Abstract
A resorbable composite material (40% PLLA and 60% beta TCP) with a high breaking strength and capacity to withstand plastic and elastic strain has been developed for cervical interbody fusion. This is a prospective study to evaluate clinical and radiological results of 20 patients implanted with 27 cages (mean follow-up, 27 months). Clinical (neck disability index, VAS, neurological evaluation) and radiological (anteroposterior, lateral, bending X-rays) data were assessed before and after surgery. At the end of the study, CT scan was performed to evaluate fusion, resorption of the cage and density of the new tissue substituting the cage. The mean patient age was 50.3 years (range, 18–79 years). The average improvement was 55% for neck pain, 83% for arm pain and 65% for NDI, with 85% good or excellent results at final outcomes. Radiologically, lordosis was significantly improved (mean gain of 5.4° and 3.7° for overall and segmental lordosis, respectively). This correction was conserved in 95% of cases. Fusion was obtained in 96% (CT evaluation). Resorption was started in all cases and completed in an average of 36 months after surgery. The mean density of tissue substituting the cage was 659 UH with a range, of 455–911 UH (compatible with bone nature). Over time, the amount of bony tissue increased and the graft remodelled with an increase in density value. This demonstrates a biological activity and changing bone mineral content of this tissue. The new composite cage under investigation provides long-term fusion without loss of correction or inflammatory reaction. The ceramic block guarantees the maintenance of the disc height and its slow resorption allows long-term fusion and stability with good and reliable clinical and radiological outcomes.
Keywords: TCP/PLLA cage, Anterior cervical fusion, Clinical outcome, Radiological outcome, Cage resorption
Introduction
Anterior cervical discectomy and fusion has gained immense popularity since its introduction in the 1950s by Smith Robinson and Cloward [10, 29]. Autograft is the most commonly used fusion material with good or excellent results in literature [4, 8, 10, 11, 19, 24, 29, 33]. However, the use of autologous grafts induces a second operation, generally on the iliac crest, and may be associated with an important morbidity. Moreover, such complications as long-term pain syndrome, femorocutaneous nerve damage, infection or secondary fracture have been reported [3, 14, 26, 35].
Beta tricalcium phosphate (beta TCP) is an osteoconductive, resorbable material, which, in porous ceramic form, is used as bone substitute since many years. It has proved its clinical effectiveness in many indications [5, 16, 17, 20]. TCP is completely resorbable in variable period depending on the porosity but pure TCP ceramics are brittle materials, as any ceramic material.
The addition to beta-TCP particles of a plastic material, such as resorbable lactic acid polymer (PLLA), increases breaking strength and provides real capacity to withstand plastic and elastic strain [1]. Such composite materials can retain high strength over time. A composite material containing 60% beta TCP and 40% PLLA has demonstrated similar performance to pure TCP in terms of osteogenesis and improved significantly the extent and the quality of bone healing [1, 20]. Thus, it has the major advantage that it can be used under conditions of high biomechanical stress with a low risk of rupture.
The purpose of this study was to evaluate radiological and clinical results of a new cervical interbody cage elaborated from this composite material. Radiological evaluation was used to assess fusion, preservation of correction and resorption of this implant.
Materials and methods
Patient population
At our institution between January 2005 and December 2007, 20 patients (12 men 60% and 8 women 40%) underwent an anterior cervical discectomy and fusion with a composite cage containing 60% beta TCP and 40% PLLA (porosity, 45%) (Duocage®, SBM, Lourdes, France).
The average age of the patients was 50.3 years (range, 18–79 years).
Surgery was performed for one- or two-level soft disc herniation and trauma. The exclusion criteria were that the patient had more than two level stenosis, continuous or combined ossification of the posterior longitudinal ligament, prior cervical spine surgery and significant comorbidities associated with poor general health.
Clinical and radiological evaluation
All patients were evaluated before the surgery by clinical examination, conventional radiography and MRI. They were followed up at regular intervals of 6 weeks, 3, 6 and 12 months, and each year postoperatively; last visit was carried out at the time of this study. At pre-op, post-op and each follow-up visit were included neck disability index (NDI), neck and arm pain evaluation (VAS) and neurological evaluation.
AP, lateral and bending X-rays were performed before the surgery and at each follow-up visit.
