Abstract
Sacral insufficiency fractures (SIFs) are an increasingly recognised cause of back pain in the elderly. They can cause significant pain and disability in the elderly population and until recently, the mainstay of treatment has been analgesia and physical therapy. We undertook a review of the literature looking at the outcome with various operative techniques currently used in the treatment of SIF. A thorough literature search was undertaken to identify the various techniques used in the surgical treatment of SIF and their outcome. Keywords used included sacroplasty, SIF and cement augmentation. We analysed the number of cases presented, surgical technique, follow-up and clinical outcome. The techniques described include sacroplasty (injection of cement into fractured sacrum) and augmented iliosacral (trans-sacral) screws. Fifteen papers were published in the English literature between 2002 and 2008. No Level I, II or III evidence was available. In total, 108 patients were included. Computerised tomography combined with fluoroscopy was the most common image guidance technique used (80 patients). Where documented, there was significant improvement in mean visual analogue score (VAS) from 8.9 to 2.6 (P < 0.001, paired Student’s t test). In conclusion, cement augmentation techniques such as sacroplasty with or without iliosacral screw fixation can produce significant improvements in VAS scores. They appear to be a suitable alternative to analgesia and rehabilitation. However, more robust evidence is required to validate these promising early results with cement augmentation techniques.
Keywords: Sacral insufficiency fracture, Sacroplasty, Osteoporosis, Cement augmentation
Introduction
Sacral insufficiency fractures (SIFs) are not uncommon in the elderly population and there is increasing awareness of the burden on healthcare attributable to these fractures [8, 15]. Traditional management has involved analgesia, bed rest and formal rehabilitation. The disadvantages of conservative management can include intractable pain, with significant morbidity and mortality occurring due to periods of prolonged bed rest or impaired mobility [2, 20].
Sacroplasty for SIF is a technique first described by Garant [12]. The technique evolved from the success of vertebroplasty and kyphoplasty in the treatment of insufficiency fractures of thoracic and lumbar vertebrae. Sacroplasty is essentially the same technique where PMMA cement is injected into the fracture zone of the sacral ala with the aim of achieving an analgesic effect through presumed restoration of mechanical integrity. However, the risks and benefits of this procedure are not yet fully understood. Sacroplasty remains an evolving technique and numerous methods have been described in the form of technical reports with accompanying case reports or small case series [4, 6, 7, 9, 11, 12, 14, 16, 19, 23, 25–28, 30]. So far, no consensus has been agreed with regard to best practise.
We, therefore, decided that it would be beneficial to the medical and surgical practitioners if the various techniques and results were amalgamated into one paper for comparison; specifically focusing on clinical outcome.
We have undertaken a review of the published literature that has reported results of sacroplasty between 2002 and 2008. In this paper, we summarise the techniques described and their clinical outcome. We discuss the issues that are controversial, suggesting direction for future studies.
Materials and methods
We performed a literature search of Medline via PubMed, and of Ovid Online via Athens, for articles published on sacroplasty. Keywords used included sacroplasty, SIF and cement augmentation. We excluded the following studies: fractures due to primary or secondary bone tumours, non-English language papers. Biomechanical studies were considered where appropriate. These exclusion criteria were suggested in order to capture only those papers where osteoporosis was the cause of the fracture. This would allow a comparable patient group. The time period incorporated was because of the paper by Garant [12] which has initiated the interest in sacroplasty.
The studies were analysed for the number of cases presented, surgical technique performed, follow-up period and clinical outcome. We performed simple statistics where feasible.
Results
Fifteen papers were published in the English literature between 2002 and 2008. Seven were case series (one performed prospectively), five papers were technical reports and three were case reports. No Level I, II or III evidence was available. Frey et al. [11] constitutes Level IV evidence because although it is described as a prospective observational cohort study, there is neither an independent observer, nor a comparative group. It is more in-keeping with a large prospective case series.
There are only five papers which include follow-up of 1 year or more [6, 11, 14, 16, 27, 30], despite the fact that most journals require at least 12 months of follow-up for reports of surgical outcomes. It is, therefore, unusual to see so many case reports on a subject, without these requirements met. We have, however, discussed the short-term outcomes to highlight the early results, which can guide future research.
