Abstract
CMA values have been effectively used to evaluate the amount of BI, the brainstem and medulla compression, and the amount of postoperative decompression. However, the reliability and reproducibility of this measurement have yet to be determined. In addition, the information that is available concerning CMA values in normal individuals has been limited to small series of patients. We recruited 200 patients that underwent MR imaging of the craniovertebral junction (CVJ) for unrelated reasons. None of the patients had evidence of abnormalities at the CVJ. Two senior spine surgeons then measured the CMAs of these patients in a blind manner on three separate occasions. The CMA values ranged from 139.0° to 175.5°, with an average value of 158.46°, and a 95% confidence interval from 144.8° to 172.1°. Overall, the CMA had excellent intraobserver repeatability and interobserver reliability. The CMA also had excellent intraobserver repeatability based on both the age and gender of the patients (P = 0.87 and 0.93, respectively). At the same time, the CMA also demonstrated excellent interobserver reliability based on gender (P = 0.97), while good interobserver reliability based on patients age (P = 0.23). No significant correlation between the actual CMA values and the patients’ gender (P = 0.17), age (P = 0.058), or spin-echo series used (P = 0.342). This study demonstrated that CMA values obtained from midsagittal T1 MR images were a highly reliable and repeatable measurement. The data reported in this study can be used as baseline parameters for normal individuals.
Keywords: Cervicomedullary angle, Reproducibility, Basilar invagination, Cervical spine
Introduction
Basilar invagination (BI) and compression of the brainstem and medulla are typically diagnosed using plain radiographs. Several radiographic parameters, including Chamberlain’s line, McGregor’s line, clivus-canal angle, etc., have been described to quantify its amount in relation to the hard palate, the tip of odontoid, and the cranium [6, 10, 11, 20]. However, because of the overlying structures seen on the lateral X-ray, these anatomical landmarks are unreliable and difficult to identify in normal patients, and increasingly so in cases of BI. Using reconstructed images obtained from computerized tomography (CT) scans, the relationship between the odontoid and cranium can be more easily identified on the midline sagittal slices, as can the degree of BI. Despite the improved resolution obtained with CT imaging, all these methods remain limited in their ability to demonstrate compression of the brainstem and upper cervical spinal cord directly. In addition, absence of these anatomical landmarks following decompressive procedures can further complicate the aforementioned methods.
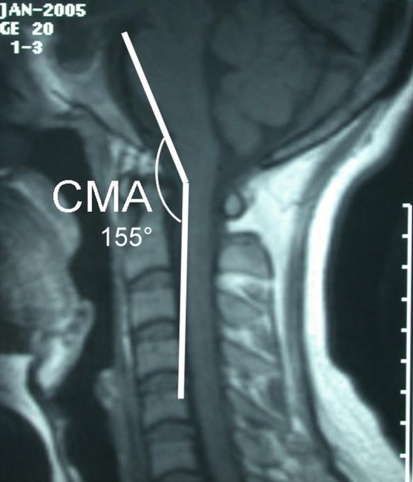
The cervico-medullary angle (CMA) was first described by Bundschuh et al. [5], as the angle subtended by the lines drawn parallel to the ventral surfaces of the medulla and upper cervical cord on magnetic resonance imaging (MRI) (Fig. 1). CMA values have been found to have important clinical relevance [2, 5, 15, 16, 21]. The CMA can be used to quantify the amount of BI, with an angle less than 135° suggestive of neurologic deterioration [18]. In his classic article, Bunschuh et al. [5] reported that there was a strong correlation between CMA values less than 135° and clinical evidence of myelopathy and/or brainstem compression. Furthermore, it has been recommended by some authors that C1–C2 fusion (with possible decompression) be performed in RA patients with CMA values less than 135° [3, 13]. Lastly, CMA values have also been shown to be useful for predicting prognosis in BI and AAD patients treated surgically [2, 21], with increasing mean values being correlated with improved results following decompression (Fig. 2) [1, 4].
Fig. 1.
