Abstract
Reconstruction of the highly unstable, anteriorly decompressed cervical spine poses biomechanical challenges to current stabilization strategies, including circumferential instrumented fusion, to prevent failure. To avoid secondary posterior surgery, particularly in the elderly population, while increasing primary construct rigidity of anterior-only reconstructions, the authors introduced the concept of anterior transpedicular screw (ATPS) fixation and plating. We demonstrated its morphological feasibility, its superior biomechanical pull-out characteristics compared with vertebral body screws and the accuracy of inserting ATPS using a manual fluoroscopically assisted technique. Although accuracy was high, showing non-critical breaches in the axial and sagittal plane in 78 and 96%, further research was indicated refining technique and increasing accuracy. In light of first clinical case series, the authors analyzed the impact of using an electronic conductivity device (ECD, PediGuard) on the accuracy of ATPS insertion. As there exist only experiences in thoracolumbar surgery the versatility of the ECD was also assessed for posterior cervical pedicle screw fixation (pCPS). 30 ATPS and 30 pCPS were inserted alternately into the C3–T1 vertebra of five fresh-frozen specimen. Fluoroscopic assistance was only used for the entry point selection, pedicle tract preparation was done using the ECD. Preoperative CT scans were assessed for sclerosis at the pedicle entrance or core, and vertebrae with dense pedicles were excluded. Pre- and postoperative reconstructed CT scans were analyzed for pedicle screw positions according to a previously established grading system. Statistical analysis revealed an astonishingly high accuracy for the ATPS group with no critical screw position (0%) in axial or sagittal plane. In the pCPS group, 88.9% of screws inserted showed non-critical screw position, while 11.1% showed critical pedicle perforations. The usage of an ECD for posterior and anterior pedicle screw tract preparation with the exclusion of dense cortical pedicles was shown to be a successful and clinically sound concept with high-accuracy rates for ATPS and pCPS. In concert with fluoroscopic guidance and pedicle axis views, application of an ECD and exclusion of dense cortical pedicles might increase comfort and safety with the clinical use of pCPS. In addition, we presented a reasonable laboratory setting for the clinical introduction of an ATPS-plate system.
Keywords: Cervical pedicle screw fixation, Accuracy, Electronic conductivity device, Insertion technique, ATPS
Introduction
A recent nationwide analysis in the US revealed that in a 3-year period the number of multilevel (≥4) cervical spine surgeries averaged more than 8,500 with a significant morbidity rate related to anterior and in particular to posterior procedures [64]. Surgical rates at the cervical spine increase [20, 80], and extensive surgery at the anterior cervical spine has created a challenge with regard to ensuring stability after reconstruction [4]. Consequently, with the advent of modern generation instrumentations, we observe a growing interest in the techniques, outcomes and also complications of multilevel cervical surgeries [4, 7, 11, 23, 43, 45, 64, 65]. Reconstruction of the multilevel or three-column destabilized cervical spine imposes significant biomechanical challenges on anterior constructs, as is reflected by a significant rate of failures and complications compared with routine cervical procedures [4, 7, 11, 36–38, 41, 54, 65].
In light of the fact that most cervical spine pathologies, including injuries, tumors, infections, and degeneration, cause instability of the anterior portion of the column there is concern that in anterior constructs the screws are anchored in the vertebral body that mainly consists of cancellous bone with a thin cortex [53]. Biomechanical studies of rigid plates demonstrate the dependence on the posterior elements for the maintenance of stability [14, 30, 57] and the number of high quality fixation points, especially the longer the length of the constructs [38]. The high degree of failure rates in anteriorly plated more than two-level corpectomies is a testimony to these challenges, indicating a posterior supplement to prevent failure [4, 11, 17, 25, 37, 38, 45].
Recent clinical series and reviews stress that there are situations that require more immediately rigid techniques of anterior cervical fixation [11], counteracting the translational and rotational forces that act on long constructs and, thus, reducing the need for posterior stabilization. Coincidently, there is an increasing long-term experience with posterior cervical pedicle screw (pCPS) techniques that offer highest anchorage characteristics and construct stability [31, 32, 60, 62, 63, 81, 82]. Consequently, the demands for increased construct rigidity with anterior reconstructions in biomechanical challenging settings and the development of pCPS fixations warranted the introduction of the anterior transpedicular screw (ATPS) plate concept in this Journal [37]. In part I of the project, we demonstrated the anatomical feasibility of ATPS insertion, its implications for a rigid screw-plate system and a set of indications. Part II analyzed pull-out strength characteristics of ATPS and were shown 2.5-fold that of vertebral body screws. Analysis of accuracy using a manual, fluoroscopically assisted technique with the use of pedicle axis views [83] showed that 78% of screws inserted showed no or non-critical screw placements in the axial plane. Although there were no critical breaches at the biomechanically most challenging levels in multi-level constructs, C6–T1 [38, 49, 65], the first results warranted further research.