We also had to exclude three articles from the German and French literatures which we were unable to analyse further because of the language barrier. Reviewing the abstracts, this would have allowed a further three cases to be included in our results, which may have improved the strength of our results [17, 18, 24].
In total, 108 patients (range 1–52 per study, median 3) with osteoporotic SIF were included. Mean age was 75.5 years. With regard to follow-up, Tjardes et al. [28], Smith and Dix [26] and Binaghi et al. [4] have only mentioned immediate post-operative results. Butler et al. [7] had a follow-up period of between 2 and 8 weeks in four patients, but did not clarify this further and so could not be included in the calculations of the mean. All the other papers had a minimum follow-up of 2.5 months with an average of 9.1 months (range 2.5–41.7).
Amongst the 108 patients included, 47 patients had sustained bilateral sacral fractures whilst 36 had only unilateral fractures. Three patients sustained H-type fractures [4, 6, 23] with each undergoing sacroplasty without concomitant iliosacral screw fixation. Binaghi et al. [4] describe an H-type fracture in a patient with a history of lymphoma. They do not specifically state that it is a pathological fracture and so the patient has been included in our data. Type of fracture was not documented in 22 patients [4, 7, 28, 30].
Procedures were performed under conscious sedation or general anaesthetic. Computerised tomography (CT) combined with fluoroscopy was the most commonly used image guidance technique (80 patients). All other procedures were performed with either CT or fluoroscopic guidance only. Needle insertion techniques described include the long- and short-axis approaches (Table 1; Fig. 1), the principles of which we explain later in the discussion.
Table 1.
Summary of articles published with clinical results of sacroplasty
| References | Type of study | Total patients | Mean follow-up period (months) | Imaging used | Technique | Outcome | Complications |
|---|---|---|---|---|---|---|---|
| Garant [12] | CR | 1 | 9 | F | Short axis | Immediate pain relief | Nil |
| Pommersheim et al. [23] | CS [r] | 3 | 3.5 | CT + F | Short axis | Immediate + long term | Cement extrusion: clinically insignificant |
| Butler et al. [7] | CS [r] | 4 | NK | CT + F | Short axis | Mild: significant relief | Cement extrusion; Failure of procedure |
| Brook et al. [6] | CR | 2 | 12 | CT | Short axis | VAS 10–0 | Nil |
| Deen and Nottmeier [9] | CS [r] | 3 | 6 | CT + F | Balloon assisted, short axis | VAS 9–5 (ave) | Cement extrusion: clinically insignificant |
| Binaghi et al. [4] | TR | 6 | Immediate | CT | Long axis | VAS 10–2 | Nil |
| Layton et al. [19] | TR | 1 | 2.5 | CT | Long and short axis | Significant pain relief | Nil |
| Smith and Dix [26] | TR | 1 | NK | F | Long axis | NK | Cement extrusion: clinically insignificant |
| Heron et al. [16] | CR | 3 | 3 | CT | Short axis | VAS 8–1 (ave) | Cement extrusion: clinically insignificant |
| Sciubba et al. [25] | TR/CR | 1 | 6 | CT | Iliosacral screws | VAS 7–2 (ave) | Nil |
| Strub et al. [27] | CS [r] | 13 | 15 | CT + F | Long axis | Immediate pain relief | Nil |
| Whitlow et al. [30] | CS [r] | 12 | 21.5 | Not described | Significant pain relief | Not discussed | |
| Frey et al. [11] | CS [p] | 52 | 12 | CT + F | Short axis | VAS 8.1–0.8 (ave) | S1 radicular pain |
| Gjertsen et al. [14] | CS [r] | 5 | 6 | CT | Long axis | Immediate pain relief | 1 case: intractable pain |
| Tjardes et al. [28] | TR | 1 | Immediate | CT + F | Iliosacral screws | Immediate pain relief | Nil |
TR technical report, CR case report, CS [r] retrospective case series, CS [p] prospective case series, CT computerised tomography, F fluoroscopy, NK not known, ave average, VAS visual analogue score
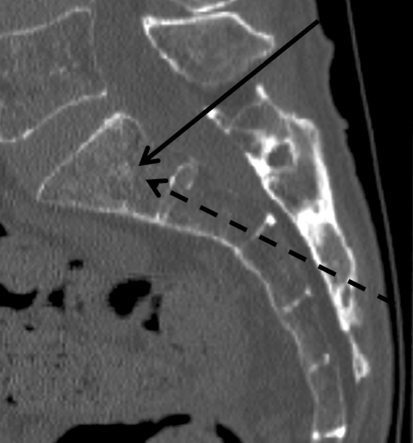
Fig. 1.
