 7 (8) | 8 (3) | 8 (3) | 6 (2) |
| Level L5 | 85 (94) | 83 (33) | 84 (31) | 88 (30) |
| Level L4 | 13 (14) | 15 (6) | 14 (5) | 9 (3) |
| Levels L4 and L5 | 3 (3) | 3 (1) | 3 (1) | 3 (1) |
| Grade 1 slip | 60 (67) | 68 (27) | 54 (20) | 59 (20) |
| Grade 2 slip | 38 (42) | 30 (12) | 43 (16) | 41 (14) |
| Grade 3 slip | 2 (2) | 3 (1) | 3 (1) | 0 |
| Sick-leave or disability pension | 71 (79) | 68 (27) | 84 (31) | 62 (21) |
| Mean sick-leave before treatment (months) | 16 | 15 | 14 | 18 |
| Blue collar | 80 (87) | 90 (36) | 75 (27) | 73 (24) |
| Immigrants | 32 (35) | 30 (12) | 27 (10) | 38 (13) |
| Married | 74 (82) | 75 (30) | 76 (28) | 71 (24) |
| Smokers | 54 (60) | 63 (25) | 57 (21) | 41 (14) |
| Medication for other than back pain | 21 (23) | 30 (12) | 16 (6) | 15 (5) |
The operating technique included fusion in situ with autologous bone transplantation harvested from the right iliac crest. A one level posterolateral fusion was used in all except 2 patients with grade 3 slips and in 15 patients with pronounced disc degeneration or retrolisthesis at the adjacent level, a two level fusion was performed. Removal of the loose lamina and nerve root decompression were performed in all patients with sciatica, i.e., in 73% of patients operated on (Table 1). The non-instrumented patients wore a daytime lumbar brace for 6 months post-operatively.
The patients in the exercise group had a one-year exercise program including 12 different exercises based on strength and postural training, with emphasis on back and abdominal muscle strength. This was done under supervision by a physiotherapist for 45 min 3 times a week during the first 6 months and twice a week at 6–12 months. 2/3 of the patients complied to the exercise program during the first year. After that the patients were recommended a home exercise program consisting of 8 exercises not needing any special equipment. It is not known to what extent the patients followed the recommendation. Most likely, the majority of the patients gradually discontinued the training after the first supervised year.
Radiographic analysis
Standard A-P and lateral radiographs with minimum 10 years follow-up were obtained from 80 finally included patients (72%). Six patients died before radiographs could be obtained. In the EX group, 9 patients had been operated on and were therefore excluded, resulting in 17 patients with long-term radiographs and outcome data available. Thus, the intention to treat principle was not followed. The reason for this being that keeping patients fused at an early stage in the conservative group would have seriously flawed the analysis.
Out of the 63 patients with long-term radiographs in the PLF group, pre-operative radiographs were missing in 8. These 8 patients were kept in the study, as the missing pre-operative radiographs do not influence the most important analysis in the study, the comparison at long-term follow-up between the EX and PLF groups. Out of the 32 patients operated on with instrumentation, 12 patients had their instrumentation removed before the long-term follow-up, but were, analysed in the instrumentation group according to the intention to treat principle.
At 2 years, 72% of patients were classified as definitely fused (Grade A) according to the Lenke scale and 92% as definitely or possibly fused (Grade A or B) [27, 32]. Patients not fused (Grade C or D) were at long-term re-evaluated by 3 experienced spine surgeons. The fusion rate at long term was determined according to Lenke, and the additional criteria of bony bridging between vertebral bodies indicating fusion, and resorbtion around screws indicating pseudarthrosis. This resulted in exclusion of three patients (5% of available long term radiographs) with pseudarthrosis from the study. Thus, the number of patients included in the final analysis were 63 in the PLF group and 17 in the EX group.
In 4 patients, all non-instrumented, the fusion had extended one more level than initially intended. In these 4 patients the level above the extended fusion was analysed.
The average follow-up time was 12.6 years (range 10–17). The mean age at the start of the study was 39 years and at the end of the study was 52 years.
Radiographic measurements
Three different methods of quantification of disc degeneration were used, all based on AP and lateral radiographs:
Digital radiographic measurement method.
Quantitative Motion Analysis (QMA™), a computerised FDA approved radiographic technique [54].
UCLA grading scale of disc degeneration, a semiquantitative morphological disc degeneration scale [16].
All measurements of the disc height and degenerative changes were made at the level above the slip in the EX group and at the level above the fusion in the PLF group.
-
The digital radiographic measurements included disc height and slip on digitalised radiographs using our hospitals standard software system for viewing and measuring distances in radiographs (IDS5, Sectra PACS™).
The pictures were magnified to increase precision after which the corners of the vertebrae were marked [14]. If the radiograph was oblique, the spot mid distance between the two identifiable corners was used. To control for magnification, the disc height was measured relative to anterior vertebral height (Fig. 1). For validation, measurements were performed twice by 3 independent observers, two experienced spine surgeons and one radiologist.
QMA™ was used to measure anterior and posterior disc height, disc angle and slip. The computer-assisted QMA™ is a measurement technique performed by Medical Metrics, Inc (TX, USA). The corners of the vertebrae are marked manually, software calibrates the pictures via a formula derived from normal data on vertebral dimensions and the results for disc height are given in mm. The method has been validated and reported to be accurate and reproducible in measurements of the lumbar spine, and has also received FDA approval (K022585) [38, 54].
The University of California Los Angeles grading scale of disc degeneration [16] is a semi quantitative method, based on disc-height reduction and the presence of osteophytes and end plate sclerosis. The pre-treatment and long-term radiographs were compared and graded by 2 independent observers to consensus and classified as: Normal (Grade 1), general narrowing of the disc space (Grade 2), presence of osteophytes (Grade 3) and presence of end plate sclerosis (Grade 4). In the 8 patients with missing pre-op radiographs, the UCLA grading was performed as originally described without comparison of pre-operative X-ray.
Fig. 1.