Thus, in concert with the first clinical case series by one of the authors (YY) the current study targeted at increasing the accuracy of ATPS placement in an in vitro setting. For the purpose of the study, selective instrumentation of non-sclerotic pedicles with the use of an electric conductivity device (PediGuard) was chosen.
Materials and methods
The authors acquired five fresh-frozen cervical spines C0–T2 harvested from one female and four male cadavers (mean age 65 years, range 58–69 years; Science Care, Phoenix, USA). To rule out any structural pathology and to determine morphometry of the vertebrae and pedicles intended for screw placement, the specimens were subjected to multi-slice computerized tomography scanning. The cervical spine CT scans were performed on a 16-row helical CT scanner (Somatom Volume Zoom, Siemens) using a 14–18 cm field of view with 4 × 1 mm collimation and overlapping axial slice thickness of 1 mm. Reformations in sagittal plane were obtained from 1-mm axial reconstructions and stored digitally (PACS Magic View VC 42, Siemens). Excluding the deep lateral neck muscles, capsules and ligaments the specimens were freed from all soft tissues and the spinal processes, laminae and the lateral masses C2–C7 were exposed. The specimens were further procured at the C2–C3 level and the remaining spine was used for assessment of accuracy with ATPS and pCPS.
None of the specimens had evidence of infectious, neoplastic or traumatic disease, nor congenital cervical spine deformation. However, all specimens showed advanced degenerative changes. Using the cursor, digital CT measurements (0.1 mm increments) were performed on the reconstructed CT scans with a commercially available software program (Escape Medical Viewer V3, Escape). The authors measured anatomical parameters prior to ATPS for the purpose of referencing the anatomical dimensions entailed in our specimens compared with normal [37]. The definition of parameters measured were described previously [37]. The description and the results of the measurement process are illustrated in Tables 1 and 2. The CT images were scanned for sclerosis at the anterior or posterior pedicle entrance, center and lateral mass.
Table 1.
Description of anatomical parameters measured
| Parameter | Measurement | Description |
|---|---|---|
| OPW | Outer Pedicle Width | Distance medial border of transverse foramen to medial border of pedicle |
| OPH | Outer Pedicle Height | Distance upper to lower pedicle surface in sagittal plane |
| ltPA | Left transverse Pedicle Angle | Angle formed between transverse pedicle axis and mid-sagittal line |
| lsPA | Left sagittal Pedicle Angle | Angle formed between plane of anterior vertebral body wall at mid-sagittal line and sagittal pedicle axis |
| DltIP/DrtIP | Left/right transverse Intersection Point | Transverse intersection point of transverse pedicle axis with anterior vertebral body wall |
| DrsIP/DlsIP | Left/right sagittal Intersection Point | Sagittal intersection point of transverse pedicle axis with anterior vertebral wall |
Table 2.