Sagittal CT sacrum showing the trajectory of the long-axis (dashed arrow) and short-axis approaches (solid arrow). In either approach, the needle is placed into the sacral ala lateral to the neuroforamina
Garant [12] was the first to describe the short-axis technique using 7 ml of PMMA cement. Subsequent studies that have used the short-axis approach have utilised 4–20 ml of cement in total, across unilateral or bilateral fractures. Butler et al. [7] described using 4–8 ml per side, with Pommersheim et al. [23] and Deen and Nottmeier [9] using between 2 and 6 ml per side. Heron et al. [16] injected 4 ml per fracture and Frey et al. [11] 2–5 ml per vertebral level.
Long-axis techniques have also incorporated 4–20 ml of cement per patient with Binaghi et al. using 6 ml per side [4]. Strub et al. [27] limited the cement volume to 8 ml per patient, whereas Gjertsen et al. [14] used up to 10 ml per sacral side. Layton et al. [19] described injecting 2 ml of cement per vertebral body instrumented, similar to that of Tjardes et al. [28] and Frey et al. [11]. Unfortunately, Brook et al. [6], Smith and Dix [26] and Sciubba et al. [25] did not comment on the amount of cement they injected despite describing their technique.
Two patients underwent simultaneous augmented iliosacral screw fixation. Tjardes et al. [28] describe a lateral approach where PMMA cement is introduced through an 8 mm cannulated screw (DePuy Inc.). The screw is directed over a guide wire until it reaches the lateral mass of the sacrum. At this stage, the guide wire is removed and an 11 gauge trocar is placed through the axis of the screw and 2 ml of cement is injected. The trocar is subsequently removed and the guide wire is reinserted. The cannulated screw is brought to its final position in the S1 vertebra and the guide wire is removed before the cement hardens.
Sciubba et al. [25] describe a technique where the trans-iliosacral rod is positioned through a lateral incision, before injection of the cement. Although they use different incisions and approaches to each step of their procedure, they do not specifically describe their approach to the sacral vertebrae when injecting the cement.
Visual analogue score (VAS) was used as a clinical outcome measure in the follow-up of 62 patients. The average VAS improved from a pre-operative score of 8.9 to 2.6 post-operatively [4, 6, 9, 11, 16, 25]. This is statistically significant (P < 0.001, paired Student’s t test). Frey et al. [11] demonstrated that low VAS scores could be maintained at 1 year follow-up.
Whitlow et al. [30] compared the clinical outcome of patients undergoing sacroplasty (12 patients) with those of vertebroplasty procedures (21 patients). Follow-up periods in the two groups were comparable because subjects were only recruited for the vertebroplasty arm of the study if their procedure had occurred on the same day as a sacroplasty procedure. The ability to ambulate and perform activities of daily living improved in both groups following the intervention. There was a statistically significant improvement in pain scores in both groups (61.5% after sacroplasty, 61.8% after vertebroplasty) with no significant difference between the two.
Complications reported so far have included clinically insignificant cement leakage into the S1 foramen in one case [16], posteriorly in one case [23], into the sacroiliac joint [7], anteriorly in four patients [9, 26] and intravenously in one case [7]. Frey et al. [11] describe the development of an S1 radiculopathy during the procedure, at which point they stopped injecting cement, although they did not clarify whether the cement had extravasated. The symptoms settled with an S1 nerve root injection 1 week later. A further case was refractory to pain despite a repeat procedure being performed [14].
