Abstract
It has been widely reported a vascular and neurologic damage of the lumbar muscles produced in the classic posterior approach for lumbar spinal fusions. The purpose of this study is to demonstrate a better clinical and functional outcome in the postoperative and short term in patients undergoing minimal invasive surgery (“mini-open”) for this lumbar spinal arthrodesis. We designed a prospective study with a 30 individuals cohort randomized in two groups, depending on the approach performed to get a instrumented lumbar circumferential arthrodesis: “classic posterior” (CL group) or “mini-open” approach (MO group). Several clinical and functional parameters were assessed, including blood loss, postoperative pain, analgesic requirements and daily life activities during hospital stay and at the 3-month follow-up. Patients of the “mini-open approach” group had a significant lower blood loss and hospital stay during admission. They also had significant lower analgesic requirements and faster recovery of daily life activities (specially moderate efforts) when compared to the patients of the “classic posterior approach” group. No significant differences were found between two groups in surgery timing, X-rays exposure or sciatic postoperative pain. This study, inline with previous investigations, reinforces the concept of minimizing the muscular lumbar damage with a mini-open approach for a faster and better recovery of patients’ disability in the short term. Further investigations are necessary to confirm these findings in the long term, and to verify the achievement of a stable lumbar spinal fusion.
Keywords: Mini-invasive, Surgery, Lumbar fusion, Discopathy, Arthrodesis
Introduction
Lumbar spinal fusion is a standardized and widely accepted procedure in the treatment of discogenic back pain, when the conservative treatments have failed [2, 3, 11, 38, 39, 45]. The clinical results of lumbar circumferential fusions (anterior lumbar interbody fusion (ALIF) + posterior fusion [5, 43] or 360° posterolateral interbody fusion/transforaminal interbody fusion (PLIF/TLIF) fusion [1, 14, 16, 27, 45]) are substantially better than the obtained with posterolateral arthrodesis with pedicle screws. However, the high rate of complications and the surgical risks associated to these double approaches (anterior and posterior) lead us to make these circumferential fusions with a single posterior approach PLIF [6, 33, 46] or TLIF [15, 26, 38]. For the PLIF technique a bilateral approach to the disc is necessary, but the TLIF technique is available with a single one, using a one-side total or subtotal facetectomy to achieve an intersomatic fusion with guarantees [1, 40] with less neural retraction and postoperative epidural scar [1, 17, 22, 26, 33].
It has been widely reported in previous studies, a vascular and neurologic damage of the lumbar muscles produced in the classic posterior approach [11, 12, 21, 22, 29, 35, 41, 42]. With minimally invasive approaches it is possible to avoid this muscular damage and to achieve a circumferential fusion. The minimally invasive TLIF procedure was first described by Foley et al. [10] in order to minimize the paraspinous muscle injury that occurs with traditional open procedures. The intersomatic fusion can be performed using a PLIF [23, 33] or a TLIF technique [7, 8, 10, 18–20, 30, 32, 40]. The insertion of pedicle screws can be made percutaneously [18, 19, 23, 33, 40], or with a mini-open approach assisted by special retractors (Xtube and Quadrant®) (Medtronic Sofamor Danec, Memphis, TN, USA) [7, 13, 30] or others [20, 31, 32, 44].
The potential advantages of these mini-open approaches in opposition to the classic medial approach are: smaller scars, reduced intra- and postoperative blood loss, lesser transfusion requirements, reduced analgesic and narcotic use in the immediate postoperative period and shortened hospital stay [7, 10, 13, 18–20, 33, 40, 44]. All these advantages are strengthened because the short-term clinical results are usually better when compared to the ones obtained with the classical techniques in the few studies found in the literature [19, 20, 33]. The limitations of these new techniques are the learning curve, longer time of surgery and more exposition to X rays [7, 10, 18–20, 31, 40].
Mini-invasive approaches, besides a minor cutaneous incision, can produce lesser lumbar muscular damage than the classic approach, as it has been suggested [29], with laboratory enzymatic [41] and blood-analysis studies [24, 37] and with MRI controls [24, 25, 41]. Is this reduced tissular damage leading these patients to a better and faster recovery of their daily life activities?
The aims of this study are, in first place, to confirm the intra- and postoperative advantages of mini-open approaches for lumbar spinal fusion in our environment. In second place, to study the analgesic requirements once the patients have been discharged from the hospital in a three-month follow-up. And in third place, to study the recovery of daily life activities in the first 3-months after lumbar spine surgery.
Methods
We studied a consecutive series of 30 individuals, without previous medical conditions, who underwent surgery from January to October 2007, with a circumferential (360°) lumbar or lumbosacral fusion at one-level using a posterior approach.
The indication for surgery was a degenerative discopathy in all cases, with or without disc herniation in patients who had not undergone previous lumbar spine surgery. Patients with spinal stenosis or isthmic spondylolisthesis were excluded. In all cases, the surgery was offered to the patients after six or more months of back lumbar pain for which conservative treatments had been unsuccessful or in cases of previous degenerative discopathy with acute neurological deficit or pain exacerbation. None of the cases had other lumbar discs showing MRI degenerative changes. All the patients underwent one-level fusion.