Morphometrical measurements of cervical vertebra instrumented at C3 to T1 using ATPS and CPS in all specimen
| Level | OPW (mm) | OPH (mm) | tPA (°) | sPA (°) | DsIP (°) | DtIP (°) |
|---|---|---|---|---|---|---|
| Mean ± SD (range) |
Mean ± SD (range) |
Mean ± SD (range) |
Mean ± SD (range) |
Mean ± SD (range) |
Mean ± SD (range) |
|
| C3 | 5.57 ± 0.37 (4.37–5.82) |
7.47 ± 0.62 (5.9–7.98) |
49.20 ± 2.97 (40.6–51.3) |
38.47 ± 9.89 (74.5–101.7) |
1.54 ± 2.17 (0–3.48) |
2.08 ± 2.40 (0–4.34) |
| C4 | 5.73 ± 0.27 (4.55–6.53) |
7.50 ± 0.73 (5.61–8.43) |
49.60 ± 2.12 (38–54.2) |
88.00 ± 6.80 (88–108.4) |
1.28 ± 1.48 (0–4.41) |
1.75 ± 3.06 (−1.52 to 5.91) |
| C5 | 6.45 ± 0.08 (5.06–6.63) |
7.50 ± 0.48 (5.48–8.15) |
37.30 ± 4.38 37.3–49.8 |
103.5 ± 6.36 (99–117.8) |
4.24 ± 2.21 (1.44–5.97) |
2.56 ± 0.96 (1.88–4.76) |
| C6 | 6.96 ± 0.11 (4.7–7.3) |
7.82 ± 0.01 (6.62–7.83) |
38.47 ± 2.21 (36.9–51.9) |
106 ± 4.83 (103.2–117.4) |
5.60 ± 0.14 (2.8–7.47) |
2.56 ± 1.65 (−3.22 to 2.25) |
| C7 | 7.10 ± 0.02 (6.21–9.64) |
8.12 ± 0.76 (6.17–8.65) |
25.55 ± 2.90 (23.5–44.7) |
106.50 ± 2.91 (98.2–106.5) |
4.41 ± 0.72 (3.18–7.15) |
–4.33 ± 2.15 (−7.25 to 1.52) |
| T1 | 9.10 ± 0.75 (8.57–12.49) |
9.81 ± 0.30 (8.99–11.62) |
20.30 ± 1.41 (19.3–32.3) |
106.50 ± 3.54 (89.7–109.1) |
4.73 ± 1.46 (2.01–8.56) |
–5.34 ± 1.16 (−8.03 to −4.3) |
Two days prior to the screw insertion, the specimens were thawed and placed in a basin to get soaked with saline solution. Then, the specimens were subjected to manual insertion of ATPS and pCPS. As this was the first study using an electronic conductivity device (ECD = PediGuard, SpineVision, Paris, France) for cervical pedicle screws, the authors decided to insert not only ATPS, but also pCPS for the comparison of its general versatility and potential pitfalls. Standard C-arm fluoroscopy was used only for entry point determination and the ECD for intraosseous navigation. The complete process of screw insertion was performed in a radiolucent water basin with the specimen fixed by an assistant.
For the assessment of accuracy of cervical pedicle screw insertion using the ECD, we applied 3.5-mm diameter self-tapping polyaxial cervical pedicle screws (Axon, Synthes, Switzerland). Diameter of ATPS and pCPS used was not matched to the individual pedicle dimension. But according to clinical practice and experiences reported by Magerl [48], vertebrae with complete sclerotic bone formation at either the posterior or anterior pedicle entrance as well as dense cortical pedicles were noted and excluded from statistical analysis (Fig. 1; n = 3: once C3 in the ATPS group and once C3 and C4 in the pCPS group).
Fig. 1.
Illustrative case of a vertebra with dense cortical pedicles. Similar cases were excluded from cervical pedicle screw fixation in the current study
The total of five specimens enabled placement of 30 ATPS and pCPS in a total of 30 vertebrae and 60 pedicles C3–T1, similar to the first study on ATPS [36]. The vertebrae were randomly assigned to undergo ATPS on the left side anteriorly and pCPS on the right side posteriorly and vice versa.
One C3 vertebra fractured during the harvesting procedure and, thus, was excluded from study. At all, 27 screws remained for statistical analysis in the pCPS group and 28 in the ATPS group.
Application of the PediGuard
The PediGuard has been recently introduced in the Journal by Bolger et al. [10]. Further experiences are promising [9, 10]. It is an electric conductivity device (ECD) designed as a free-hand drilling instrument such as the gear shift used for thoracic pedicle screw insertion. Briefly, navigation inside the vertebra with the ECD is based on the medium-related changes of electrical conductivity that are detected and transformed audible by the ECD. Thus, in vivo (in human or in animal studies) the ECD signals homogenous tones when persuading inside cancellous bone; the signal changes when reaching sclerotic islands, cortical bone or breaching it (Fig. 2). To simulate a homogenous viscous environment in our cadaver-based study, the pedicle preparations and screw insertions were performed on saline soaked vertebrae, immerged in a saline water basin (that specific laboratory protocol was validated after pretesting).
Fig. 2.