The results are summarised in Table 1.
Discussion
Sacral insufficiency fractures were first described by Lourie [21] and their clinical interest has increased exponentially since. Sacroplasty for insufficiency fractures was first described by Garant [12], although there had been two earlier case reports where PMMA cement was injected into sacral metastases successfully [10, 22]. Since then, following the success of vertebroplasty and kyphoplasty for thoracic and lumbar vertebral fractures, there has been an increasing number of case reports based around the idea of sacroplasty for SIF. The general consensus is that sacroplasty can be performed safely on Denis Zone 1 fractures, with more central fractures increasing the risk of foraminal leakage and S1 nerve root injury.
Throughout the article, there is no consensus on optimal technique, with differences in opinion on which approach to utilise, and on whether there is a need to use balloon kyphoplasty or augment with iliosacral screws. The image guidance used is also variable, with the majority incorporating CT due to the difficulties of visualising the osteology safely [4, 6, 7, 9, 16, 23, 27]. It has previously been suggested that there is a tendency amongst interventional radiologists to use the posterior approach with spinal surgeons likely to prefer the lateral approach given their experience with posterior ring pelvic surgery [29]. This also allows the surgeon to place iliosacral screws concomitantly. In our review, the posterior approach was the commonest described, with lateral approaches only being used when iliosacral screws are being placed [26, 28] simultaneously. However, the majority of ‘simple’ sacroplasty procedures are being reported from departments of radiology [4, 6, 7, 12, 14, 16, 19, 23, 26, 27, 30], with only four papers originating from other departments (e.g. neurosurgery and orthopaedics) [9, 11, 25, 28].
Two posterior techniques for cannula placement have been described: the long- and short-axis approaches (Fig. 1). In the long-axis approach, the cannula is passed along the ala of the sacrum in a caudal–cephalad direction [4, 26], with the short axis the needle is placed in a posterior–anterior direction. The long-axis technique is considered easier to perform [4] with an added advantage of the cement being directly injected along the whole fracture line. Potential risks include entering the sacral foramen, penetration of the superior or cephalad margin of the sacral ala, or breaching of the anterior cortex [14, 26].
Deen and Nottmeier [9] have shown that balloon-assisted sacroplasty is a feasible technique with good clinical results. However, they acknowledge that it is unlikely to benefit fracture configuration in the same way that kyphoplasty in the lumbar or thoracic spine is considered to. A cadaveric study has shown that cement deposition was better confined within the cortical boundaries with a balloon-assisted technique [3]. Therefore, if multiple cases are performed, a benefit with regard to a decrease in cement extravasation may be shown, but this has not been a clinically significant complication in the cases reported so far.
Other augmentation techniques described include iliosacral screw fixation in addition to sacroplasty. This technique was supported by Tjardes et al. [28] because they believe that cement augmentation alone is not biomechanically stable enough to counteract the vertical shear and stress forces in the pelvis. This is confirmed by the cadaveric studies that show no significant difference is made to the strength or stiffness of the sacrum, even when comparing the amount of cement or approach used [29]. Sciubba et al. [25] justified their technique by suggesting that the screws provide greater stabilization of shear forces, with the cement providing stabilization between the screws and bone. Pommersheim et al. [23] also discussed the fact that wide fracture lines may be a contraindication to cement injection, and so the additions of iliosacral screws will help to bring the fracture line together, allowing the potentially safer injection of cement.
Waites et al. [29] performed sacroplasty in cadaveric osteoporotic pelves and concluded that strength or stiffness of the sacrum and pelvis was not improved by augmentation of the fracture with cement. They used both a lateral and posterior approach, with no significant difference between the two. This supports the above opinions that more biomechanical stability is needed, other than simple augmentation with cement. An important limitation to this study is that they injected only 4 ml of PMMA cement, with some clinical cases needing considerably more. (Brook et al. [6] report the injection of 10 ml of cement into each fracture.) They do, however, acknowledge that fracture micromotion may be prevented which can result in analgesic effect.