The subjects included in this study were randomized in two groups depending on their position in the “waiting list” for surgery. Patients included in the “couples” group underwent a 360° circumferential arthrodesis with the classic posterior approach (CL group); and patients included in the “odds” group underwent the same instrumented arthrodesis, but using the mini-open approach (MO group).
Surgical techniques
In the CL group (classic approach) the procedure was: medial approach, with a 12-cm length incision, one side subtotal facetectomy (symptomatic side), discectomy made from the symptomatic side and mono-portal TLIF. We added pedicle screw fixation, decortication of lateral facets and transverse processes and autologous bone graft extracted from the laminectomy and facetectomy.
In the MO group: two paramedian incisions of 2.5 cm length were performed, 3.5 cm lateral to the medial back line. Under fluoroscopic control the insertion of progressive wider dilators (METRx-MD®) was done up to the 22-mm diameter. On this dilator, we slide the tubular retractor (QUADRANT). After removing the remaining musculature and identification of articular facets and transverse process, we made the subtotal facetectomy, discectomy, mono-portal TLIF, pedicle screws placement, lateral facets and transverse processes decortication and bilateral autologous bone grafting in the same way as in the classic approach, but all across the tubular retractors. These tubular retractors allowed us to perform the surgery with binocular loupes or even under direct visualization. Two surgeons simultaneously, in each side of the patient, made the surgery (Figs. 1, 2, 3).
Fig. 1.
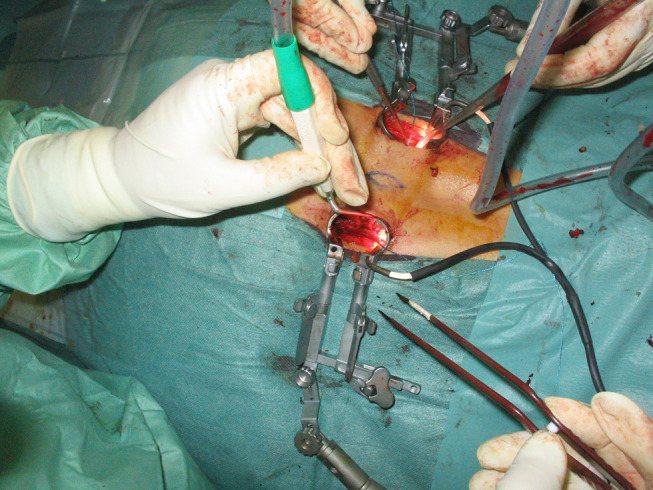
With the tubular retractors through the paramedian incisions, two spine surgeons can work simultaneously
Fig. 2.
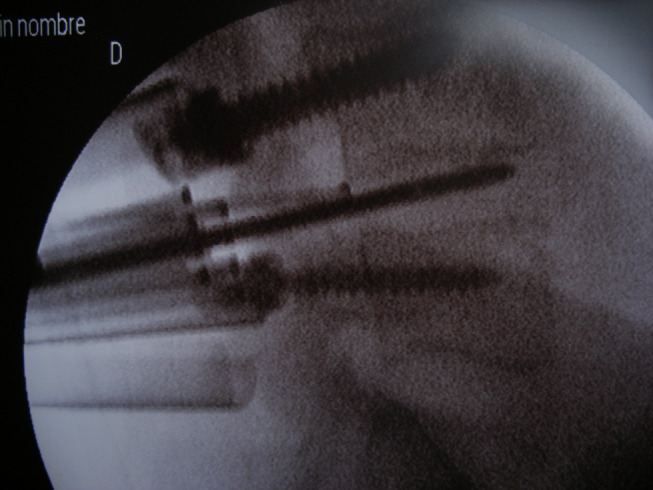
Fluoroscopic image of the same lumbar fusion
Fig. 3.
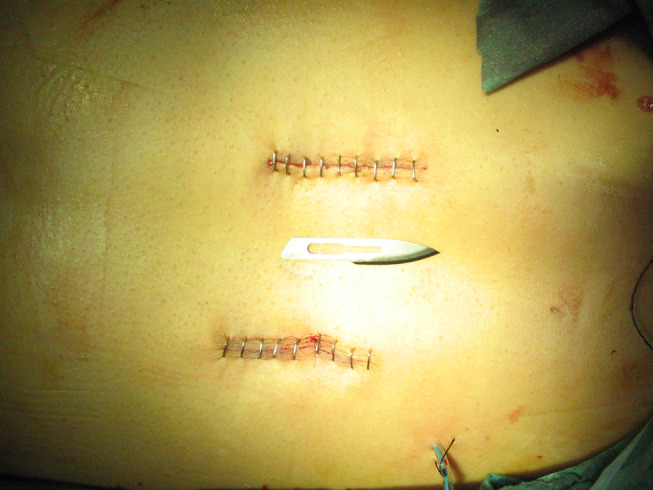
The 2.5-cm length paramedian incisions at the end of the surgery
The intersomatic device used was a 25-mm CIO (PEEK®), filled with autologous bone extracted from the resected laminas and articular processes. The height of the intersomatic device depended on the discal distraction. The sizes have varied from 9 to 12 mm. In all cases bilateral fixation was practised with pedicle screws (Legacy®). While low back pain was the main symptom, most of the patients also presented unilateral sciatica, with the exception of three individuals who suffered bilateral sciatica. In these three cases, bilateral radicular liberation was performed.