The overall (C2–C7) and segmental alignment was defined by angles Cobb technique on lateral X-ray [12]. CT scan evaluation was performed at the end of the study to evaluated fusion, resorption of the material and quality of new bone formation substituting the cage. Resorption of the material was compared with the ceramic initial seize and divided in four grades: no resorption, partial resorption (inferior to 50%); major resorption (more than 50%) and complete resorption (functions of initial cage size). Analysis of new bone formation substituting the cage was performed by CT density measurement of tissue around the cage (Hounsfield units). Values were recorded in the tissue formation at each cardinal points around the residual ceramic and only the lowest density was conserved (lowest mineralization area). Intra- and inter-operator repetitivity and reproducibility were evaluated using the analysis of variance (ANOVA).
Radiological evaluation was carried out by an independent person.
Surgical technique
General anesthesia was used in all patients; standard right-side anterior approach to the cervical spine was performed as described by Smith and Robinson (pre-sterno-cleido-mastoid approach) [29]. Classical discectomy was carried out, the disc space was opened and distraction was applied using a Caspar distractor. Subchondral bone was preserved carefully.
The posterior ligament was opened in all cases and neural structure decompressed if necessary (dura and origin of the nerve roots visualized).
The interbody fusion was performed by composite cage containing 60% beta TCP and 40% PLLA (45% of porosity) (Fig. 1).
Fig. 1.
Composite beta TCP–PLLA cage (Duocage®, Science for Bio Materials, Lourdes, France)
Before surgery, size of implant was defined with templates on anteroposterior and lateral X-rays.
Cages were first imbibed by bone marrow blood (harvested from iliac crest) for 1 min. Then, they were softly introduced in disc space with a specific device (Fig. 2) and carefully pushed in good position with a polyethylene bone impactor.
Fig. 2.
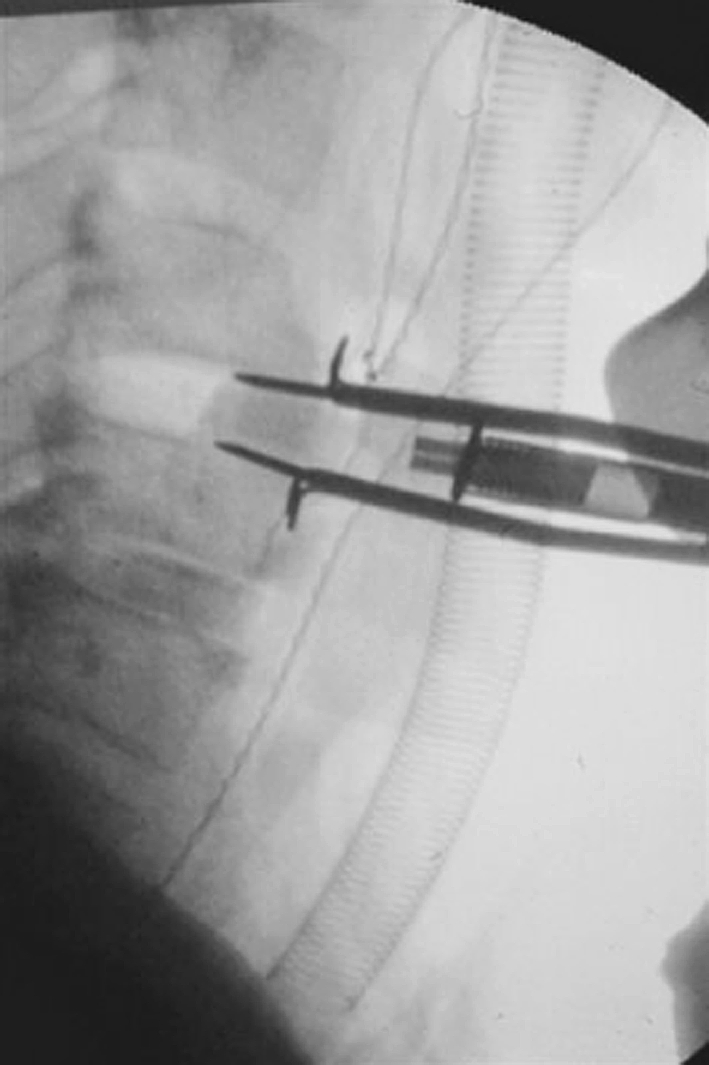
Insertion of the cage with a specific impactor
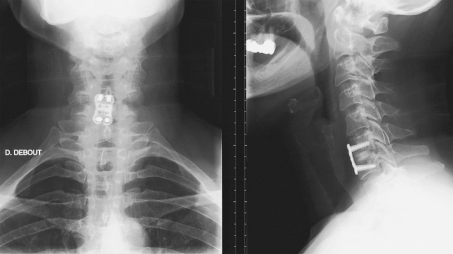
Each cage was consolidated by an anterior titanium locked plate (Zephyr plate, Medtronic, Memphis, USA) (Fig. 3).