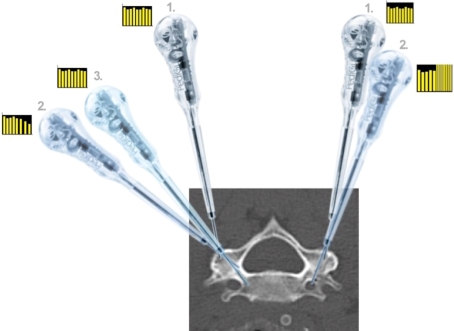
Principles in using an ECD for cervical pedicle tract preparation (Here: pCPS in C5). Right pedicle Following entry point selection and cortex perforation with the ECD (position 1), the ECD is inclined medially in direction of the anatomical pedicle axis (positions 1–2). If there is, e.g., to much a medial inclination the tip of the ECD will face cortical bone. Thus, while progressing the ECD towards the cortical wall, the ECD will emit signals with decreased frequency and sounds signaling that the ECD is at a cortex (position 2). Accordingly, as illustrated, the ECD is only 14 mm inside the vertebra and therefore the surgeon has to redirect the ECD (positions 2–3). After redirection the ECD will protrude again in cancellous bone and the ECD will emit homogenous signals indicating correct pedicle protrusion. Left pedicle Example of perforation of the transverse foramen. As on the right side, the ECD is driven into the lateral mass cancellous bone (position 1). If surgeon does not address the steep medial inclination of the anatomical pedicle axis, but rather goes straightforward he will breach the transverse foramen with the ECD (position 2). However, if this is done slowly, surgeon will face sharp signal changes emitted by the ECD with high pitching and highly frequenced signals indicating redirection of the ECD or abortion of pCPS fixation and change to lateral mass placement for that vertebra. The same principle is with ATPS insertion. The main difference is the larger corridor towards the pedicle and working area
Technique of ATPS and pCPS insertion
The anatomical and technical fundamentals of inserting ATPS and pCPS have been discussed elsewhere [37, 70], including the analysis of results with the use of a manual fluoroscopically assisted insertion technique. In the current study, all screws were inserted by the author. First, the specimens were placed in prone position and instrumented posteriorly, positioning the water basin and surgical towels as to obscure the anterior and lateral vertebral elements normally covered by deep neck muscles. At the anterior part of the cervical spine, the longitudinal muscles were bluntly removed laterally for screw insertion. For pCPS placement, a multi-planar fluoroscope was set to obtain an exact anteroposterior and lateral view of the cervical spine. Because the authors yielded to evaluate the clinical feasibility and the effect of using the ECD in cervical pedicle screw positioning the fluoroscope was only used to verify correct entry point selection.
Concerning pCPS insertion, there are several reports addressing entry point selection at the posterior surface of the lateral mass [15, 32, 40, 82]. In general, preoperative CT scans are analyzed in detail and the imaginary intersection between the pedicle axis and the lateral mass outer surface is determined as entry point. It should be as lateral as possible in the articular mass with its lateral edge serving for reliable guidance, far lateral at C3–C5, going medially to the mid-point of the lateral mass at C7 [70] and close to the superior articular surface in sagittal plane. Under fluoroscopic control, the entry point and pilot hole were determined using the fluoroscopical pedicle axis view and true-lateral view [58, 83]. Vertebral entrance was created with the tip of a small awl just perforating the posterior lateral mass cortex. Then, the ECD was introduced. Subsequent preparation of the pedicle tract was exclusively done with the ECD (Fig. 2). After completion of the procedure, the fluoroscope was brought back to the surgical field and used for documentation of screw positions. 3.5 mm pCPS (length ~20 mm) were inserted not obscuring the track for the following ATPS placement contralaterally.
With ATPS insertion the CT-based preoperative calculations of pedicle axis projection on the anterior vertebral body surface (resembled by data of DlsIP/DrsIP in sagittal plane and DltIP/DrtIP in transverse plane, Table 1) eased templating of the approximated entry points and positioning the tip of the small awl. Pedicle screw tract preparation was initialized similar to the pCPS: following real-time fluoroscopic entry point determination using pedicle axis view and true-lateral view, the anterior vertebral cortex was opened using the small awl. The awl was advanced stepwise with small circular movements for 5 mm without fluoroscopic control. The ECD was then introduced and advanced to the pedicle base. According to signal changes, the cortical entrance to the pedicle was identified and the ECD was advanced through the lateral mass and beyond its cortex. 3.5 mm pedicle screws were inserted (~30 mm).