On the other hand, two biomechanical studies of the pelvis based on finite element analysis [1, 31] have shown that the sacroplasty technique decreases micromotion between fracture segments. Anderson and Cotton [1] claimed that the sacrum was between 2 and 5% stiffer, and that strain can be reduced by between 40 and 60%. Whitlow et al. [31] showed that mechanical stress decreases by approximately 83% around the fracture site and also that fracture gap micromotion can be decreased by 48%. Anderson and Cotton injected 3–7 ml of cement on each side, whereas Whitlow et al. used 3 ml. These results may explain the clinically apparent pain reduction and improved mobility with cement augmentation, and support those who believe that iliosacral screws are not needed. The limitation to Anderson and Cotton’s study is that their finite element model did not have a fracture simulated.
Complications described specific to sacroplasty have included cement extravasation. In the eight cases where this occurred, there was no clinically apparent detriment to the patients’ well being. In the large cohort treated by Frey et al. [11], only one case of S1 radicular pain was reported. This resolved following injection of the nerve root with steroid and local anaesthetic, causing no long-term deficit. Although Gjertsen et al. [14] have cited pulmonary embolism, infection and nerve injury as potential complications, no such reports with sacroplasty have yet been made. The case refractory to pain relief was in a tetraplegic male where a repeat procedure did not help. It was felt that the significant muscle dystrophia contributed to the pain [14]. As long as the variously described landmarks are adhered to, it would seem that sacroplasty is a safe technique to perform. However, despite this being an elderly population, long-term follow-up studies are needed to assess complications and evolution of cement position.
The average follow-up is just under 10 months, although infrequently in the literature follow-up of greater than 1 year is reported [27, 30]. Analgesic effect can be instantaneous, and this benefit, along with improvements in mobility and ability to perform activities of daily living, has been shown to be maintained long term in a small number of cases [6, 11, 27, 30]. With conservative management, patients are often advised that healing will occur over 12 months [20], and admissions for rehabilitation can be prolonged. Dasgupta et al. [8] showed that an average stay was 20 days in their hospital when treating conservatively, almost as long as the average in-patient stays following a fractured neck of femur (38 days). Although no direct comparison has been made with post-operative length of stay following sacroplasty, none of the articles reviewed have suggested the need for long-term admission.
Most papers discuss the need for a combination of CT and fluoroscopic guidance during the procedure because of difficulty visualising the landmarks. Live fluoroscopy is needed to monitor cement extravasation during injection [4, 6, 7, 9, 16, 23, 27]. The obvious concern with CT-guided procedures is the increased radiation exposure. Boszczyk et al. [5] showed that with one level kyphoplasty the lifetime risk of developing cancer is theoretically increased by 0.02–0.06% (fluoroscopy guided). We can assume that the radiation dose is higher with CT-guided sacroplasty and so a patient’s risk will be increased further. This may not be clinically significant in an elderly cohort, but its effects need to be considered.
Comparisons will always be made with vertebroplasty and kyphoplasty given the similarities in technique. Success rates as high as 95% have been reported with vertebroplasty and kyphoplasty [13]. Whilst the number of sacroplasty cases currently reported in the medical literature is too low to claim such a success rate, Whitlow et al. [30] have shown that sacroplasty results are comparable with those undergoing vertebroplasty. In our review, we have only found one case refractory to treatment [14]. These results are promising but a larger cohort needs to be treated before sacroplasty can try and claim a 95% success rate.
Conclusion
Cement augmentation techniques such as sacroplasty can produce significant improvements in VAS scores and appear to be a suitable alternative to analgesia and rehabilitation. The results are promising with immediate pain relief and maintenance of benefit in the medium to long term; however, there are questions still to be answered. In particular, the optimal technique to be used and the long-term outcome with this procedure need further analysis. Future prospective clinical studies with an independent observer to analyse the long-term success rate and complications of this procedure are warranted.