The intertransverse fusion was performed with autologous bilateral bone graft in both groups. Bone extracted from the laminectomy is not much quantity, because in the transforaminal approach this laminectomy is minimal.
Bone taken from iliac crest and from the facetectomy was used. In the classic approach group iliac crest bone was extracted following the standard technique, through the posterior medial approach. In the Mini-open group the graft bone was obtained from the contralateral side of the TLIF, through the incision of this side. In all the cases there were no difficulties to reach the iliac crest because the levels affected were L4–L5 and L5–S1. No bone substitute was used in any cases.
In all the patients, drainages were placed in both sides.
Postoperative treatment
Haematocrit and haemoglobin controls were completed 3, 24 and 48 h after the surgery. Requirements for blood transfusion were assessed individually. None of the patients needed intraoperatory blood transfusion. All of them followed the same postoperatory drug treatment: 48 h of analgesic treatment (paracetamol 1 g/8 h, metamizol 2 g/8 h) and intravenous antibiotic. If the patient was suffering from pain, the rescue analgesic administered was subcutaneous methadone clorhidrate 0.1 mg/kg/12 h. Aspirative drainages were maintained during 48 h. In all cases, X-ray study was practised 48 h postsurgery, and then the patients were allowed to stand out of bed if no contraindication was found. They were discharged from hospital when they reached enough autonomy to stand up and when the pain was controlled with oral treatment. No orthesis was prescribed.
At the time of hospital discharge, all patients received the same instructions. They were allowed to a self-control of analgesic requirement, supervised by their General Practitioner, always recording the type and moment they needed the drugs. After the first month, they were encouraged to start normal daily life activities. The patients were also instructed to control these activities on their own.
Assessment of results
The following epidemiological information was recorded: age, gender, spinal level affected, preoperative VAT, SF-36 and Oswestry’s Disability Test (ODI).
The characteristics of each one of the groups are described in Table 1. We did not found statistical differences between groups in age, gender, spinal level to fuse or preoperative clinical status (VAT, ODI and SF-36).
Table 1.
Epidemiological characteristics and perioperative results in both groups
| Classic approach group (CL) | Mini-open approach group (MO) | P | |
|---|---|---|---|
| No. of patients | 15 | 15 | |
| Mean age | 42.06 ± 0.6 | 34.14 ± 8.1 | 0.78 |
| Gender (M/F) (% male) | 11/4 (73%) | 9/6 (60%) | 0.45 |
| Level L4–L5 | 5 (33%) | 4 (27%) | |
| Level L5–S1 | 10 (67%) | 11 (73%) | |
| Haematocrit preop. (%) | 45.8 ± 3.5 | 43.6 ± 4.0 | 0.45 |
| Haemoglobin preop. | 15.2 + 1.5 | 14.7 + 1.5 | 0.66 |
| Vat preop. | 7.4 ± 1.9 | 7.2 ± 1.7 | 0.87 |
| ODI preop (%) | 55.9 ± 10.3 | 59.1 ± 7.9 | 0.74 |
| Surgery time (min) | 162 ± 33 | 179 ± 35 | 0.62 |
| X-ray time (s) | 64 ± 21 | 75 ± 32 | 0.27 |
| Estimated blood loss (ml) | 757 ± 255 | 318 ± 215 | 0.002 |
| Drainages (cc) | 480 ± 326 | 269 ± 197 | 0.69 |
| Haematocrit decrease | 13.9 ± 4.8 | 8.9 ± 4.2 | 0.02 |
| Haemoglobin decrease | 5.0 ± 1.6 | 3.3 ± 0.6 | 0.04 |
| Hospital stay (day) | 5.2 ± 1.5 | 3.6 ± 0.6 | 0.000 |
| Morfic requirements [no. of (%) patients] | 6/15 (40%) | 5/15 (33%) | 0.77 |
In both groups we studied two types of parameters:
-
A.
Hospitalary parameters: duration of surgery (minutes), time of X-ray exposure (seconds), estimated intraoperative bleeding (cm3), total drainage bleeding (cm3), postoperative sciatica, narcotic requirements in the first 48 h, haematocrit and haemoglobin decrease compared to preoperative values, intra- and postoperative complications and hospital stay length.
-
B.
Three-month postoperative parameters: at this moment, the patients refilled the Oswestry’s Test of Disability (ODI) and the ten questions of the physical status scale of the SF-36 (with the following values, 1: for severe disability, 2: for moderate disability and 3: for no disability). They also showed us their analgesia requirement records of the first 3 months, subdivided in daily, occasional or none analgesia requirements.
Statistical assessment
The statistical analysis was made with the program SPSS 16.0. Besides the standard descriptive analysis we used non-parametric tests for the comparative study, mainly the Mann–Whitney U. For quantitative variables the Mann–Whitney U was used, and for ordinal variables Linear-by-linear or Chi-square tests were performed.