It is of note that, if there were high frequency signals emitted by the ECD, the position and depth of insertion of the ECD was documented and the ECD was redirected if the surgeon suspected a pedicle breach.
Assessment of accuracy of pCPS and ATPS inserted
Using the same CT-scanning protocol as applied preoperatively, post-procedural CT images were acquired to assess accuracy. Grading of ATPS and pCPS positions was performed as previously established both in the axial and the sagittal plane [36]. Initially five grades were defined according to deviations of the screws from the pedicle wall [36]. Recently, the grades were verified as useful thresholds, distinguishing correct screw position from non-critical and critical screw position [75]: analyzing 127 computer tomography angiographies, Tomasino et al. [75] calculated a safe zone between the lateral border of the cervical pedicles and the vertebral artery (VA) with a mean of 1.2 mm at C3 to 2.0 mm at C6. Finally, the grading defines screw positions as follows:
Screw centered in the pedicle, at most causing only minor plastic deformation of the pedicle cortex.
Screw threads less than one-third of the screw cross-section (≤1.2 mm with a 3.5-mm diameter screw) penetrating the cortex.
Between one-third and one-half of the screw cross-section penetrating the cortex (or deviation <2 mm).
More than one-half of the screw cross-section penetrating the cortex (or deviation ≥2 mm).
Deviation equal to or more than the screw diameter.
Non-critical pedicle breaches were determined as such with grades 1 and 2. Critical pedicle breaches with the potential for posing a risk to the vertebral artery (VA), nerve root or dural sac were determined as those of grades 3–5. Concerning the elaborated safe zone [75] around the cervical pedicles, clinicians would judge grade 3 pedicle screw positions ‘indeterminate’ or ‘borderline’ not causing injury to the VA or nerve root, but rather pushing it away [50, 75]. However, to compare the results to our previous study, we calculated grade 3 positions within critical breaches also in the current study. With the scoring system [36] assessment of pedicle screw position could be performed both in the axial and sagittal plane. Each one point was assigned to each of the five grades of screw position. Finally, the accuracy score in axial plane (range 1–5 points) and the accuracy score in sagittal plane (range 1–5 points) was summed and described as the accuracy sum score with its maximum being 10 points and the minimum being 2, delineating most accurate screw placement.
Statistical analysis
Pearson’s Chi-square test and Fisher’s exact test was used to analyze cross-tabulation tables (e.g., cervical level vs. OPW). Correlation analyses were done using Pearson’s and Spearman’s correlation coefficient (e.g., correlation of critical breach vs. OPW). An ANOVA with post hoc tests was used to analyze data. A P value <5% was considered as statistically significant. All computations and illustrations were done with Statistica 6.1 (StatSoft, Tulsa, USA). All statistical analyses were done by one of the authors (WH). Due to sample size, we did not analyze the specificity of the ECD in the cervical spine as done for the thoracolumbar spine [10]; but descriptive analyses were shown valuable.
Results
Morphological characteristics of specimen
Means, standard deviations and ranges of anatomical parameters measured in the harvested spines were within the upper percentiles of the physiological range [37, 70]. For correlative analysis of, e.g., OPW and axial accuracy, each corresponding pedicle size and pedicle screw inserted was matched. Accordingly, mean outer pedicle width (OPW) and outer pedicle height (OPH) in the pCPS group was 6.9 and 7.6 mm. In the ATPS group, OPW and OPH were 6.9 and 7.7 mm on average. Concerning the morphometrical measurements, OPW and OPH in both groups were not significantly different (P = 0.84 and P = 0.96).
Concerning all vertebrae, both the OPW and the OPH significantly increased from cephalad C3 to caudad T1 (P = 0.0001). Likewise, in the pCPS group, OPW and OPH significantly increased from cephalad C3 to caudad T1 (P < 0.0002, P < 0.000005, respectively). In the ATPS group, OPW and OPH significantly increased from cephalad C3 to caudad T1 too (P < 0.00000, P < 0.00002, respectively).
Accuracy
Concerning ATPS placement, the analysis of CT measurements revealed an accuracy score for the axial plane of 1.2 points (range 1–2) and 1.1 (range 1–2) in the sagittal plane. The accuracy sum score showed a mean of 2.3 points (range 2–4) with a possible total of 10 points. The detailed distribution is listed in Table 3. Astonishingly, the percentage of non-critical screw positions was 100% in the ATPS group using an ECD. There was no critical breach. In the ATPS group, increasing accuracy in the axial plane was reflected by an increased accuracy in sagittal plane (P = 0.02). OPW and OPH showed no significant impact on accuracy in axial or sagittal plane which has to be interpreted in perspective of only grades 1 and 2 screw positions.