Cervico-medullary angle (CMA). The angle subtended by lines drawn parallel to the ventral surfaces of the medulla and upper cervical cord
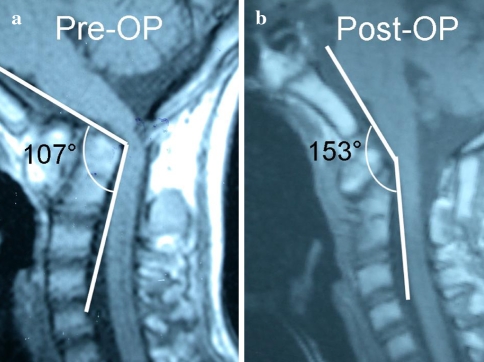
Fig. 2.
a Preoperative MRI demonstrated basilar invagination, occipitalization of the atlas, and fixed atlantoaxial dislocation with the CMA of 107°. b Postoperative MRI showed anatomic reduction of the atlantoaxial joint, complete decompression of the cervicomedullary area, and the CMA value increased to 153°
Despite its broad clinical relevance, the information that is available concerning CMA values in normal individuals has thus far been limited to small series of patients. There have been a few previous studies that have discussed CMA measurement in a small number of patients. Bunschuh et al. [5] used T1 MR imaging to measure CMA angle in 50 normal patients with no lesions present in the CVJ, as well as in 15 rheumatoid arthritis patients with known abnormalities of the CVJ. They found all patients with a CMA less than 135° to have evidence of brainstem compression, cervical myelopathy, or C2 root pain [5]. Abumi et al. [2] used MRI to measure the CMA in 50 asymptomatic individuals, as well as in 26 patients with lesions at the CVJ in order to evaluate the effectiveness of pedicle-based posterior occipitocervical reconstructive surgery [2]. These studies were both limited in their ability to establish baseline CMA values due to their small sample sizes. In addition, the interobserver reliability and intraobserver repeatability of this parameter have yet to be determined.
The purpose of this study was to determine the interobserver reliability and intraobserver repeatability of the technique for measuring the CMA using mid-sagittal MR images, and to establish a normal range for this value in individuals without cervical pathology. We obtained measurements from 200 normal patients for these purposes. We hypothesized that measurement of the CMA would have high intraobserver repeatability and interobserver reliability and that the values obtained could serve as a baseline for normal values.
Methods
Following approval by the institutional review board at our institution, we recruited 200 people from the practice of our senior surgeon. The patients were being evaluated for symptoms or lesions related regions of the spine other than the CVJ. There were 101 females and 99 males enrolled, with an age ranging from 20 to 74 years (mean: 48.1 years). None of the patients that were recruited for this study had any history of cervical spine trauma or disease at the CVJ. There was also no evidence of radiographic abnormalities, either on plain radiographs or MRI, at the CVJ in any of the patients.
The MRI examinations were performed in a routine supine natural position using a 1.5-T MRI scanner (Siemens Sonata, Germany), as has been described by Chen [7]. Although this places the head in slight extension, this represents the position in which all static clinical MRIs are obtained for the measurement of the CMA. The imaging parameters were as follows: (a) Sagittal T1-weighted spin-echo series repetition time (TR)/echo time (TE) = 587/12 ms, 2 mm thick slices with the acquisition matrix of 192 × 256; (b) Sagittal T2-weighted spin-echo series, TR/TE = 4481/130 ms, 2 mm thick slices with the acquisition matrix of 180 × 256.
Midline sagittal images of T1-weighted series were used to obtain measurements. The angle between the two lines on the ventral side of the medulla oblongata and upper cervical spinal cord constituted the CMA (Fig. 1). The CMA values were measured by two senior spine surgeons separately in a blind fashion. Each CMA value was measured on three separate occasions by both surgeons in 3-week intervals and then averaged.
The statistics results were analyzed using SAS software (SAS Institute Inc, version 9.1, Cary, NC). A multivariate fitting model with repeated measures was used to evaluate the intraobserver repeatability and interobserver reliability. We used the coefficient of variation (Cv) to determine agreement with regards to intraobserver repeatability and interobserver reliability.
Results
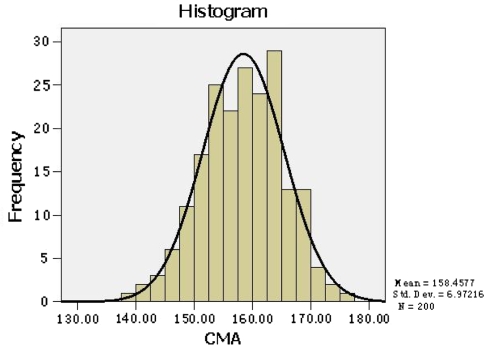
In the current study, we found the CMA values of 200 Chinese patients ranged from 139° to 175.5°, with an average value of 158.46°, and a 95% confidence interval from 144.8° to 172.1° (Table 1). The CMA values obtained met the normal distribution (Fig. 3). Based on the Cv, the CMA values demonstrated excellent agreement, with intraobserver repeatability values of 0.007 and 0.011 for observer 1 and 2, respectively, and an interobserver reliability value of 0.008.