Results
Intrahospitalary parameters
No major complications have been observed, including infections, medical complications or abnormal position of the pedicle screws or interbody devices. In the MO group there was one wound disruption that healed for second-intention.
The results are described in Tables 1, 2 and 3, with significant differences in bold values. In the MO group, the frequency of estimated operative blood loss, the difference between pre- and postoperative haematocrit levels and haemoglobin values and the hospital stay were all significantly lower than in the CL group. The time of exposition to X-ray was higher in the MO group, when compared to the CL group, but differences were not statistically significant.
Table 2.
Oswestry Disability Index (ODI) scores at the 3 month follow-up
| Classic approach group (CL) | Mini-open approach group (MO) | P | |
|---|---|---|---|
| Surgery time (min) | 162 ± 33 | 179 ± 35 | 0.62 |
| X-ray time (s) | 64 ± 21 | 75 ± 32 | 0.27 |
| Estimated blood loss (ml) | 757 ± 255 | 318 ± 215 | 0.002 |
| Drainages (cc) | 480 ± 326 | 269 ± 197 | 0.69 |
| Haematocrit decrease | 13.9 ± 4.8 | 8.9 ± 4.2 | 0.02 |
| Haemoglobin decrease | 5.0 ± 1.6 | 3.3 ± 0.6 | 0.04 |
| Hospital stay (day) | 5.2 ± 1.5 | 3.6 ± 0.6 | 0.000 |
| Morfic requirements [no. (%) patients] | 6/15 (40%) | 5/15 (33%) | 0.77 |
A higher score means more disability
Table 3.
SF-36 physical scale scores at the 3-month follow-up
| Oswestry disabled index | Classic approach group (CL) | Mini-open approach group (MO) | P |
|---|---|---|---|
| Analgesics | 1.8 ± 1.5 | 0.7 ± 1.2 | 0.016 |
| Getting dressed | 0.3 ± 0.7 | 0.2 ± 0.5 | 0.56 |
| Lifting | 2.5 ± 0.9 | 1.9 ± 1.0 | 0.15 |
| Walking | 1.2 ± 1.1 | 0.4 ± 0.6 | 0.03 |
| Sitting | 0.9 ± 1.1 | 0.9 ± 1.1 | 0.8 |
| Standing | 1.2 ± 1.1 | 0.4 ± 0.6 | 0.016 |
| Sleeping | 1.0 ± 1.3 | 0.8 ± 0.2 | 0.07 |
| Social and recreational life | 1.6 ± 0.9 | 0.8 ± 0.7 | 0.013 |
| Travelling | 2.7 ± 1.6 | 1.1 ± 1.2 | 0.006 |
| Sex life | 2.7 ± 1.2 | 1.5 ± 1.1 | 0.009 |
| ODI (%) | 31.5 ± 14.2 | 16.0 ± 12.1 | 0.005 |
Similarly, we found no significant differences in the duration of surgery, in the postoperative bleeding (drainage), in the presence of sciatic pain or in narcotic requirements in the immediate postoperative period.
Analgesia in the first 3 months post-discharge
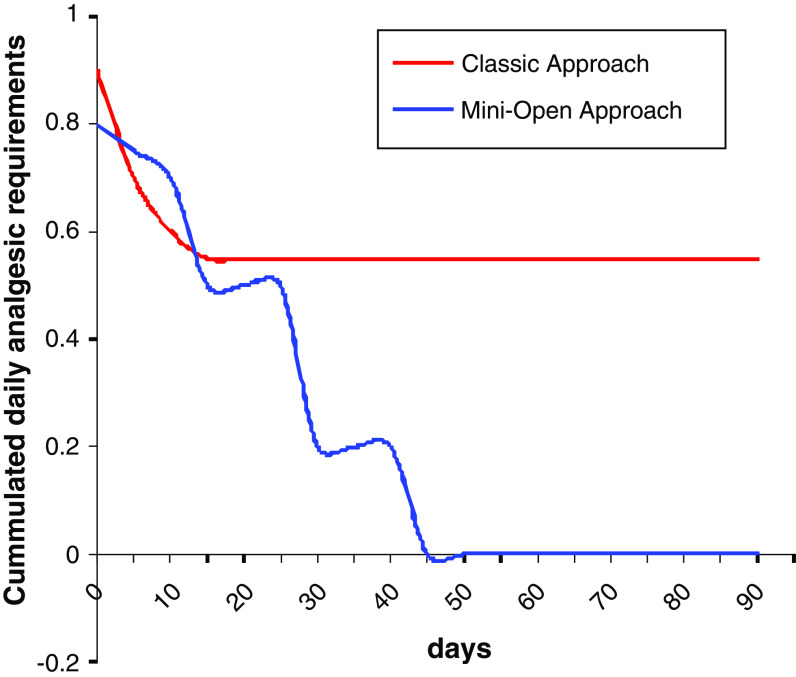
The need of analgesics was much less frequent in MO group during the first 3 months postsurgery (Fig. 4).
Fig. 4.
Cumulated daily analgesic requirements
“Daily analgesia requirements” diminished remarkably in both groups in the first days after hospital discharge. Nevertheless, beyond 20 days from the surgery, some differences were observed. This decrease continued in the MO group patients, and 40 days after the surgery no patients in this group needed daily analgesia. On the contrary, patients in the CL group needed daily analgesia up to 3 months as minimum (Fig. 4).