Fig. 3.
Anteroposterior and lateral postoperative X-rays
Stand up was allowed the day after surgery and a soft collar should be worn for 10 days.
A 24-h postoperative radiographic control was performed.
Statistical analysis
Statistical comparisons of preoperative and postoperative results were performed using paired Student’s t test. The chosen level of significance was 0.05.
Results
A total of 20 patients underwent an anterior cervical discectomy and fusion with 27 cervical cages was implanted.
Single-level fusion was performed in 13 patients (65%) for soft disc herniation (11 patients) or trauma (2 patients). Two-level fusions were accomplished at contiguous levels in 7 patients (35%). Surgery was carrying out at the C4–C5 level in 8%, at the C5–C6 in 44% and at the C6–C7 in 48%. The mean follow-up was 27 months (range, 18–42 months).
Clinical outcomes
Preoperative average VAS score was 5.3 (SD, 1.9) for neck pain and 7.5 (SD, 2.6) for arm pain. At the final follow-up, these scores became 2.4 (SD, 1.6) and 1.3 (SD, 1) for neck and arm pain, respectively. The average improvement rate was 55% for neck pain and 83% for arm pain.
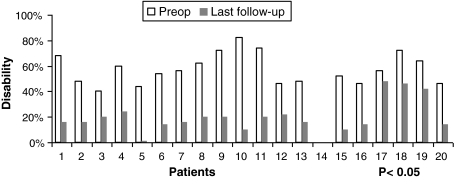
The preoperative mean NDI was 57% (SD, 17%) and values ranged between 40 and 82%. At the end of this study, this index was equal to 20% (SD, 12%) and the average improvement rate was 32.5 points (65%) (Fig. 4).
Fig. 4.
Preoperative and postoperative NDI assessment
Results were significant (P < 0.05) and no clinical complications were reported.
Radiological outcomes
The average overall and segmental lordosis before surgery was 33.6° (SD, 9.1°) and 1.2° (SD, 2.6°), respectively.
After surgery these lordosis angles significantly improved (P < 0.05), overall and segmental mean gain of 5.4° (SD, 2.9°) and 3.7° (SD, 2.4°) were found, respectively.
At the last visit, 95% of the patients conserved initial correction with no curve modification (kyphotic or lordotic evolution) higher than 2°, the mean gain became 6° (SD, 2.4°) and 4° (SD, 2.3°), respectively. These measures are displayed in Table 1.
Table 1.
Overall and segmental sagittal curve assessment
| Patients | Overall lordosis | Segmental lordosis | ||||
|---|---|---|---|---|---|---|
| Pre-op. (°) | Post-op. change (°) | Final follow-up change (°) | Pre-op. (°) | Post-op. change (°) | Final follow-up change (°) | |
| 1 | 34 | +6 | +4 | −1 | +3 | +3 |
| 2 | 54 | 0 | +1 | 3 | −4 | −2 |
| 4 | +2 | +2 | ||||
| 3 | 34 | +4 | +5 | −1 | +4 | +5 |
| 4 | 38 | +8 | +9 | −2 | +6 | +7 |
| 5 | 18 | +8 | +7 | −1 | +4 | +6 |
| 6 | 20 | +7 | +8 | 4 | +7 | +8 |
| 7 | 32 | +5 | +6 | 1 | +2 | +2 |
| 3 | +2 | +3 | ||||
| 8 | 50 | +2 | +4 | 8 | 0 | 0 |
| 3 | +5 | +6 | ||||
| 9 | 26 | +6 | +7 | −2 | +4 | +4 |
| 10 | 40 | +4 | +5 | 4 | +6 | +5 |
| 11 | 20 | +6 | +5 | 1 | +3 | +3 |
| −2 | +4 | +4 | ||||
| 12 | 38 | +8 | +8 | −2 | +5 | +6 |
| 13 | 30 | +4 | +4 | 2 | +3 | +5 |
| 2 | +4 | +3 | ||||
| 14 | 24 | +6 | +6 | −1 | +5 | +5 |
| 15 | 28 | +5 | +6 | −3 | +6 | +7 |
| 16 | 31 | +6 | +7 | 1 | +5 | +6 |
| 17 | 35 | +7 | +9 | 3 | +4 | +3 |
| 18 | 41 | +6 | +5 | 5 | +3 | +3 |
| 2 | +4 | +2 | ||||
| 19 | 37 | +4 | +6 | 0 | +4 | +3 |
| 20 | 38 | +6 | +6 | −1 | +3 | +3 |
| 2 | +5 | +7 | ||||
CT scan performed at the end of the study evidenced fusion rate of 96% (19 patients), while flexion/extension X-ray showed no mobility of the grafted level(s) for all patients 6 months after surgery (successful bone fusion criteria). One pseudarthrosis occurred in an excessive smoker who had a two-level procedure (non-union risks factors).