Table 1.
The CMA values of 200 cases
| No. | Min | Max | Mean | SD | 95% confidence (X ± 1.96 SD) | |
|---|---|---|---|---|---|---|
| CMA | 200 | 139.0 | 175.5 | 158.46 | 6.97 | 158.46 ± 13.66 |
Fig. 3.
The CMA values obtained met the normal distribution
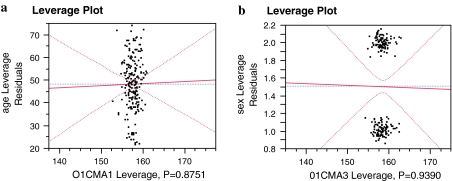
We also found that the CMA had excellent intraobserver repeatability based on both the age and gender of the patients (Fig. 4a, b) (P = 0.87 and 0.93), respectively. At the same time, the CMA also demonstrated excellent interobserver reliability based on gender (Fig. 5b) (P = 0.97), while good interobserver reliability based on patients age (Fig. 5a) (P = 0.23).
Fig. 4.
a Intraobserver repeatability based on age and b intraobserver repeatability based on gender
Fig. 5.
a Interobserver reliability based on age and b interobserver reliability based on gender
Finally, our results demonstrated that there was no significant correlation between the actual CMA values and the patients’ gender (Table 2) (P = 0.17), age (Table 3) (P = 0.058), or spin-echo series used (Table 4) (P = 0.342).
Table 2.
Gender and the CMA values
| Sex | N | Mean | Standard deviation | Standard error mean | |
|---|---|---|---|---|---|
| CMA | Male | 99 | 159.1426 | 6.85877 | 0.68933 |
| Female | 101 | 157.7863 | 7.05084 | 0.70158 |
P = 0.17 (Independent-samples t test)
Table 3.
Age and the CMA values
| 20+ (n = 12) | 30+ (n = 31) | 40+ (n = 61) | 50+ (n = 66) | 60+ (n = 30) | P value | |
|---|---|---|---|---|---|---|
| The CMA values | 157.1792 | 156.7559 | 157.6536 | 158.7452 | 161.7300 | 0.058 |
One-way ANOVA, Student-Newman-Keuls test
Table 4.
Different spin-echo series and the CMA values
| Mean | N | Standard deviation | Standard error mean | ||
|---|---|---|---|---|---|
| Pair 1 | |||||
| T1 | 158.6150 | 200 | 6.91622 | 0.48905 | |
| T2 | 158.7310 | 200 | 7.08422 | 0.50093 | |
P = 0.342 (Paired samples t test)
Discussion
Certain anomalies of the CVJ, including BI and AAD, have the potential to cause ventral compression of the cervicomedullary junction [8, 12]. In such conditions, superior migration of the odontoid, and/or the horizontal clivus, can lead to direct compression of the brainstem and upper cervical spinal cord [15]. These changes place the cervicomedullary junction in excessive kyphosis, thus compromising the blood supply and resulting in chronic neurological injury [15, 17]. With disease progression, the ventral compression from the displaced odontoid becomes more severe, increasing distortion of the medulla and upper cervical cord, as well as exacerbating neurological dysfunction. In the past, several radiographic parameters have been used to quantitatively describe these pathologic changes that occur. The majority of these, including Chamberlain’s line, McRae’s line, McGregor’s line, clivus-canal angle, and the basal angle, were all initially described using plain radiographs [6, 10, 11, 20]. Recently Kulkarni and Goel [9] have described the vertical atlantoaxial index (VAAI). This index is obtained from CT imaging, and measures the vertical relationship between the atlas and the axis to quantitatively describe and classify BI. Although an improvement from prior radiographic parameters, the VAAI has limited application in cases involving either os odontoideum or rheumatoid arthritis [9], and it has the potential to be miscalculated in individuals with occipitalization of the atlas or C2–C3 assimilation. In addition, the VAAI is similar to previous methods based on plain radiographs in that they can only assess BI and cervicomedullary compression indirectly. Furthermore, the majority of individuals with BI (80%) have an occipitalized atlas that is fused with the clivus [8], which will be partially removed during the anterior decompressive procedure, and the aforementioned methods will thereby lose this anatomic landmark on postoperative imaging, making it difficult to evaluate the results of decompression.