Requirements of “some kind of analgesia” also diminished progressively in both groups. But, while in the MO group only 20% of the patients required analgesics in the 3-month follow-up, in the CL group this percentage was 53.8%, with statistically significant differences.
Clinical results three months postsurgery
In Tables 1 and 2, descriptive and comparative statistics of the parameters studied are shown.
In Table 3 we observe that the Oswestry Disability Index (ODI) scores in patients of the MO group are much lower than in the CL group, with a mean difference of 15.5 points, being statistically significant (P = 0.005).
Mean values in all the parameters of disability assessed are lower in the MO group, when compared to the CL group in the first 3 months postsurgery. Remarkably, the differences were statistically significant in activities requiring “moderate” efforts (walking, standing, social and recreational Life, travelling, sex life), but not in activities requiring “hard” efforts (lifting) or “light” efforts (getting dressed).
An isolated evaluation, as well as the scores in the SF-36 physical scale (ten parameters addition; Table 4), confirm the Oswestry’s test results. We found very significant differences between both groups in the SF-36 physical scale scores (percentage difference of 15%, P = 0.001). Furthermore, the biggest differences remained in the recovery of activities requiring “moderate” efforts (climbing several flights of stairs, walking several blocks). We have not found significant differences in activities requiring “great” efforts (vigorous activities, lifting or carrying groceries, bending, kneeling or stooping) or “light” efforts (walking one block, bathing or dressing).
Discussion
The 360° single-level lumbar fusion for degenerative discopathy producing chronic lumbar pain is an alternative treatment widely accepted, once the conservative therapies have been unsuccessful. The possibility of achieving this 360° single level fusion using mini-invasive approaches has been developed recently [10, 13, 33] and different techniques have been published to obtain it: mini-invasive ALIF [28, 36] and posterior percutaneous pedicle fixation, PLIF and pedicle fixation [33], uniportal TLIF and percutaneous [10, 19, 23, 24, 32, 40] or “mini-open” [7, 30, 32] pedicle fixation, uniportal TLIF and unilateral pedicle fixation with or without fixation of contralateral facet [4, 7, 20, 44]. We have chosen the Quadrant retractor because it allows us to make a bilateral “mini-open” approach. Through this double approach a discectomy and decompression can be made, either in the symptomatic side or in both sides, to place an intersomatic device uni- or bilateral, to decorticate the articular facet and transverse process and to place pedicle screws under direct vision or with binocular loupes, with no need of microscope or endoscopy. Besides, through one of these small approaches it is possible to extract autologous graft from iliac bone. In our “mini-open” MO group, just in one case bilateral radicular decompression was made, in the rest of the patients we have placed uniportal TLIF from the symptomatic side. A mini-approach in the non-symptomatic side was made to place pedicle screws under direct vision, decortication of articular facets and autologous bone grafting. A correct insertion of screws is easier for us in identifying the facets and transverse processes under direct vision than making it percutaneously. This bilateral mini-approach can be made with two surgeons working simultaneously. In our opinion, that is the reason because of our surgery duration is not as long as in other studies, in spite of the learning curve.
No major complication has been reported in our group, although we agree with Foley et al. [10, 13] who say this is a difficult procedure in which you need a good 3-D knowledgement of lumbar spine. We also confirm that L5–S1 space is more “uncomfortable” than the L4–L5 one. In our sample, with a one-level degenerative discopathy, the most frequent affected disc was the L5–S1.
The first advantage of this mini-invasive technique is a lesser operative and postoperative blood loss [19, 33, 40, 45]. This leads to a significantly smaller haematocrit and haemoglobin decrease in the “mini-open” group. As in other studies [7, 19, 33, 40], none of the patients with a mini-open approach needed blood transfusion.
During the surgery we used radioscopic control. The average time of exposure has been greater in the mini-invasive surgery that in the classic one. We think that it will be decreasing as we have more experience, but objectively in the same conditions it is necessary to use more time of X-rays in the mini-invasive techniques than in the open ones [33, 45]. Our operative and postoperative results (length of surgery, X-rays exposure, bleeding and hospital stay) do not differ from the wide range of results previously reported in these surgeries [7, 19, 20, 33, 40, 44].
We had neither intraoperative complications (dural tears) nor wrong-positioned devices (screws or intersomatic devices). We attribute that to the exclusion of any patient with previous surgery or spinal stenosis. The TLIF is safer than the PLIF technique because of the risks of dural tears, since a minimum resection of the ligamentum flavum and a smaller separation of the nerve root are needed [15, 19, 40]. On the other hand, with a uniportal TLIF technique, a subtotal excision of the joint facets of one side is made, so it would be more exact accurate to name call it a 270° instead a 360° fusion.
Other advantages attributed to the mini-invasive approaches are the lesser postoperative requirements of analgesic drugs, and a shorter hospital stay [7, 19, 33, 40, 44]. In our group we confirm that hospital stay has been significant minor in the “mini-open” group; nevertheless, the narcotic demand in the first days after surgery has not been significantly different in both groups. The lesser blood loss, the smaller requirements of transfusion and the shorter hospital stay of the mini-invasive surgeries can support their economic advantages [47].