Complete resorption of cages was found in 19% (mainly beyond 36 months) while major (>50%) or partial (<50%) resorption in 48 and 33%, respectively. This process was started among all patients observed. The ceramic has spherical form and an initial seize of 500 mm³ (V = 4/3μR³). The average resorption of the material was 64% (SD, 29%) at latest follow-up.
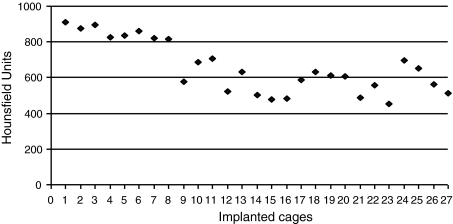
CT evaluation of tissue density substituting the cage (UH) is shown in Fig. 5.
Fig. 5.
CT evaluation of tissue density substituting the cage (Hounsfield Units)
The average density was 659 UH (SD, 147 UH) with a range of 455–911 UH.
The mean standard deviation of repetitiveness was 3.37%, and the mean standard deviation of reproducibility was 5.27% of the measured value.
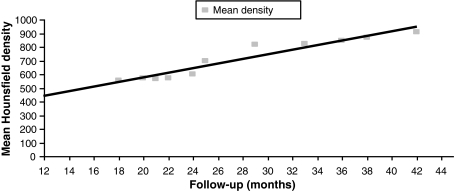
Evolution of this density with time (mean Hounsfield density/follow-up) is assessed and is displayed in the Fig. 6.
Fig. 6.
Evolution of the mean tissue density with time
In 100% of our observations, no measurable implant displacement or other material complication was observed, no cyst or lysis where detected on CT scan analysis at latest follow-up.
Discussion
Interbody fusion cages have been developed to avoid the complications related to bone harvesting from the donor site while increasing immediate stability and promoting bony fusion [7, 18, 21]. Elaborated from pure chemical compounds, such materials seemed safer for patients in comparison with allografts or xenografts [20, 22].
Satisfactory results were obtained with bone substitute regarding the fusion rate although fusion was delayed as compared with that in a cage containing autograft [9].
Tricalcium phosphate (TCP) is a completely resorbable substitute; the advantage of this ceramic is that resorption and bone healing are simultaneous (creeping substitution), leading to complete fusion and new bone formation [5, 16, 17, 20]. However, pure TCP ceramic materials are brittle and can fracture without prior elastic deformation, leading to the formation of hard debris presenting risk of spinal cord or nerve root lesion [1]. A composite material composed of b-TCP and lactic acid polymer (PLLA) was recently developed. This biodegradable polymer presented a modulus of elasticity closer to that of natural cortical bone and can retain high strength over time. This material has a high breaking strength and a real capacity to withstand plastic and elastic strain as compared to pure beta-TCP ceramics. It thus has the major advantage that it can be used under conditions of high biomechanical stress with a low risk of rupture.
In vivo results indicate that, in comparison with pure PLLA, TCP-containing composite material has faster degradation kinetics, caused less inflammatory reaction and promoted contact osteogenesis [1]. This could be a serious advantage but there is no study published to date evaluating the clinical and radiological results such composite device.