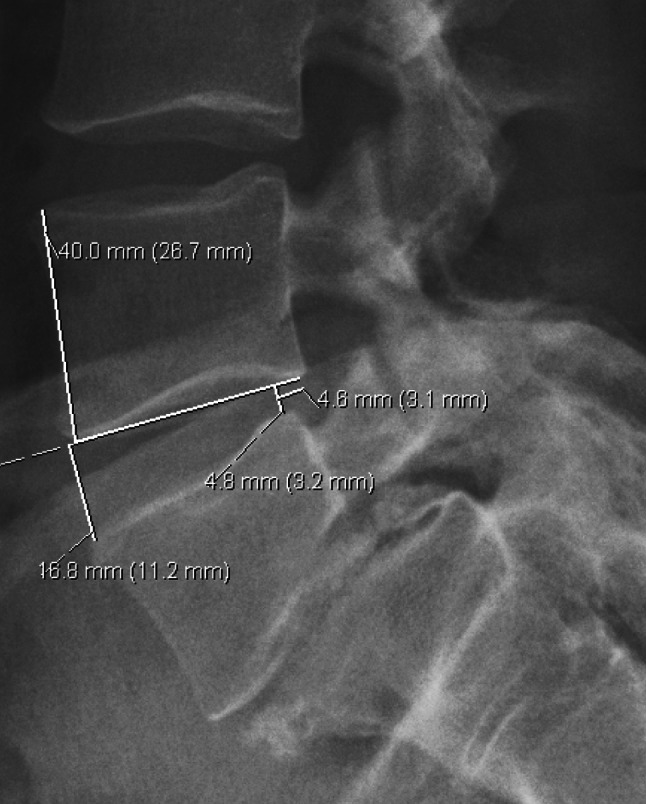
Measurements according to the digital radiographic measurement method: The anterior and posterior disc heights are measured relative to the anterior vertebral body height, and olisthesis relative to the vertebral body antero-posterior depth. The disc heights are measured at a 90° angle to a straight line going through the two lower corners of the vertebra above. The olisthesis is measured from the posterior disc height line to the posterior corner of the vertebra above
Outcome measurements
The patients completed questionnaires concerning functional disability, pain and global outcome. Long-term outcome questionnaires were obtained from 99% (79/80) of patients with long-term radiographs.
Pain was assessed as Pain index which is the mean of the VAS-scores for “pain right now” and “worst pain last week”. Functional Disability was measured by the disability rating index (DRI) and the oswestry disability index (ODI).
The DRI was chosen as the primary measurement of functional outcome. It was developed in the 1980s and later validated by a group including a member of the spinal research group at Karolinska University Hospital, Huddinge, Sweden [43]. The score is the mean of 12 functional VAS concerning different daily activities (dressing, outdoor walking, climbing stairs, sitting for longer time, standing bent over a sink, carrying a bag, making a bed, running, light work, heavy work, lifting heavy objects and participation in exercise/sports). The form is self-administered according to oral and written instructions. In the VAS, the patient marks on a 100-mm scale the ability to perform the respective activity, with anchor points; 0 = without difficulty and 100 = impossible.
The ODI is a validated disease specific instrument for assessment of spinal disorders consist’s of a 10-item ordinal scale with 6 response alternatives for each item [13]. The total score ranges from 0 to 100, where 100 is worst disability.
Global outcome, as compared with the pre-treatment situation, was assessed by the patient into “much better”, “better”, “unchanged” or “worse”.
Statistical methods
The prevalence of ASD at long-term follow-up was determined using four different diagnostic criteria: a/ disc height reduction > 2SD over the mean reduction as observed in the EX group, which was considered as natural history, b/ remaining mean disc height less than 20% of anterior vertebral height, c/ worsening of the UCLA score from pre-treatment and d/ totally reduced posterior disc height (0 mm) at long-term follow-up.
To test for differences in disc height reduction and slip between and within treatment groups the unpaired and paired t-tests were used. The Mann–Whitney U test was used to test for differences in Pain index, DRI and ODI between groups. P < 0.05 was considered as statistically significant. The chi-square test, Fisher′s exact test and the chi-square test for trend were used to analyse frequency distributions.
Inter- and intraobserver reliability was estimated by calculation of the Shrout-Fleiss intraclass correlation coefficient (ICC), and by calculation of the mean error.
Ethical approval
The study was approved by the Medical Ethical Committee of Karolinska University Hospital, Huddinge.
Results
Validity
The intraobserver reliability of the digital radiographic measurement using our own hospital based digital technique was 0.93–0.97 and the interobserver reliability was 0.87–0.99 (ICC).
The mean intraobserver error of the different disc heights measured pre-operatively and at long term twice was 2.1% for the anterior disc height and 1.95% for the posterior disc height. The mean interobserver error was 10.6% for the anterior disc height and 3.4% for the posterior disc height.
Prevalence
ASD defined as a posterior disc height reduction more than 2 standard deviations below the mean, as observed in the EX group, resulted in a prevalence of ASD of 6% (1/17), and 14% (9/63) in the EX and PLF groups, respectively. Similarly, ASD defined as a remaining mean disc height less than 20% of anterior vertebral height resulted in a prevalence of 6% (1/17) in the EX group and of 11% (7/63) in the PLF group. ASD defined as any deterioration of the UCLA grading scale resulted in a prevalence of 0% in the EX group and in 38% (24/63) in the PLF group. ASD defined as a totally reduced posterior disc height resulted in a prevalence of 0% in the EX group and in 6% (4/63) in the PLF group (Tables 4, 5, 7).
Table 4.
Number of patients with ASD according to 4 different definitions in patients with (n = 32) and without instrumentation (n = 31)
| No. of patients | ASD: >2SD disc height reduction | ASD: <20% remaining disc height | ASD: UCLA grade 2–4 | ASD: no remaining post disc height |
|---|---|---|---|---|
| Instrumented | 4 | 2 | 11 | 1 |
| Non-instrumented | 5 | 5 | 13 | 3 |
Table 5.
Number of patients with accelerated disc degeneration according to the UCLA grading scale in the exercise and fusion group (p = 0.026)
| Patients | Exercise | Fusion |
|---|---|---|
| Grade 1 (=Normal) | 17 | 39 |
| Grade 2 | 0 | 11 |
| Grade 3 | 0 | 12 |
| Grade 4 | 0 | 1 |
Table 7.