The smaller scar can be a cosmetic and psychological advantage for the patient. Nevertheless, once the patient is discharged from hospital, the clinical results in the short- and mid-term rewarded the effort of the surgical team. For us, it was very important to appreciate if a smaller vascular and neurological injury of the lumbar musculature in the mini-invasive surgery provoked less pain in the first months and a faster and better recovery of daily life activities.
Although we have not confirmed a smaller analgesic demand during the hospital stay [19, 33, 40], we have proved that in the first 3 months after surgery these analgesic requirements are significantly lower in the “mini-open” group. This could be explained by a minimum injury of the lumbar musculature.
In our study we have documented that patients in both groups had a fast recovery of activities requiring “light” efforts (getting dressed, sitting, sleeping, walking one block, having bath); and that a few patients in both groups could perform activities requiring “great” efforts (lifting heavy things, running, participating in sports, bending, kneeling or stooping) 3 months after surgery. Nevertheless, activities requiring “moderate” efforts (walking more than a mile, standing, social life, travelling, having sex) were significantly sooner recovered in patients of the mini-open (MO) group. Further studies must reinforce these findings, suggesting a faster muscular recovery in patients undergoing mini-invasive approaches.
There are no evidences about the clinical results of the 360° single level lumbar fusions being better with mini-invasive techniques than with conventional open surgery. Several studies of mini-invasive techniques have been published, reporting hopeful early results in a 1-year follow-up [33, 40]. Nevertheless we do not have knowledge of any comparative report studying functional recovery in the first months after surgery.
Although to evaluate definitive results of spinal fusion, a minimum follow-up of 1 year is necessary, and the recovery of the daily activities must be an objective of the lumbar surgery, in our study we observed that 3 months postsurgery, the recovery of activities of daily life is significantly better in the “mini-open” group, measured by Oswestry’s test and the ten parameters of the physical scale in the SF-36, validated as standard outcome measures [9, 34].
This fact, added to a lower ingestion of analgesic drugs, in our opinion, is the result of a smaller vascular and neurological damage of lumbar musculature with a mini-invasive approach. This is not in contradiction with studies demonstrating comparable clinical results in a 1-year follow-up between classic and mini-open approaches, since the lumbar musculature recovery takes place throughout several months in patients undergoing classic approaches.
We are conscious about the limitations of this study. The size of both groups is short to draw definitive conclusions; the period of follow-up is short and there can be differences in the long-term, specially if the intersomatic fusion is not achieved; and the better and faster recovery of physical activity does not predict the same rate of fusion with mini-invasive than with open approaches techniques.
Although we cannot predict comparable clinical results in both groups in the long term, we have confirmed that the hospitalary parameters (operative bleeding, haemoglobin and haematocrit decrease and hospital stay) are significantly better in the “mini-open” approach patients than in the classic approach ones. The smaller analgesic requirements during the first three months and the better recovery of the activities of daily life, as well as the satisfaction of the patient in the “mini-open” group, is, to our opinion, the result of a smaller vascular and neurological injury in the lumbar musculature caused with a “mini-open” in comparison to the classic approach of the lumbar spine. Finally, we think that the technical effort of undergoing the “learning curve” is really worthy.
Conflict of interest statement
None of the authors has any potential conflict of interest.
References
- 1.Anand N, Hamilton JF, Perri B, Miraliakbar H, Goldstein T. Cantilever TLIF with structural allograft and RhBMP2 for correction and maintenance of segmental sagittal lordosis: long-term clinical, radiographic, and functional outcome. Spine. 2006;31(20):E748–E753. doi: 10.1097/01.brs.0000240211.23617.ae. [DOI] [PubMed] [Google Scholar]
- 2.Atlas SJ, Keller RB, Robson D, Deyo RA, Singer DE. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the maine lumbar spine study. Spine. 2000;25(5):556–562. doi: 10.1097/00007632-200003010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine. 2005;30(8):934–936. doi: 10.1097/01.brs.0000158953.57966.c0. [DOI] [PubMed] [Google Scholar]