In the present study, we report prospectively the clinical and radiological outcomes of anterior cervical fusion with interbody composite cage containing 60% beta TCP and 40% PLLA following one- to two-level discectomy.
Moreover, autologous bone marrow cells from iliac crest aspirate is a source mesenchymal stem cell and ultimately osteoblasts to promote fusion. This addition improves fusion rate mainly in multilevel procedure (similar to autograft) [34].
Clinical results show 90 and 60% of good or excellent results concerning radicular and neck pain, respectively (VAS < 3). Better results are found for arm pain than neck pain as frequently describe by patients in cervical surgery.
The neck disability index is considered good or excellent in 85% with no or mild disability using criteria of Vernon and Mior [32]. None of the patients had severe disability with a score >50%. The NDI average improvement rate is 65%, which is similar to literature data [4, 8, 10, 11, 13, 19, 24, 29, 33].
Radiographic outcome demonstrates correction of the kyphotic deformation with improvement of the lordotic overall and segmental alignment after surgery (except one patient with a smaller cage). Over time, this correction is conserved in 95% of cases [2]. Since no loss of correction or screw facture was observed, authors admit that the mechanical properties of the cage were maintained during the fusion period.
Radiological evaluation of successful bone fusion is achieved in every case 6 months after surgery (flexion/extension plains), but the last follow-up fine cut CT scan show fusion rate of 96%. Fine cut CT scan with reconstruction have been shown to be more reliable and sensitive than plain radiography for detection of pseudoarthrosis [7, 25, 27, 28].
Analysis of tissue density substituting the cage shows values compatible with natural bone in all the cases. Moreover, over time the amount of bony tissue increases and the graft remodels with an increase in density. This demonstrates a biological activity and evolution of bone mineral content of this tissue [30].
Resorption of this composite material occurred in every patient and seemed to start early (before 18 months). Major resorption (>50%) is acquired 2 years after surgery and is complete in an average of 36 months.
Finally, no inflammatory tissue response was found around the cage in opposition to pure PLLA [3, 6, 15, 23, 27, 31] and this procedure leads to a complete fusion with only bone tissue (like autologous grafts).
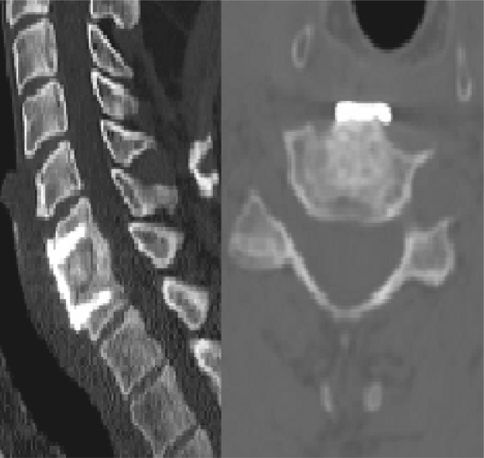
The advantage of this cage is that resorption occurs in the same time of bone healing and this leads to a complete fusion with at the end only bone in the same way as a Cloward procedure (Fig. 7).
Fig. 7.
CT sagittal and coronal views: full resorption of the cage, complete fusion with bone 36 months after surgery
Conclusion
New composite cage under investigation (60% beta TCP–40% PLLA) allows a long-term fusion without loss of correction or inflammatory reaction. The ceramic block guarantees the maintenance of the disc height and its slow resorption allows long-term fusion and stability. At the end, this combination provides the same results as tri-cortical iliac crest graft without the disadvantages of the bone harvesting site. Further clinical studies are requested to confirm these results.