The difference in pain index, DRI and ODI between fused patients with and without ASD according to 4 different definitions of ASD
| Difference ASD-pat vs. the rest | ASD: >2SD post disc height reduction (n = 9) | ASD: <20% mean disc height (n = 7) | ASD: UCLA grade 2–4 (n = 24) | ASD: no remaining post disc height (n = 4) |
|---|---|---|---|---|
| Pain | 6 (p = 0.77) | 19 (p = 0.09) | 0 (NS) | 24 (p = 0.09) |
| DRI | 8 (p = 0.45) | 20 (p = 0.08) | 0 (NS) | 20 (p = 0.11) |
| ODI | 4 (p = 0.64) | 13 (p = 0.09) | 2 (NS) | 5.5 (p = 0.24) |
Subgroup analysis: Instrumentation vs no instrumentation
As previously reported for pain, DRI, ODI and Global outcome [11], no significant differences in any radiological variable could be demonstrated between patients operated on with or without instrumentation. The posterior disc height reduction was 6.6% of anterior vertebral height in the instrumented group compared to 6.5% in the non-instrumented group (ns).
In both groups, a tendency of retrolisthesis of the vertebra above the slip level was observed, the change in sagittal translation was 0.8% in the instrumented group and 1.2% in the non-instrumented group (ns). The distribution of retrolisthesis and anterolisthesis in the two groups was also similar: In instrumented patients, 91% (29/32) had a retrolisthesis and 9% (3/32) an anterolisthesis, compared to 84% (26/31) of the non-instrumented patients with retrolisthesis and 13% (4/31) with anterolisthesis of the adjacent vertebra (ns).
Instrumentation did not affect the prevalence of ASD, using any definition of ASD (Table 4). Furthermore, Pain Index, DRI, ODI and Global outcome were not significantly different between patients with ASD in the instrumented and the non-instrumented groups. Therefore, to improve statistical power, the main analyses comparing fusion vs exercise are based on the combined, instrumented and non-instrumented PLF group.
Subgroup analysis: Laminectomy vs non-laminectomy
In the PLF group, all patients had a PLF and all patients with sciatica had a laminectomy and decompression. Patients without sciatica had no laminectomy and no decompression. Pre-operatively, there were no differences in disc heights between the laminectomy and non-laminectomy groups. In patients operated on with combined PLF and laminectomy, 22/47 developed ASD, as defined by the UCLA criteria (grade 2–4). In contrast, only 2/16 patients operated on with PLF without laminectomy developed ASD (p = 0.015). Using other criteria, however, the difference in ASD incidence was not statistically significant between laminectomised and non-laminectomised patients.
Digital radiographic method
There were no statistical differences in the disc heights before treatment between the EX group and the PLF group. The mean pre-operative posterior disc height was 21.8% in the EX group and 20.5% in the PLF group (p = 0.245). The posterior disc height reduction was more pronounced in the PLF group than in the EX group at long term (p = 0.0072).
The posterior disc height reduction was significantly more pronounced than the anterior disc height reduction in the PLF group (p = 0.0015). In the EX group the posterior disc height decreased by 11%, whereas the anterior disc height increased by 3% (p = 0.029).
In the longitudinal analysis, the anterior as well as the posterior disc height was reduced in the PLF group (p < 0.0001), whereas only the posterior disc height changed over time in the EX group (p = 0.0175). The change in slip (regardless of antero- or retrolisthesis) over time within the EX and PLF groups, as well as between the groups, was not significant (Table 2).
Table 2.
The mean change in relative (% units) anterior, posterior and mean (ant + post/2) disc height and change in amount of slip (regardless of antero- or retrolisthesis) according to the Digital radiographic measurement method
| Change (%) | Conserv (%). | Fusion (%) | p (Between groups) |
|---|---|---|---|
| Ant Disc height | 3 | −7*** | 0.0098 |
| Post disc height | −11* | −30*** | 0.0079 |
| Mean disc height | −2 | −15*** | 0.0016 |
| Sagittal translation | 6 | 15 | 0.64 |
*Significant change in longitudinal analysis within group
The QMA-method
The pre-treatment posterior disc heights were 6.42 mm in the EX group and 6.76 mm in the PLF group (ns). Similar to the findings using the digital radiographic method, at long term the QMA™-method resulted in a more pronounced decrease of posterior disc height in both groups. In the PLF group, the mean posterior disc height reduction was 1.3 mm and the anterior disc height reduction was 0.33 mm (p = 0.0002). In the EX group, there was a 0.83 mm reduction in the posterior disc height and a 0.21 mm increase of the anterior disc height (p = 0.0086). The decrease of posterior disc height was significant in the EX group (p = 0.034) as well as in the PLF group (p < 0.0001).
Reflecting the decrease in posterior disc height, the disc angle increased significantly and similarly in both groups, 1.8° in the EX group and 1.7° in the PLF group. There was a mean slip into retrolisthesis of 0.55 mm (p < 0.01) in the PLF group. In contrast, the EX group showed very limited change in sagittal translation (Table 3). Although the change in translation was larger in the PLF group, the difference to the EX group was not significant.
Table 3.
The mean change in adjacent level absolute anterior and posterior disc height and sagittal translation (mm, neg. value = retrolisthesis) and disc angle (°) according to the QMA™-method
| Change in | Conserv. | Fusion | p (Between groups) |
|---|---|---|---|
| Anterior disc height | 0.21 | −0.33 | NS |
| Posterior disc height | −0.84* | −1.28*** | NS |
| Disc angle | 1.8** | 1.7*** | NS |
| Sagittal translation | 0.08 | −0.55** | NS |
*Significant change in longitudinal analysis within group
The UCLA grading scale
None of the patients in the EX group presented visually demonstrable decrease in mean disc height at follow-up. In contrast, in the PLF group there were 24 patients (38%) with ASD (p = 0.026) (Table 5).