References
- 1.Anderson DE, Cotton JR. Mechanical analysis of percutaneous sacroplasty using CT image based finite element model. Med Eng Phys. 2007;29(3):316–325. doi: 10.1016/j.medengphy.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Babayev M, Lachmann E, Nagler W. The controversy surrounding sacral insufficiency fractures: to ambulate or not to ambulate? Am J Phys Med Rehabil. 2000;79(4):404–409. doi: 10.1097/00002060-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Betts A. Sacral vertebral augmentation: confirmation of fluoroscopic landmarks by open dissection. Pain Physician. 2008;11(1):57–65. [PubMed] [Google Scholar]
- 4.Binaghi S, Guntern D, Schnyder P, Theumann N. A new, easy, fast, and safe method for CT-guided sacroplasty. Eur Radiol. 2006;16(12):2875–2878. doi: 10.1007/s00330-006-0467-z. [DOI] [PubMed] [Google Scholar]
- 5.Boszczyk BM, Biershneider M, Panzer S, Panzer W, Harstall R, Schmid K, Jaksche H. Fluoroscopic radiation exposure of the kyphoplasty patient. Eur Spine J. 2006;15(3):347–355. doi: 10.1007/s00586-005-0952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook AL, Mirsky DM, Bello JA. Computerized tomography guided sacroplasty: a practical treatment for sacral insufficiency fracture. Case report. Spine. 2005;30(15):E450–E454. doi: 10.1097/01.brs.0000172182.35619.d1. [DOI] [PubMed] [Google Scholar]
- 7.Butler CL, Given CA, Michel SJ, Tibbs PA. Percutaneous sacroplasty for the treatment of sacral insufficiency fractures. Am J Roentgenol. 2005;184(6):1956–1959. doi: 10.2214/ajr.184.6.01841956. [DOI] [PubMed] [Google Scholar]
- 8.Dasgupta B, Shah N, Brown H, Gordon TE, Tanqueray AB, Mellor JA. Sacral insufficiency fractures: an unsuspected cause of low back pain. Br J Rheumatol. 1998;37(7):789–793. doi: 10.1093/rheumatology/37.7.789. [DOI] [PubMed] [Google Scholar]
- 9.Deen HG, Nottmeier EW. Balloon kyphoplasty for treatment of sacral insufficiency fractures. Report of three cases. Neurosurg Focus. 2005;18(3):E7. [PubMed] [Google Scholar]
- 10.Dehdashti AR, Martin JB, Jean B, Rüfenacht DA. PMMA cementoplasty in symptomatic metastatic lesions of the S1 vertebral body. Cardiovasc Intervent Radiol. 2000;23(3):235–237. doi: 10.1007/s002700010052. [DOI] [PubMed] [Google Scholar]
- 11.Frey ME, DePalme MJ, Cifu DX, Bhagia SM, Carne W, Daitch JS. Percutaneous sacroplasty for osteoporotic sacral insufficiency fractures: a prospective, multicenter, observational pilot study. Spine J. 2008;8(2):367–373. doi: 10.1016/j.spinee.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Garant M. Sacroplasty: a new treatment for sacral insufficiency fracture. J Vasc Interv Radiol. 2002;13(12):1265–1267. doi: 10.1016/S1051-0443(07)61976-9. [DOI] [PubMed] [Google Scholar]
- 13.Garfin SR, Yuan HA, Riley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26(14):1511–1515. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- 14.Gjertsen O, Schellhorn T, Nakstad PH. Fluoroscopy-guided sacroplasty: special focus on preoperative planning from three-dimensional computed tomography. Acta Radiol. 2008;49(9):1042–1048. doi: 10.1080/02841850802350659. [DOI] [PubMed] [Google Scholar]
- 15.Gotis Graham I, McGuigam L, Diamond T, Portek I, Quinn R, Sturgess A, Tulloch R. Sacral insufficiency fractures in the elderly. J Bone Joint Surg Br. 1994;76(6):882–886. [PubMed] [Google Scholar]