- 4.Beringer WF, Mobasser JP. Unilateral pedicle screw instrumentation for minimally invasive transforaminal lumbar interbody fusion. Neurosurg Focus. 2006;20(3):E4. [PubMed] [Google Scholar]
- 5.Bono CM, Lee CK. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine. 2004;29(4):455–463. doi: 10.1097/01.BRS.0000090825.94611.28. [DOI] [PubMed] [Google Scholar]
- 6.Cloward RB. The treatment of ruptured lumbar intervertebral disc by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10:154–168. doi: 10.3171/jns.1953.10.2.0154. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch H, Musacchio MJ., Jr Minimally invasive transforaminal lumbar interbody fusion with unilateral pedicle screw fixation. Neurosurg Focus. 2006;20(3):E10. doi: 10.3171/foc.2006.20.3.11. [DOI] [PubMed] [Google Scholar]
- 8.Eck JC, Hodges S, Humphreys SC. Minimally invasive lumbar spinal fusion. J Am Acad Orthop Surg. 2007;15(6):321–329. doi: 10.5435/00124635-200706000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 10.Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(15 Suppl):S26–S35. doi: 10.1097/00007632-200308011-00006. [DOI] [PubMed] [Google Scholar]
- 11.Fritzell P, Hägg O, Wessberg P, Nordwall A. Swedish Lumbar Spine Study Group. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine. 2002;27(11):1131–1141. doi: 10.1097/00007632-200206010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Gejo R, Matsui H, Kawaguchi Y, Ishihara H, Tsuji H. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine. 1999;24(10):1023–1028. doi: 10.1097/00007632-199905150-00017. [DOI] [PubMed] [Google Scholar]
- 13.German JW, Foley KT. Minimal access surgical techniques in the management of the painful lumbar motion segment. Spine. 2005;30(16 Suppl):S52–S59. doi: 10.1097/01.brs.0000174501.53285.9d. [DOI] [PubMed] [Google Scholar]
- 14.Hackenberg L, Halm H, Bullmann V, Vieth V, Schneider M, Liljenqvist U. Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine J. 2005;14(6):551–558. doi: 10.1007/s00586-004-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author’s transl) Z Orthop Ihre Grenzgeb. 1982;120(3):343–347. doi: 10.1055/s-2008-1051624. [DOI] [PubMed] [Google Scholar]
- 16.Harms JG, Jeszenszky D. The unilateral transforaminal approach for posterior lumbar interbody fusion. Oper Orthop Traumatol. 1998;10(2):90–102. doi: 10.1007/s00064-006-0112-7. [DOI] [PubMed] [Google Scholar]
- 17.Hee HT, Castro FP, Jr, Majd ME, Holt RT, Myers L. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: analysis of complications and predictive factors. J Spinal Disord. 2001;14(6):533–540. doi: 10.1097/00002517-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Holly LT, Schwender JD, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion: indications, technique, and complications. Neurosurg Focus. 2006;20(3):E6. doi: 10.3171/foc.2006.20.3.7. [DOI] [PubMed] [Google Scholar]
- 19.Isaacs RE, Podichetty VK, Santiago P, Sandhu FA, Spears J, Kelly K, Rice L, Fessler RG. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine. 2005;3(2):98–105. doi: 10.3171/spi.2005.3.2.0098. [DOI] [PubMed] [Google Scholar]
- 20.Jang JS, Lee SH. Minimally invasive transforaminal lumbar interbody fusion with ipsilateral pedicle screw and contralateral facet screw fixation. J Neurosurg Spine. 2005;3(3):218–223. doi: 10.3171/spi.2005.3.3.0218. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi Y, Yabuki S, Styf J, Olmarker K, Rydevik B, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. Topographic evaluation of intramuscular pressure and blood flow in the porcine back muscle during surgery. Spine. 1996;21(22):2683–2688. doi: 10.1097/00007632-199611150-00019. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi Y, Matsui H, Tsuji H. Changes in serum creatine phosphokinase MM isoenzyme after lumbar spine surgery. Spine. 1997;22(9):1018–1023. doi: 10.1097/00007632-199705010-00015. [DOI] [PubMed] [Google Scholar]
- 23.Khoo LT, Palmer S, Laich DT, Fessler RG. Minimally invasive percutaneous posterior lumbar interbody fusion. Neurosurgery. 2002;51(5 Suppl):S166–S181. [PubMed] [Google Scholar]
- 24.Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine. 2005;30(1):123–129. doi: 10.1097/01.brs.0000157172.00635.3a. [DOI] [PubMed] [Google Scholar]
- 25.Kim KT, Lee SH, Suk KS, Bae SC. The quantitative analysis of tissue injury markers after mini-open lumbar fusion. Spine. 2006;31(6):712–716. doi: 10.1097/01.brs.0000202533.05906.ea. [DOI] [PubMed] [Google Scholar]
- 26.Lowe TG, Tahernia AD, O’Brien MF, Smith DA. Unilateral transforaminal posterior lumbar interbody fusion (TLIF): indications, technique, and 2-year results. J Spinal Disord Tech. 2002;15(1):31–38. doi: 10.1097/00024720-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Madan S, Boeree NR. Outcome of posterior lumbar interbody fusion versus posterolateral fusion for spondylolytic spondylolisthesis. Spine. 2002;27(14):1536–1542. doi: 10.1097/00007632-200207150-00011. [DOI] [PubMed] [Google Scholar]