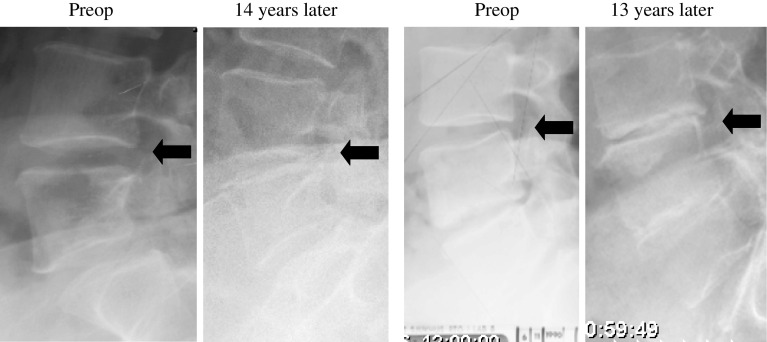
A totally reduced (0 mm) posterior disc height was found only in the PLF group. This was observed in 4/63 (6%) of the patients, one with and 3 without instrumentation (Fig. 2; Table 4).
Fig. 2.
Examples of 2 patients operated on with non-instrumented PLF with total posterior disc height reduction at long term follow up. Arrows indicate the level adjacent to the fusion
Outcome
Only 11% of PLF patients with ASD (defined as > mean +2SD disc height reduction observed in the EX group) classified themselves as “much better” compared to 49% of PLF patients without ASD (p = 0.036) (Table 6). However, no statistically significant correlation between global outcome and disc degeneration could be demonstrated using the other definitions of ASD used in the study, i.e., UCLA grading 2–4, <20% relative remaining mean disc height and totally reduced posterior disc height.
Table 6.
Global outcome in fused patients with and without ASD, defined as > 2SD posterior disc height reduction as observed in the exercise group (p = 0.036)
| ASD patients | Non ASD patients | |
|---|---|---|
| Much better | 11% (1/9) | 49% (22/45) |
| Better, unchanged or worse | 89% (8/9) | 51% (23/45) |
Nonetheless, it should be noted that there was a tendency towards a worse Pain Index, DRI and ODI in PLF patients with a <20% relative remaining mean disc height (n = 7). The mean pain was 56 versus 37 (p = 0.09), mean DRI was 51 versus 31 (p = 0.08) and mean ODI 38 versus 25 (p = 0.09) in patients with less compared to more than 20% relative mean disc height, respectively. Similarly, the outcome for the 4 PLF patients with total posterior disc height reduction tended to be worse than for the rest of the group. The mean pain was 65 versus 41 (p = 0.09), mean DRI 53 versus 33 (p = 0.11) and mean ODI 39 vs 26 (p = 0.24) in patients with totally reduced posterior disc height compared with the rest of the PLF patients respectively.
Thus, the outcome measurements of Pain Index, DRI, ODI and Global outcome were in general insignificantly worse for the patients defined as having ASD, regardless of definitions used. An exception was mild disc degeneration according to the UCLA grading scale for which the outcome was almost identical for fused patients with and without ASD (Table 7).
Discussion
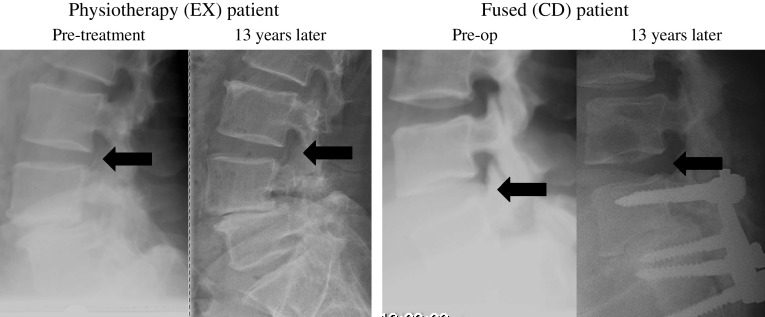
This long-term study on spinal fusion is the only RCT prospectively investigating and comparing the prevalence and clinical importance of adjacent segment degeneration in non-operated and fused patients. The study demonstrates that fusion, particularly combined with laminectomy, does accelerate adjacent disc degeneration. The strongest argument for this conclusion is that the semi-quantitative UCLA grading scale showed a 38% frequency of increased disc degeneration among fused patients compared with no increase in disc degeneration in conservatively treated patients (Table 5; Fig. 3). The main conclusion of the study is also supported by the finding of a significantly more pronounced posterior disc height reduction in the fused group compared to the conservatively treated group, and by a significant slip of the vertebra above the spondylolisthesis into retrolisthesis in the fused group, but not in the conservatively treated group. A total disc height reduction was a less frequent finding, and was found in only a small proportion (6%) of fused patients, but never found in non-operated patients (Fig. 2).
Fig. 3.
Examples of one physiotherapy patient (EX) and one fused patient with pedicel fixation (PLF) without disc height decrease at long term follow up 13 years later. Arrows indicate the level adjacent to the slip-level and the fusion, respectively
A somewhat surprising finding was that among the fused patients, ASD was almost exclusively observed in those who had had a laminectomy. The pre-operative scores of patients with laminectomy were not different from that of patients without laminectomy, suggesting that the observed difference is a cause of the laminectomy rather than an effect of a pre-treatment difference in biomechanical disc properties. The reason for this is unclear. It could be that the loss of posterior tension band function creates instability and, hence, accelerated disc degeneration. The finding is in accordance with radiological data showing that a disruption of the posterior ligamentous complex leads to instability of the motion segment [23].
An alternative explanation is that the mechanical removal of the complete loose lamina can be compared with loss of posterior column support and a tendency to mechanical failure in compression. Such a mechanism is compatible with our findings of a much more pronounced reduction of the posterior disc height. In addition to the possible explanation of a “laminectomy effect”, as discussed above, the loss of predominantly posterior disc height may be a reflection of a compensatory hyperextension mechanism triggered by fusion of the spondylolytic segment in a non-physiological kyphotic position [24, 34, 47]. It may be hypothesised that fusion in kyphosis leads to compensatory cranial hyperlordosis and ASD. There is, however, no reason to believe that laminectomy patients were fused in less lordosis than non-laminectomised patients. Although the results support the idea of a negative laminectomy effect rather than fusion with or without kyphosis, as a risk factor for ASD, the limited number of patients and the methodological problems certainly prevent us from excluding fusion and/or muscle dissection as pathogenetic factors for ASD.