Table 3.
Assessment score for cervical transpedicular screw placement
Accuracy of insertion of ATPS C3-T1 using the PediGuard
Critical pedicle breaches shadowed gray
Concerning pCPS placement, mean accuracy in axial plane was scored as 1.4 points (range 1–4) and 1.2 (range 1–4) in sagittal plane. The accuracy sum score showed a mean of 2.6 points (range 2–4) with a possible total of 10 points. The percentage of non-critical breaches in axial plane was 88.9%. Critical breaches occurred in 11.1%. In the sagittal plane, non-critical breaches were detected in 96.3% and critical in 3.7%. The detailed distributions are listed in Table 4. Critical perforations occurred twice at C5 and once at C6 in vertebrae with small pedicle anatomy in terms of pedicle square diameter. In the pCPS group, the level of screw placement showed significant impact on accuracy in axial plane (P = 0.004) with higher accuracy at the lower cervical levels. Whereas we did not find a correlation between accuracy in axial plane, accuracy in sagittal plane was correlative with increased OPH (P = 0.02).
Table 4.
Assessment score for cervical transpedicular screw placement. Accuracy of insertion of pCPS C3-T1 using the PediGuard
Critical pedicle breaches shadowed gray
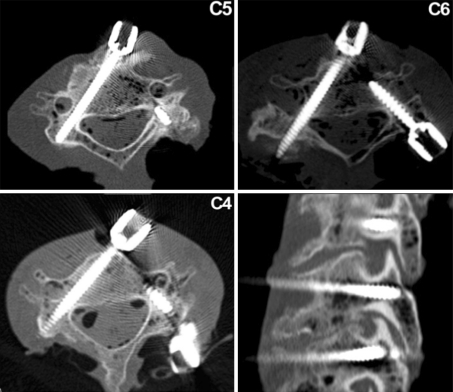
Examples of screw placement on postoperative CT scans are illustrated in Fig. 5.
Fig. 5.
Examples of pCPS and ATPS placements in three different specimens. Axial CT scans and one sagittal view (grade 1 of all screws shown)
In both the groups, the order of screw placement had no impact on accuracy. There was no statistical significant difference in the accuracy rates of ATPS and pCPS in both planes, probably due to the limited sample size. Comparing morphometric and accuracy parameters of the ATPS and the pCPS group, there were also no significant differences in both concerning accuracy scores (P = 0.3/P = 0.6), incidence of non-critical and critical screw placements in axial and sagittal plane (P = 0.1/P = 0.1, P = 0.3/P = 0.3) and the accuracy sum score (P = 0.6/P = 0.6).
We also calculated the results for all cervical pedicle screws placed (ATPS and pCPS). Statistical analysis did not show statistical correlations between accuracy rate and the cervical level. The order of screw placement had no effect on accuracy; the learning curve with ATPS had been climbed previously [36]. Notably, increasing accuracy in the axial plane was reflected by an also significantly increased accuracy in the sagittal plane confirming the trend we observed if the ATPS and pCPS groups were analyzed separately: the larger the pedicle, the easier the screw placement was. However, increasing OPW did not correlate with the accuracy in the axial plane, whereas OPH and accuracy in the sagittal plane did so (P = 0.02). Finally, concerning all screws, the total score as well as the descriptive classification of ‘non-critical’ and ‘critical breaches’ showed no relation to morphometric measurements of OPW, OPH or their product ([OPH × OPW]).
Study report
This study was the authors first time using the ECD. Although the specific saline bathing environment rendered the study conditions difficult, it is of note that after adaptation to the transmitted signal, the navigation inside the cervical vertebrae using the ECD was comfortable. In 28 of 54 pedicle screw placements analyzed, changes of ECD signals indicated redirection needs of the ECD during pedicle tract preparation. In this context, differences between the usage of the ECD in ATPS and pCPS have to be noted. Owing to the smaller arch of possible entry points at the lateral mass and the subsequently smaller safe corridor for pCPS insertion (Figs. 3 and 4), as well as frequently seen small islands of sclerosis inside the lateral mass and at the posterior pedicle entrance, the posterior route to the cervical pedicle was not as convenient and straightforward as was the anterior one. But compared with navigation using anatomical landmarks in combination with fluoroscopy only, the pedicle screw placement was significantly supplemented and eased by the use of the ECD, while providing high-accuracy rates.