References
- 1.Aunoble S, Clément D, Frayssinet P, Harmand MF, Le Huec JC. Biological performance of a new beta-TCP/PLLA composite material for applications in spine surgery: in vitro and in vivo studies. J Biomed Mater Res A. 2006;78(2):416–422. doi: 10.1002/jbm.a.30749. [DOI] [PubMed] [Google Scholar]
- 2.Bartels RH, Donk R, van Azn RD (2001) Height of cervical foramina after anterior discectomy and implantation of a carbon fiber cage. J Neurosurg 95(1)(Suppl):40–42 [DOI] [PubMed]
- 3.Bergsma JE, Bruijn WC, Rozema FR, Bos RR, Boering G. Late degradation tissue response to poly(l-lactide) bone plates and screws. Biomaterials. 1995;16(1):25–31. doi: 10.1016/0142-9612(95)91092-D. [DOI] [PubMed] [Google Scholar]
- 4.Bishop RC, Moore KA, Hadley MN. Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg. 1996;85:206–210. doi: 10.3171/jns.1996.85.2.0206. [DOI] [PubMed] [Google Scholar]
- 5.Bonnevialle P, Abid A, Mansat P, Verhaeghe L, Clement D, Mansat M (2002) Tibial valgus osteotomy using a tricalcium phosphate medial wedge: a minimally invasive technique. Rev Chir Orthop Repar Appar Mot 88(5):486–492 (in French) [PubMed]
- 6.Bos RR, Rozema FR, Boering G, Nijenhuis AJ, Pennings AJ, Verwey AB, Nieuwenhuis P, Jansen HW. Degradation of and tissue reaction to biodegradable poly(l-lactide) for use as internal fixation of fractures: a study in rats. Biomaterials. 1991;12(1):32–36. doi: 10.1016/0142-9612(91)90128-W. [DOI] [PubMed] [Google Scholar]
- 7.Carreon LY, Glassman SD, Djurasovic M. Reliability and agreement between fine-cut CT scans and plain radiography in the evaluation of posterolateral fusions. Spine J. 2007;7:39–43. doi: 10.1016/j.spinee.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Cauthen JC, Kinard RE, Vogler JB, Jackson DE, DePaz OB, Hunter OL, et al. Outcome analysis of noninstrumented anterior cervical discectomy and interbody fusion in 348 patients. Spine. 1998;23:188–192. doi: 10.1097/00007632-199801150-00008. [DOI] [PubMed] [Google Scholar]
- 9.Cho DY, Lee WY, Sheu PC, Chen CC. Cage containing a biphasic calcium phosphate ceramic (Triosite) for the treatment of cervical spondylosis. Surg Neurol. 2005;63:497–504. doi: 10.1016/j.surneu.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Cloward RB (2007) The anterior approach for removal of ruptured cervical disks. 1958. J Neurosurg Spine 6(5):496–511. doi:10.3171/spi.2007.6.5.496 [DOI] [PubMed]
- 11.Connolly PJ, Esses SI, Kostuik JP. Anterior cervical fusion: outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord. 1996;9:202–206. [PubMed] [Google Scholar]
- 12.Côté P, Cassidy JD, Yong-Hing K, Sibley J, Loewy J. Apophysial joint degeneration, disc degeneration, and sagittal curve of the cervical spine. Can they be measured reliably on radiographs? Spine. 1997;22(8):859–864. doi: 10.1097/00007632-199704150-00007. [DOI] [PubMed] [Google Scholar]
- 13.Dai LY, Jiang LS. Anterior cervical fusion with interbody cage containing beta-tricalcium phosphate augmented with plate fixation: a prospective randomized study with 2-year follow-up. Eur Spine J. 2008;17(5):698–705. doi: 10.1007/s00586-008-0643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fielding WJ. Complications of anterior cervical disc removal and fusion. Clin Orthop Relat Res. 1992;284:10–13. [PubMed] [Google Scholar]
- 15.Fuchs M, Köster G, Krause T, Merten HA, Schmid A. Degradation of and intraosseous reactions to biodegradable poly-l-lactide screws: a study in minipigs. Arch Orthop Trauma Surg. 1998;118(3):140–144. doi: 10.1007/s004020050334. [DOI] [PubMed] [Google Scholar]
- 16.Galois L, Mainard D, Cohen P, Pfeffer F, Traversari R, Delagoutte JP (2000) Filling of bone defects with tricalcium phosphate beta in traumatology. Ann Chir 125(10):972–981. doi:10.1016/S0003-3944(00)00406-5 (in French) [DOI] [PubMed]