The randomisation resulted in an equal distribution of radiological as well as demographical characteristics. Except from the long observation time, the strength of the study is the use of a conservatively treated group which allows an estimation of the natural history of disc degeneration at the level above a spondylolytic slip. The average normal disc degeneration at this level 13 years later was in the non-operative group reflected by a decrease of posterior disc height by 1 mm. The fact that in 17 patients more than one segment was fused does not invalidate the results of the study as, consequently, the discs measured in the fused group were on average at a slightly higher level than in the control group. This one should expect to result in a lesser degree of ASD observed in the fused group since degenerative changes generally are more severe in caudal discs. As the opposite was found, this rather strengthens the conclusion of the study.
The results of the study reflect standard treatment procedures, including laminectomy if sciatica and an extra level fused if definite degenerative changes at the adjacent level. Although this makes interpretation more difficult from a biological point of view it reflects the risk of ASD after clinically relevant surgical intervention.
The pseudoarthosis rate in the study was low (5%), and possibly underestimated. Most likely this does not, however, invalidate the results as a pseudarthrosis biomechanically may protect the fusion group from ASD.
The fact that radiographs were missing in 8 patients pre-operatively does not invalidate the comparison at 13 years follow-up between the groups. There is no indication that loss of the radiographs was associated with any particular circumstance, such as degenerative changes at adjacent levels.
The major weakness of the study is the limited number of patients in the control group, as well as in the subgroup analysis of laminectomised and non-laminectomised patients. The exclusion of patients from the EX group because of subsequent fusion, however, does not seem to invalidate the results, as they were operated on at a relatively early stage after inclusion in the study, i.e., after 20, 21, 25, 26, 27, 28, 32, 34 and 54 months (range 20–54, median 27 months). They were all operated on because of unaltered symptoms from inclusion into the study. Furthermore, 7/9 were operated on only at the slip level. In 2/9, one extra segment was fused, L4 to S1, because of a high degree of slip and easier instrumentation respectively. These factors all suggest that these “cross over” patients all were operated on for the basic pathology, spondylolisthesis, and not for ASD, limiting the risk for bias.
Larger number of patients in the conservative group would of course have improved the potential for strong conclusions. Adequately powered future studies may provide such data. It must, however, be realised that the difficulties with large RCTs with adequate long-term follow-up rates are monumental and that we most likely will have to depend on less than ideal data also in the future.
Although a correlation was found between radiological ASD and clinical outcome, the limited number of patients with severe ASD does not allow any strong conclusion on its clinical relevance. The commonly used definition for disease, i.e., >2 standard deviations outside the normal range of disc height reduction, resulted in an ASD frequency of 14% among fused patients. An excellent global outcome was reported by 49% of patients without ASD, defined accordingly, compared with 11% of patients with ASD (Table 6). On the other hand, slight disc height reduction, as observed in the UCLA scale in 38% of fused patients, resulted in a similar outcome compared to patients with normal adjacent discs (Table 7). Nonetheless, the study lends some support to the conclusion that more severe degrees of ASD may adversely affect outcome.
Two recent review articles reported very variable rates of radiological ASD, with prevalences from 5.2 to 100% and symptomatic ASD from 5.2 to 36% [29, 37]. Reflecting the large variability of the risk of developing ASD reported in the review articles, our study showed that the prevalence of ASD varies widely with the definition used.
The fact that the observed differences in disc height between the groups were small, and for the QMA™ method non-significant, reflects the need for precise methods of quantification. Some other authors have earlier concluded that disc height measurements on radiographs show a great deal of inconsistency and that radiographical methods therefore may not be appropriate [3, 16, 42]. Our validation of the manual measurements showed, however, a high inter- and intraobserver reliability, higher than previously reported for the FDA approved QMA™ method. Compared to our own radiographic method, based on standard digital measurement programme (IDS5, Sectra), the QMA method has been reported to have similar intraobserver reliability, but slightly worse interobserver reliability (ICC 0.70–0.79) [38].
Although it is well known that the precision of disc height measurements is limited, our digital method showed to be of acceptable precision at least for group comparisons. This is particularly true for intraobserver precision with an observed mean error of around 2%. The intraobserver error for this study is more relevant than interobserver error as the results are based on measurements by the same observer. This measurement error can be compared with the observed mean loss of 30% of posterior disc height in PLF group disc heights. Furthermore, to compensate for the limited precision, we used three different methods of assessment of disc degeneration, with fairly consistent results, despite a non-significant difference with the digital method based on absolute measurements of disc height (QMA™). Not surprisingly, the results suggest the use of relative rather than absolute disc heights.
Furthermore, the semiquantitative UCLA grading scale showed major differences between the groups, strongly supporting the conclusion that fusion accelerates DDD at the adjacent level. Although some bias cannot be excluded, two observers were used and, furthermore, only the lateral radiographs were used for assessment, not revealing the posterolateral fusion mass in non-instrumented patients. In this study the UCLA grading scale was used slightly different from the originally described UCLA method, not using serial radiographs. As longitudinal radiographs were available in the great majority of patients, a decrease in disc height was more easily detected, with, most likely, increased precision.
Interestingly, the semiquantitative UCLA grading scale, largely based on “eye balling” disc height, sclerosis and osteophytes showed the most convincing differences between the PLF and EX groups (Table 5). The finding that no patient in the exercise group presented with a worsening of disc degeneration with the use of the UCLA scale was surprising. Although the possibility of a protective effect of exercise on disc degeneration cannot be excluded, this seems unlikely. Considering the age of the patients, some deterioration in conservatively treated patients should be expected, and was also found with the digital radiographic method showing an 11% loss of posterior disc height at long term (Table 2).