With its excellent visualization of the neural tissues, MRI is an ideal diagnostic imaging method for evaluating brainstem and spinal cord compression. Unlike the previous measurements described, measuring the CMA with MRI has the ability to directly demonstrate brainstem and upper cervical cord compression and spinal cord distortion. In addition, the CMA has the advantage of not being an absolute number, but an angle between the medulla oblongata and upper cervical spinal cord, and it is thus unaffected by osseous anomalies of the CVJ and magnification errors. Measurement of the CMA is helpful to both quantify and classify the severity of BI or ventral brainstem compression, and can help determine the treatment strategy [2, 5, 15, 16, 21]. In the present study, CMA measurements obtained from the MRIs of 200 patients demonstrated extremely high intraobserver and interobserver correlation coefficients. We also found that these values had no significant correlation with age, gender or MRI sequence.
The relationship between the medulla and spinal cord changes considerably during cervical motion, with the CMA decreasing significantly with flexion [14]. At the same time, the medulla has a pyramidal shape, and measurements taken from the para-sagittal MR images will result in a miscalculation of this value. Taking these points into consideration, we used midline sagittal MR images obtained in the neutral position to establish the standard value of the CMA in the present study. MR images can also vary based on the sequence utilized, with T2-weighted spin-echo being larger than T1 images because of the Gibb’s phenomenon [19]. Being not a number; however, but an angle, the values of the CMA were unaffected by this phenomenon. In order to verify this, we compared values obtained from T1 and T2 images and found no difference based on type of spin-echo sequence. Despite this, we chose to use the T1-weighted series to establish the standard value in the present study to standardize our data and allow them to be compared to earlier work.
The CMA measurements published by Bunschuh et al. [5] revealed a mean of 155.2°, with a range of 135–175°, in 50 normal patients. In their report, Abumi et al. [2], found the average CMA in 50 normal adults to be 163°, with a range from 154° to 179°. In the current study, we found the CMA values of 200 Chinese patients ranged from 139.0° to 175.5°, with an average value of 158.46°, and a 95% confidence interval from 144.8° to 172.1°. Possible reasons for the differing results are multiple. First, the CMA values we obtained may not be representative of different races. Second, our data were based on a much larger number of cases than the previous reports. Third, as stated the results of CMA measurements will change during the cervical motion. MR images obtained in the routine supine neutral position were used in the present study, while neither of the previous authors mentioned the cervical position in their studies.
There are several limitations to this study. Although the patients enrolled in this study had no evidence of abnormalities present in the CVJ, as determined by both experienced radiologists and surgeons, many were being evaluated for symptoms or abnormalities in other regions of their spine and this may have conceivably affected the CMA measurements. Another clear limitation is that we were unable to normalize our measurements based on the heights and weights of the patients due to a lack of available data. In addition, the MRI images that were used to obtain CMA measurements were taken with the head in the routine supine natural position, which is effectively in slight extension. However, in order to allow these values to be used as a baseline for comparison we chose not to change this head position. Finally, CMA measurements were performed by highly experienced spine surgeons and the high repeatability and reproducibility seen may not be reproduced by less experienced physicians.
In summary, this study demonstrated that CMA values obtained from midsagittal T1 MR images were a highly reliable and repeatable measurement. In addition, the data reported in this study can be used as baseline parameters for normal individuals. Further study is needed to directly compare our results with those obtained from individuals with known abnormalities of the CVJ.
Conflict of interest statement
No Funding was received for this project.