- 28.Mayer TG, Vanharanta H, Gatchel RJ, Mooney V, Barnes D, Judge L, Smith S, Terry A. Comparison of CT scan muscle measurements and isokinetic trunk strength in postoperative patients. Spine. 1989;14(1):33–36. doi: 10.1097/00007632-198901000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine. 1997;22(6):691–699. doi: 10.1097/00007632-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 30.Mummaneni PV, Rodts GE., Jr The mini-open transforaminal lumbar interbody fusion. Neurosurgery. 2005;57(4 Suppl):256–261. doi: 10.1227/01.NEU.0000176408.95304.F3. [DOI] [PubMed] [Google Scholar]
- 31.Ozgur BM, Yoo K, Rodriguez G, Taylor WR. Minimally-invasive technique for transforaminal lumbar interbody fusion (TLIF) Eur Spine J. 2005;14(9):887–894. doi: 10.1007/s00586-005-0941-3. [DOI] [PubMed] [Google Scholar]
- 32.Ozgur BM, Hughes SA, Baird LC, Taylor WR. Minimally disruptive decompression and transforaminal lumbar interbody fusion. Spine J. 2006;6(1):27–33. doi: 10.1016/j.spinee.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537–543. doi: 10.1097/01.brs.0000256473.49791.f4. [DOI] [PubMed] [Google Scholar]
- 34.Perkins AJ, Stump TE, Monahan PO, McHorney CA. Assessment of differential item functioning for demographic comparisons in the MOS SF-36 health survey. Qual Life Res. 2006;15(3):331–348. doi: 10.1007/s11136-005-1551-6. [DOI] [PubMed] [Google Scholar]
- 35.Pradhan BB, Nassar JA, Delamarter RB, Wang JC. Single-level lumbar spine fusion: a comparison of anterior and posterior approaches. J Spinal Disord Tech. 2002;15(5):355–361. doi: 10.1097/00024720-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Regan JJ, Yuan H, McAfee PC. Laparoscopic fusion of the lumbar spine: minimally invasive spine surgery. A prospective multicenter study evaluating open and laparoscopic lumbar fusion. Spine. 1999;24(4):402–411. doi: 10.1097/00007632-199902150-00023. [DOI] [PubMed] [Google Scholar]
- 37.Sasaoka R, Nakamura H, Konishi S, Nagayama R, Suzuki E, Terai H, Takaoka K. Objective assessment of reduced invasiveness in MED. Compared with conventional one-level laminotomy. Eur Spine J. 2006;15(5):577–582. doi: 10.1007/s00586-005-0912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheufler KM, Dohmen H, Vougioukas VI. Percutaneous transforaminal lumbar interbody fusion for the treatment of degenerative lumbar instability. Neurosurgery. 2007;60(4 Suppl 2):203–212. doi: 10.1227/01.NEU.0000255388.03088.B7. [DOI] [PubMed] [Google Scholar]
- 39.Schofferman J, Slosar P, Reynolds J, Goldthwaite N, Koestler M. A prospective randomized comparison of 270 degrees fusions to 360 degrees fusions (circumferential fusions) Spine. 2001;26(10):E207–E212. doi: 10.1097/00007632-200105150-00019. [DOI] [PubMed] [Google Scholar]
- 40.Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(Suppl):S1–S6. doi: 10.1097/01.bsd.0000132291.50455.d0. [DOI] [PubMed] [Google Scholar]
- 41.Stevens KJ, Spenciner DB, Griffiths KL, Kim KD, Zwienenberg-Lee M, Alamin T, Bammer R. Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J Spinal Disord Tech. 2006;19(2):77–78. doi: 10.1097/01.bsd.0000193820.42522.d9. [DOI] [PubMed] [Google Scholar]
- 42.Styf JR, Willén J. The effects of external compression by three different retractors on pressure in the erector spine muscles during and after posterior lumbar spine surgery in humans. Spine. 2005;23(3):354–358. doi: 10.1097/00007632-199802010-00014. [DOI] [PubMed] [Google Scholar]
- 43.Thalgott JS, Chin AK, Ameriks JA, Jordan FT, Giuffre JM, Fritts K, Timlin M. Minimally invasive 360 degrees instrumented lumbar fusion. Eur Spine J. 2000;9(Suppl 1):S51–S56. doi: 10.1007/PL00010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuttle J, Shakir A, Choudhri HF. Paramedian approach for transforaminal lumbar interbody fusion with unilateral pedicle screw fixation. Technical note and preliminary report on 47 cases. Neurosurg Focus. 2006;20(3):E5. doi: 10.3171/foc.2006.20.3.6. [DOI] [PubMed] [Google Scholar]
- 45.Videbaek TS, Christensen FB, Soegaard R, Hansen ES, Høy K, Helmig P, Niedermann B, Eiskjoer SP, Bünger CE. Circumferential fusion improves outcome in comparison with instrumented posterolateral fusion: long-term results of a randomized clinical trial. Spine. 2006;31(25):2875–2880. doi: 10.1097/01.brs.0000247793.99827.b7. [DOI] [PubMed] [Google Scholar]
- 46.Villavicencio AT, Burneikiene S, Bulsara KR, Thramann JJ. Perioperative complications in transforaminal lumbar interbody fusion versus anterior–posterior reconstruction for lumbar disc degeneration and instability. J Spinal Disord Tech. 2006;19(2):92–97. doi: 10.1097/01.bsd.0000185277.14484.4e. [DOI] [PubMed] [Google Scholar]
- 47.Whitecloud TS, III, Roesch WW, Ricciardi JE. Transforaminal interbody fusion versus anterior-posterior interbody fusion of the lumbar spine: a financial analysis. J Spinal Disord. 2001;14(2):100–103. doi: 10.1097/00002517-200104000-00002. [DOI] [PubMed] [Google Scholar]