Contrary to our findings, Seitsalo reported that fusion does not significantly increase the rate of disc degeneration in the adjacent disc above the fusion after a mean post-operative follow-up of 13.8 years [45]. All patients in the finish study, however, were under 20 years at inclusion, with most likely a lower risk of developing DDD irrespective of treatment.
Whether the results can be generalised to populations without spondylolisthesis is difficult to assess. Spondylolisthesis may induce abnormal motion at adjacent levels and thereby increase the risk of DDD. If so, the conclusion may not apply to DDD, fusion in spondylolisthesis would tend to protect from ASD, not increase the risk of it. Axelsson et al. using RSA technique, however, showed that the spondylolytic defect in pars interarticularis does not cause permanent instability or hypermobility in the adult patient with low back pain [2]. Furthermore, the prevalence of ASD in the control group was almost nil, strongly opposing the existence of such a mechanism. Although far fetched, one may also, on the other hand, argue that the spondylolysis protects from adjacent segment degeneration. There seems, however, little reason to believe that it does.
A stronger arguement against generalisation of the results is that DDD may be a specific disorder, genetically determined [5] which would tend to accelerate disc degeneration more severely than what is observed in spondylolisthesis, also at adjacent segments. However, if this is the case, it would be the genetic predisposition increasing the risk of ASD, not the fusion. Thus, this study may be less affected by the well-known genetic influence on the risk of disc degeneration, and, therefore, better reflect the effect of fusion.
The similarity between ordinary DDD and spondylolisthesis was underlined by our previous findings of similar symptoms and clinical findings in degenerative lumbar spine disorders and isthmic spondylolisthesis [33]. In fact, one may argue that the use of a more homogenous patient population, such as one with spondylolisthesis, most likely including less non-specific chronic pain syndrome patients, may be theoretically advantageous when correlating DDD to clinical outcome variables. It is well known that spondylolisthesis patients perform better and more consistently after fusion, and this will result in less variability of outcome, facilitating analysis.
There are a few prospective randomised studies on the long-term effect of lumbar fusion. We have earlier shown a slight deterioration of the outcome 9 years after lumbar fusion in this group of patients [11]. One could speculate that the reason for this decline in outcome for the fused patients is the development of ASD. However, the low prevalence of severe ASD and the limited effect on outcome observed in this study do not seem to fully explain the previously demonstrated decline of outcome at long-term follow-up. In contrast to our results, two long term Danish studies on DDD without non-operated controls showed no deterioration of outcome 10–13 and 5–9 years post-operatively, respectively [1, 51]. The reason for this discrepancy is unclear, but differences in diagnosis as well as type of fusion may apply. It cannot be excluded that 360° fusion results in a more lordotic fused segment, and thereby less need of compensatory lordosis, and, hence, less accelerated adjacent segment degeneration.
Earlier reports have indicated a higher rate of symptomatic ASD in patients operated on with PLF with, compared to without, pedicle fixation [12, 37]. We could not confirm this finding. Somewhat in contrast, we found ASD to be non-significantly more frequent among non-instrumented than instrumented patients (Table 4).
This study extends previous findings showing no difference in long-term outcome with or without instrumentation [11], now additional results showing no difference in the risk for adjacent segment degeneration. Thus, neither clinical outcome nor adjacent segment degeneration seems to be affected by spinal instrumentation in PLF, the latter was also reported by Wiltse [52].
Conclusions
One can conclude that adjacent segment disease for the first time is demonstrated using a scientifically valid design. The results of the present long-term RCT comparing the effect of fusion with natural history in isthmic spondylolisthesis show that laminectomy and fusion accelerates degenerative changes at the adjacent level. The effect, however, is marginal in most patients and affects outcome only in the less frequent, severe forms of ASD.
References
- 1.Andersen T, Videbaek TS, Hansen ES, Bunger C, Christensen FB. The positive effect of posterolateral lumbar spinal fusion is preserved at long-term follow-up: a RCT with 11–13 year follow-up. Eur Spine J. 2008;17:272–280. doi: 10.1007/s00586-007-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axelsson P, Johnsson R, Stromqvist B. Is there increased intervertebral mobility in isthmic adult spondylolisthesis? A matched comparative study using roentgen stereophotogrammetry. Spine. 2000;25:1701–1703. doi: 10.1097/00007632-200007010-00014. [DOI] [PubMed] [Google Scholar]
- 3.Axelsson P, Johnsson R, Stromqvist B. The spondylolytic vertebra and its adjacent segment: mobility measured before and after posterolateral fusion. Spine. 1997;22:414–417. doi: 10.1097/00007632-199702150-00012. [DOI] [PubMed] [Google Scholar]
- 4.Axelsson P, Jr, Stromqvist B. Adjacent segment hypermobility after lumbar spine fusion: no association with progressive degeneration of the segment 5 years after surgery. Acta Orthop. 2007;78:834–839. doi: 10.1080/17453670710014635. [DOI] [PubMed] [Google Scholar]