References
- 1.Abumi K, Ito M, Kotani Y. Cervical pedicle screw fixation. New York, NY: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 2.Abumi K, Takada T, Shono Y, Kaneda K, Fujiya M. Posterior occipitocervical reconstruction using cervical pedicle screws and plate-rod systems. Spine. 1999;24:1425–1434. doi: 10.1097/00007632-199907150-00007. [DOI] [PubMed] [Google Scholar]
- 3.Boden SD, Dodge LD, Bohlmann HH, Rechtine GR. Rheumatoid arthritis of the cervical spine. A long-term analysis with predictors of paralysis and recovery. J Bone Joint Surg Am. 1993;75:1281–1297. doi: 10.2106/00004623-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Botelho R, Neto E, Patriota G, Daniel J, Dumont P, Rotta J. Basilar invagination: craniocervical instability with cervical traction and occipitocervical fixation. Case report. J Neurosurg Spine. 2007;7:444–449. doi: 10.3171/SPI-07/10/444. [DOI] [PubMed] [Google Scholar]
- 5.Bundschuh C, Modic MT, Kearney F, Morris R, Deal C. Rheumatoid arthritis of the cervical spine: surface-coil MR imaging. Am J Roentgenol. 1988;151:181–187. doi: 10.2214/ajr.151.1.181. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain WE. Basilar impression (platybasia): a bizarre developmental anomaly of the occipital bone and upper cervical spine with striking and misleading neurologic manifestations. Yale J Biol Med. 1939;11:487–496. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C-J. Cervical degenerative disease at flexion-extension MR imaging: prediction criteria. Radiology. 2003;227:136–142. doi: 10.1148/radiol.2271020116. [DOI] [PubMed] [Google Scholar]
- 8.Goel A, Bhatjiwale M, Desai K. Basilar invagination: a study based on 190 surgically treated patients. J Neurosurg. 1998;88:962–968. doi: 10.3171/jns.1998.88.6.0962. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni AG, Goel AH. Vertical atlantoaxial index: a new craniovertebral radiographic index. J Spinal Disord Tech. 2008;21:4–10. doi: 10.1097/BSD.0b013e31804856d7. [DOI] [PubMed] [Google Scholar]
- 10.McGregor M. The significance of certain measurements of the skull in the diagnosis of basilar impression. Br J Radiol. 1948;21:171–181. doi: 10.1259/0007-1285-21-244-171. [DOI] [PubMed] [Google Scholar]
- 11.McRae DL, Barnum AS. Occipitalization of the atlas. Am J Roentgenol Radium Ther Nucl Med. 1953;70:23–46. [PubMed] [Google Scholar]
- 12.Menezes AH. Craniovertebral junction database analysis: incidence, classification, presentation, and treatment algorithms. Childs Nerv Syst. 2008;24:1101–1108. doi: 10.1007/s00381-008-0605-9. [DOI] [PubMed] [Google Scholar]
- 13.Puschak TJ, Anderson PA. Posterior C1–C2 transarticular screws. Tech Orthop. 2002;17:296–305. doi: 10.1097/00013611-200209000-00005. [DOI] [Google Scholar]
- 14.Reijnierse M, Breedveld FC, Kroon HM, Hansen B, Pope TL, Bloem JL. Are magnetic resonance flexion views useful in evaluating the cervical spine of patients with rheumatoid arthritis? Skeletal Radiol. 2000;29:85–89. doi: 10.1007/s002560050015. [DOI] [PubMed] [Google Scholar]
- 15.Reijnierse M, Bloem JL, Dijkmans BA, Kroon HM, Holscher HC, Hansen B, Breedveld FC. The cervical spine in rheumatoid arthritis: relationship between neurologic signs and morphology of MR imaging and radiographs. Skeletal Radiol. 1996;25:113–118. doi: 10.1007/s002560050046. [DOI] [PubMed] [Google Scholar]
- 16.Reijnierse M, Dijkmans BA, Hansen B, Pope TL, Kroon HM, Holscher HC, Breedveld FC, Bloem JL. Neurologic dysfunction in patients with rheumatoid arthritis of the cervical spine. Predictive value of clinical, radiographic and MR imaging parameters. Eur Radiol. 2001;11:467–473. doi: 10.1007/s003300000557. [DOI] [PubMed] [Google Scholar]
- 17.Reiter MF, Boden SD. Inflammatory disorders of the cervical spine. Spine. 1998;23:2755–2766. doi: 10.1097/00007632-199812150-00017. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt KM, Grauer JN. Rheumatoid arthritis of the cervical spine. Stuttgart: Thieme; 2007. [Google Scholar]
- 19.Sherman JL, Nassaux PY, Citrin CM. Measurements of the normal cervical spinal cord on MR imaging. Am J Neuroradiol. 1990;11:369–372. [PMC free article] [PubMed] [Google Scholar]
- 20.Smoker WR, Khanna G. Imaging the craniocervical junction. Childs Nerv Syst. 2008;24:1123–1145. doi: 10.1007/s00381-008-0601-0. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Yang M, Zhou HT, Wang SL, Dang GT. Open reduction of irreducible atlantoaxial dislocation by transoral anterior atlantoaxial release and posterio internal fixation. Spine. 2006;31:E306–E313. doi: 10.1097/01.brs.0000217686.80327.e4. [DOI] [PubMed] [Google Scholar]