- 17.Galois L, Mainard D, Delagoutte JP. Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int Orthop. 2002;26(2):109–115. doi: 10.1007/s00264-001-0329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hacker RJ, Cauthen JC, Gilbert TJ, Griffith SL. A prospective randomized multicenter clinical evaluation of an anterior cervical fusion cage. Spine. 2000;25:2646–2655. doi: 10.1097/00007632-200010150-00017. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser MG, Haid RW, Jr, Subach BR, Barnes B, Rodts GE., Jr Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50:229–238. doi: 10.1097/00006123-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Le Huec JC, Lesprit E, Delavigne C, Clement D, Chauveaux D, Le Rebeller A. Tri-calcium phosphate ceramics and allografts as bone substitutes for spinal fusion in idiopathic scoliosis as bone substitutes for spinal fusion in idiopathic scoliosis: comparative clinical results at four years. Acta Orthop Belg. 1997;63(3):202–211. [PubMed] [Google Scholar]
- 21.Matge G. Anterior interbody fusion with the BAK-cage in cervical spondylosis. Acta Neurochir (Wien) 1998;140:1–8. doi: 10.1007/s007010050049. [DOI] [PubMed] [Google Scholar]
- 22.Päivärinta U, Böstman O, Majola A, Toivonen T, Törmälä P, Rokkanen P. Intraosseous cellular response to biodegradable fracture fixation screws made of polyglycolide or polylactide. Arch Orthop Trauma Surg. 1993;112(2):71–74. doi: 10.1007/BF00420258. [DOI] [PubMed] [Google Scholar]
- 23.Peltoniemi HH, Hallikainen D, Toivonen T, Helevirta P, Waris T. SR-PLLA and SR-PGA miniscrews: biodegradation and tissue reactions in the calvarium and dura mater. J Craniomaxillofac Surg. 1999;27(1):42–50. doi: 10.1016/s1010-5182(99)80009-2. [DOI] [PubMed] [Google Scholar]
- 24.Samartzis D, Shen FH, Lyon C, Phillips M, Goldberg EJ, An HS. Does rigid instrumentation increase the fusion rate in one-level anterior cervical discectomy and fusion? Spine J. 2004;4:636–643. doi: 10.1016/j.spinee.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Santos ER, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interbody fusion using carbon fiber cages. Spine. 2003;28:997–1001. doi: 10.1097/00007632-200305150-00007. [DOI] [PubMed] [Google Scholar]
- 26.Sasso R, Huec JC, Shaffrey C. Iliac crest graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(Suppl):S77–S81. doi: 10.1097/01.bsd.0000112045.36255.83. [DOI] [PubMed] [Google Scholar]
- 27.Schakenraad JM, Hardonk MJ, Feijen J, Molenaar I, Nieuwenhuis P. Enzymatic activity toward poly(l-lactic acid) implants. J Biomed Mater Res. 1990;24(5):529–545. doi: 10.1002/jbm.820240502. [DOI] [PubMed] [Google Scholar]
- 28.Shah RR, Mohammed S, Saifuddin A, Taylor BA. Comparison of plain radiographs with CT scan to evaluate interbody fusion following the use of titanium interbody cages and transpedicular instrumentation. Eur Spine J. 2003;12:378–385. doi: 10.1007/s00586-002-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith GW, Robinson RA (1958) The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Jt Surg Am 40-A(3):607–624 [PubMed]
- 30.Spruit M, Meijers H, Obradov M, Anderson PG. CT density measurement of bone graft within an intervertebral lumbar cage: increase of Hounsfield units as an indicator for increasing bone mineral content. J Spinal Disord Tech. 2004;17(3):232–235. doi: 10.1097/00024720-200406000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Elst M, Klein CP, Blieck-Hogervorst JM, Patka P, Haarman HJ. Bone tissue response to biodegradable polymers used for intra medullary fracture fixation: a long-term in vivo study in sheep femora. Biomaterials. 1999;20(2):121–128. doi: 10.1016/S0142-9612(98)00117-3. [DOI] [PubMed] [Google Scholar]
- 32.Vernon H, Mior S. The neck disability index: a study of reliability and validity. J Manip Physiol Ther. 1991;14(7):409–415. [PubMed] [Google Scholar]
- 33.Wang JC, McDonough PW, Endow KK, Delamarter RB. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine. 2000;25:41–45. doi: 10.1097/00007632-200001010-00009. [DOI] [PubMed] [Google Scholar]
- 34.Wang T, Dang G, Guo Z, Yang M. Evaluation of autologous bone marrow mesenchymal stem cell–calcium phosphate ceramic composite for lumbar fusion in rhesus monkey interbody fusion model. Tissue Eng. 2005;11:1159–1167. doi: 10.1089/ten.2005.11.1159. [DOI] [PubMed] [Google Scholar]
- 35.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]