- 5.Battie MC, Videman T, Levalahti E, Gill K, Kaprio J. Heritability of low back pain and the role of disc degeneration. Pain. 2007;131:272–280. doi: 10.1016/j.pain.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Bushell GR, Ghosh DP, Taylor TK, Sutherland JM, Braund KG. The effect of spinal fusion on the collagen and proteoglycans of the canine intervertebral disc. J Surg Res. 1978;25:61–69. doi: 10.1016/0022-4804(78)90159-2. [DOI] [PubMed] [Google Scholar]
- 7.Cheh G, Bridwell KH, Lenke LG, Buchowski JM, Daubs MD, Kim Y, Baldus C. Adjacent segment disease followinglumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine. 2007;32:2253–2257. doi: 10.1097/BRS.0b013e31814b2d8e. [DOI] [PubMed] [Google Scholar]
- 8.Chen CS, Cheng CK, Liu CL, Lo WH. Stress analysis of the disc adjacent to interbody fusion in lumbar spine. Med Eng Phys. 2001;23:483–491. doi: 10.1016/S1350-4533(01)00076-5. [DOI] [PubMed] [Google Scholar]
- 9.Dekutoski MB, Schendel MJ, Ogilvie JW, Olsewski JM, Wallace LJ, Lewis JL. Comparison of in vivo and in vitro adjacent segment motion after lumbar fusion. Spine. 1994;19:1745–1751. doi: 10.1097/00007632-199408000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Eck JC, Humphreys SC, Hodges SD. Adjacent-segment degeneration after lumbar fusion: a review of clinical, biomechanical, and radiologic studies. . Am J Orthopedics (Belle Mead NJ) 1999;28:336–340. [PubMed] [Google Scholar]
- 11.Ekman P, Moller H, Hedlund R. The long-term effect of posterolateral fusion in adult isthmic spondylolisthesis: a randomized controlled study. Spine J. 2005;5:36–44. doi: 10.1016/j.spinee.2004.05.249. [DOI] [PubMed] [Google Scholar]
- 12.Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999;90:163–169. doi: 10.3171/spi.1999.90.2.0163. [DOI] [PubMed] [Google Scholar]
- 13.Fairbank JCTDJ, Couper J, O’Brien JP. The Oswestry low back pain disability questionaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 14.Frobin W, Brinckmann P, Biggemann M, Tillotson M, Burton K. Precision measurement of disc height, vertebral height and sagittal plane displacement from lateral radiographic views of the lumbar spine. Clin Biomech (Bristol, Avon) 1997;12(Suppl 1):S1–S63. doi: 10.1016/S0268-0033(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 15.Frymoyer JW, Hanley EN, Jr, Howe J, Kuhlmann D, Matteri RE. A comparison of radiographic findings in fusion and nonfusion patients ten or more years following lumbar disc surgery. Spine. 1979;4:435–440. doi: 10.1097/00007632-197909000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86-A:1497–1503. doi: 10.2106/00004623-200407000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Goto K, Tajima N, Chosa E, Totoribe K, Kubo S, Kuroki H, Arai T. Effects of lumbar spinal fusion on the other lumbar intervertebral levels (three-dimensional finite element analysis) J Orthop Sci. 2003;8:577–584. doi: 10.1007/s00776-003-0675-1. [DOI] [PubMed] [Google Scholar]
- 18.Ha KY, Schendel MJ, Lewis JL, Ogilvie JW. Effect of immobilization and configuration on lumbar adjacent-segment biomechanics. J Spinal Disord. 1993;6:99–105. doi: 10.1097/00002517-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Hambly MF, Wiltse LL, Raghavan N, Schneiderman G, Koenig C. The transition zone above a lumbosacral fusion. Spine. 1998;23:1785–1792. doi: 10.1097/00007632-199808150-00012. [DOI] [PubMed] [Google Scholar]
- 20.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4:190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Korovessis P, Papazisis Z, Koureas G, Lambiris E. Rigid, semirigid versus dynamic instrumentation for degenerative lumbar spinal stenosis: a correlative radiological and clinical analysis of short-term results. Spine. 2004;29:735–742. doi: 10.1097/01.BRS.0000112072.83196.0F. [DOI] [PubMed] [Google Scholar]
- 22.Kumar MN, Jacquot F, Hall H. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur Spine J. 2001;10:309–313. doi: 10.1007/s005860000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai PL, Chen LH, Niu CC, Fu TS, Chen WJ. Relation between laminectomy and development of adjacent segment instability after lumbar fusion with pedicle fixation. Spine. 2004;29:2527–2532. doi: 10.1097/01.brs.0000144408.02918.20. [DOI] [PubMed] [Google Scholar]
- 24.Lazennec JY, Ramare S, Arafati N, Laudet CG, Gorin M, Roger B, Hansen S, Saillant G, Maurs L, Trabelsi R. Sagittal alignment in lumbosacral fusion: relations between radiological parameters and pain. Eur Spine J. 2000;9:47–55. doi: 10.1007/s005860050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine. 1988;13:375–377. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann TR, Spratt KF, Tozzi JE, Weinstein JN, Reinarz SJ, el-Khoury GY, Colby H. Long-term follow-up of lower lumbar fusion patients. Spine. 1987;12:97–104. doi: 10.1097/00007632-198703000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Lenke LG, Bridwell KH, Bullis D, Betz RR, Baldus C, Schoenecker PL. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord. 1992;5:433–442. doi: 10.1097/00002517-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Leong JC, Chun SY, Grange WJ, Fang D. Long-term results of lumbar intervertebral disc prolapse. Spine. 1983;8:793–799. doi: 10.1097/00007632-198310000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Levin DA, Hale JJ, Bendo JA. Adjacent segment degeneration following spinal fusion for degenerative disc disease. Bull NYU Hosp Jt Dis. 2007;65:29–36. [PubMed] [Google Scholar]
- 30.Luk KD, Lee FB, Leong JC, Hsu LC. The effect on the lumbosacral spine of long spinal fusion for idiopathic scoliosis: a minimum 10-year follow-up. Spine. 1987;12:996–1000. doi: 10.1097/00007632-198712000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Miyakoshi N, Abe E, Shimada Y, Okuyama K, Suzuki T, Sato K. Outcome of one-level posterior lumbar interbody fusion for spondylolisthesis and postoperative intervertebral disc degeneration adjacent to the fusion. Spine. 2000;25:1837–1842. doi: 10.1097/00007632-200007150-00016. [DOI] [PubMed] [Google Scholar]