- 16.Heron J, Connell DA, James SL. CT-guided sacroplasty for the treatment of sacral insufficiency fractures. Clin Radiol. 2007;62(11):1094–1100. doi: 10.1016/j.crad.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Hess GM. Sacroplasty for the treatment of sacral insufficiency fractures. Orthopade. 2006;35(8):865–870. doi: 10.1007/s00132-006-0984-0. [DOI] [PubMed] [Google Scholar]
- 18.Hess GM. Sacroplasty for the treatment of sacral insufficiency fractures. Unfallchirurg. 2006;109(8):681–686. doi: 10.1007/s00113-006-1102-9. [DOI] [PubMed] [Google Scholar]
- 19.Layton KF, Thielen KR, Wald JT. Percutaneous sacroplasty using CT fluoroscopy. Am J Neuroradiol. 2006;27(2):356–358. [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Lachmann E, Nagler W. Sacral insufficiency fractures: a report of two cases and a review of the literature. J Womens Health Gend Based Med. 2001;10(7):699–705. doi: 10.1089/15246090152563588. [DOI] [PubMed] [Google Scholar]
- 21.Lourie H. Spontaneous osteoporotic fracture of the sacrum. An unrecognised syndrome of the elderly. JAMA. 1982;248(6):715–717. doi: 10.1001/jama.248.6.715. [DOI] [PubMed] [Google Scholar]
- 22.Marcy PY, Palussière J, Descamps B, Magné N, Bondiau PY, Ciais C, Bruneton JN. Percutaneous cementoplasty for pelvic bone metastasis. Support Care Cancer. 2000;8(6):500–503. doi: 10.1007/s005200000138. [DOI] [PubMed] [Google Scholar]
- 23.Pommersheim W, Huang-Hellinger F, Baker M, Morris P. Sacroplasty: a treatment for sacral insufficiency fractures. Am J Neuroradiol. 2003;24(5):1003–1007. [PMC free article] [PubMed] [Google Scholar]
- 24.Ryska P, Malek V, Renc O, Rehak S, Hrubesova J. Percutaneous sacroplasty in sacral insufficiency fracture treatment. Cas Lek Cesk. 2006;145(3):215–217. [PubMed] [Google Scholar]
- 25.Sciubba DM, Wolinsky JP, Than KD, Gokaslan ZL, Witham TF, Murphy KP. CT fluoroscopically guided percutaneous placement of transiliosacral rod for sacral insufficiency fracture: case report and technique. Am J Neuroradiol. 2007;28(8):1451–1454. doi: 10.3174/ajnr.A0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DK, Dix JE. Percutaneous sacroplasty: long axis injection technique. Am J Roentgenol. 2006;186(5):1252–1255. doi: 10.2214/AJR.05.0823. [DOI] [PubMed] [Google Scholar]
- 27.Strub WM, Hoffmann M, Ernst RJ, Bulas RV. Sacroplasty by CT and fluoroscopic guidance: is the procedure right for your patient? Am J Neuroradiol. 2007;28(1):38–41. [PMC free article] [PubMed] [Google Scholar]
- 28.Tjardes T, Paffrath T, Baethis H, Shafizadeh S, Steinhausen E, Steinbuechel T, Rixen D, Buoillon B. Computer assisted percutaneous placement of augmented iliosacral screws: a reasonable alternative to sacroplasty. Spine. 2008;33(13):1497–1500. doi: 10.1097/BRS.0b013e318175c25c. [DOI] [PubMed] [Google Scholar]
- 29.Waites MD, Mears SC, Richards AM, Mathis JM, Belkoff SM. A biomechanical comparison of lateral and posterior approaches to sacroplasty. Spine. 2008;33(20):E735–E738. doi: 10.1097/BRS.0b013e31817ecc22. [DOI] [PubMed] [Google Scholar]
- 30.Whitlow CT, Mussat-Whitlow BJ, Mattern CWT, Baker MD, Morris PP. Sacroplasty versus vertebroplasty: comparable clinical outcomes for the treatment of fracture-related pain. Am J Neuroradiol. 2007;28(7):1266–1270. doi: 10.3174/ajnr.A0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitlow CT, Yazdani SK, Reedy ML, Kaminsky SE, Berry JL, Morris PP. Investigating sacroplasty: technical considerations and finite element analysis of polymethylmethacralate infusion into cadaveric sacrum. Am J Neuroradiol. 2007;28(6):1036–1041. doi: 10.3174/ajnr.A0500. [DOI] [PMC free article] [PubMed] [Google Scholar]