- 32.Moller H, Hedlund R. Instrumented and noninstrumented posterolateral fusion in adult spondylolisthesis—a prospective randomized study: part 2. Spine. 2000;25:1716–1721. doi: 10.1097/00007632-200007010-00017. [DOI] [PubMed] [Google Scholar]
- 33.Moller H, Sundin A, Hedlund R. Symptoms, signs, and functional disability in adult spondylolisthesis. Spine. 2000;25:683–689. doi: 10.1097/00007632-200003150-00006. [DOI] [PubMed] [Google Scholar]
- 34.Oda I, Cunningham BW, Buckley RA, Goebel MJ, Haggerty CJ, Orbegoso CM, McAfee PC. Does spinal kyphotic deformity influence the biomechanical characteristics of the adjacent motion segments? An in vivo animal model. Spine. 1999;24:2139–2146. doi: 10.1097/00007632-199910150-00014. [DOI] [PubMed] [Google Scholar]
- 35.Okuda S, Iwasaki M, Miyauchi A, Aono H, Morita M, Yamamoto T. Risk factors for adjacent segment degeneration after PLIF. Spine. 2004;29:1535–1540. doi: 10.1097/01.BRS.0000131417.93637.9D. [DOI] [PubMed] [Google Scholar]
- 36.Olsewski JM, Schendel MJ, Wallace LJ, Ogilvie JW, Gundry CR. Magnetic resonance imaging and biological changes in injured intervertebral discs under normal and increased mechanical demands. Spine. 1996;21:1945–1951. doi: 10.1097/00007632-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 37.Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine. 2004;29:1938–1944. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 38.Pearson ASD, Wharton N, Genuario J, McGough W, Spratt K. Precision of lumbar intervertebral measurements: does a computer assisted technique improve reliability? Abstract at: The International Society for the Study of the Lumbar Spine (ISSLS) China: Hong Kong; 2007. [DOI] [PubMed] [Google Scholar]
- 39.Pellise F, Hernandez A, Vidal X, Minguell J, Martinez C, Villanueva C. Radiologic assessment of all unfused lumbar segments 7.5 years after instrumented posterior spinal fusion. Spine. 2007;32:574–579. doi: 10.1097/01.brs.0000256875.17765.e6. [DOI] [PubMed] [Google Scholar]
- 40.Penta M, Sandhu A, Fraser RD. Magnetic resonance imaging assessment of disc degeneration 10 years after anterior lumbar interbody fusion. Spine. 1995;20:743–747. doi: 10.1097/00007632-199503150-00018. [DOI] [PubMed] [Google Scholar]
- 41.Phillips FM, Reuben J, Wetzel FT. Intervertebral disc degeneration adjacent to a lumbar fusion: an experimental rabbit model. J Bone Joint Surg Br. 2002;84:289–294. doi: 10.1302/0301-620X.84B2.11937. [DOI] [PubMed] [Google Scholar]
- 42.Pope MH, Wilder DG, Matteri RE, Frymoyer JW. Experimental measurements of vertebral motion under load. Orthop Clin North Am. 1977;8:155–167. [PubMed] [Google Scholar]
- 43.Salen BA, Spangfort EV, Nygren AL, Nordemar R. The disability rating index: an instrument for the assessment of disability in clinical settings. J Clin Epidemiol. 1994;47:1423–1435. doi: 10.1016/0895-4356(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 44.Schulte TL, Leistra F, Bullmann V, Osada N, Vieth V, Marquardt B, Lerner T, Liljenqvist U, Hackenberg L. Disc height reduction in adjacent segments and clinical outcome 10 years after lumbar 360 degrees fusion. Eur Spine J. 2007;16(12):2152–2158. doi: 10.1007/s00586-007-0515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seitsalo S, Schlenzka D, Poussa M, Osterman K. Disc degeneration in young patients with isthmic spondylolisthesis treated operatively or conservatively: a long-term follow-up. Eur Spine J. 1997;6:393–397. doi: 10.1007/BF01834066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Throckmorton TW, Hilibrand AS, Mencio GA, Hodge A, Spengler DM. The impact of adjacent level disc degeneration on health status outcomes following lumbar fusion. Spine. 2003;28:2546–2550. doi: 10.1097/01.BRS.0000092340.24070.F3. [DOI] [PubMed] [Google Scholar]
- 47.Umehara S, Zindrick MR, Patwardhan AG, Havey RM, Vrbos LA, Knight GW, Miyano S, Kirincic M, Kaneda K, Lorenz MA. The biomechanical effect of postoperative hypolordosis in instrumented lumbar fusion on instrumented and adjacent spinal segments. Spine. 2000;25:1617–1624. doi: 10.1097/00007632-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 48.Wai EK, Santos ER, Morcom RA, Fraser RD. Magnetic resonance imaging 20 years after anterior lumbar interbody fusion. Spine. 2006;31:1952–1956. doi: 10.1097/01.brs.0000228849.37321.a8. [DOI] [PubMed] [Google Scholar]
- 49.Van Horn JR, Bohnen LM. The development of discopathy in lumbar discs adjacent to a lumbar anterior interbody spondylodesis: a retrospective matched-pair study with a postoperative follow-up of 16 years. Acta Orthop Belg. 1992;58:280–286. [PubMed] [Google Scholar]
- 50.Weinhoffer SL, Guyer RD, Herbert M, Griffith SL. Intradiscal pressure measurements above an instrumented fusion: a cadaveric study. Spine. 1995;20:526–531. doi: 10.1097/00007632-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 51.Videbaek TS, Christensen FB, Soegaard R, Hansen ES, Hoy K, Helmig P, Niedermann B, Eiskjoer SP, Bunger CE. Circumferential fusion improves outcome in comparison with instrumented posterolateral fusion: long-term results of a randomized clinical trial. Spine. 2006;31:2875–2880. doi: 10.1097/01.brs.0000247793.99827.b7. [DOI] [PubMed] [Google Scholar]
- 52.Wiltse LL, Radecki SE, Biel HM, DiMartino PP, Oas RA, Farjalla G, Ravessoud FA, Wohletz C. Comparative study of the incidence and severity of degenerative change in the transition zones after instrumented versus noninstrumented fusions of the lumbar spine. J Spinal Disord. 1999;12:27–33. doi: 10.1097/00002517-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Yang JY, Lee JK, Song HS. The impact of adjacent segment degeneration on the clinical outcome after lumbar spinal fusion. Spine. 2008;33:503–507. doi: 10.1097/BRS.0b013e3181657dc3. [DOI] [PubMed] [Google Scholar]
- 54.Zhao K, Yang C, Zhao C, An KN. Assessment of non-invasive intervertebral motion measurements in the lumbar spine. J Biomech. 2005;38:1943–1946. doi: 10.1016/j.jbiomech.2004.07.029. [DOI] [PubMed] [Google Scholar]