Fig. 3.
Differences of safe screw trajectories, corridor of pedicle screw insertion and arch of possible entry points. Closed circle resembles possible entry points for ATPS at the anterior vertebral cortex and for pCPS at the posterior lateral mass
Fig. 4.
Differences of safe pedicle screw angulations in ATPS and pCPS insertions. Note, there is a larger arch of possible screw entry points anteriorly and the safe screw corridor being larger too
Anterior transpedicular screw placement with additional use of the ECD was straightforward. In comparison with the pCPS insertion, there is a wide cancellous working area inside the vertebral body that slightly narrows towards the anterior cortically surrounded pedicle base. Hence, the navigation is easier from anterior as slight changes in signaling along the slowly narrowing pathway towards the pedicle base can be addressed easily by redirecting the protruding ECD. In comparison, from posterior navigation, inside the smaller lateral mass with less cancellous area is more difficult and redirection of the ECD due to signal changes have to be performed in a smaller field. In addition, from anterior, the protruding trajectory of the tip of the ECD directs laterally away from the spinal cord.
Discussion
Reconstruction of most cervical spine pathologies does not require pedicle screw constructs. But, of the techniques available, transpedicular anchorage provides most stability, making it attractive in biomechanically challenging deformities [3, 47, 82]; in selected patients with clear indication and in the hand of experienced surgeons pCPS fixation is a sound treatment [13, 24, 35, 82].
Pedicle perforations with pCPS fixation cause major concerns due to the potential risk of neurovascular injury. The recently reviewed low incidence of serious complications [37, 56] does not suggest that the technique is purely benign. Strong efforts are indicated to increase the accuracy: In 2008, Choi et al. [15] reported a 100% accuracy with the direct visualization of the pedicle entrance in 52 patients with 371 pCPS placed. Ito et al. [32] reported no critical breach beyond 2 mm in 176 pCPS. One of the authors [82] reported the results of 620 pCPS placed in 144 patients using the pedicle axis view technique. The definition of serious pedicle perforations equaled our grades 4–5 [36]. 9.2% of screws placed demonstrated screw exposure (grades 2–3) and only 3.9% showed breaches of grades 4–5. There was one vertebral artery injury (VAI) and radiculopathy without causing long-term sequelae. Hacker et al. [22] demonstrated the contralateral lamina to be a reliable guide in pCPS placement paralleling the contralateral pedicle by direction, with the strong correlation for C3–C5. In addition to a sufficient lateral muscle dissection allowing for correct screw tract orientation in the axial plane at C3–C7 (50°), entry point selection is crucial [6, 70]. Su et al. [70] found that the distances from the projection point of the pedicles on the lateral masses to the horizontal line drawn through the most inferior point of the superior facet did not change significantly from C3 to C7 (2.5–3.5 mm). The distance from the projection point to the vertical line through the most lateral margin of the lateral mass, however, consistently increased from cephalad to caudad (3.5–6.1 mm). Finally, Namikawa’s study [48] stressed that not only the pedicle diameter but also the presence of a cancellous pedicle core is one of the keys to avoid pCPS misplacement. In 560 pedicles (C3–C6), dense and sclerotic ones were identified in up to 15–30%. Dense pedicles were supposed to contraindicate CPS [48]. These most recent studies emphasize that proper pCPS placement is very much dependent on the experience and a detailed understanding of anatomy. Our current results with pCPS using the ECD and excluding dense pedicles showed high accuracy in axial and sagittal plane (88.9 and 96.3%) even though fluoroscopy was not used during the screw tract preparation. Consequently, using fluoroscopical assistance and an ECD, we might increase our accuracy in pCPS placement.
In contrast to the experience with pCPS, the ATPS concept cannot refer to large clinical series. Therefore, we first analyzed the morphometric fundamentals of ATPS [37]. The second step included the biomechanical assessment of ATPS compared with vertebral body screws and the accuracy of insertion of ATPS using a fluoroscopically assisted manual insertion technique [36]. Even during its first usage, we achieved high accuracy in both planes, but the accuracy was supposed to get better with technical refinements proposed in the current study: we chose an ECD instead of computer navigation for practical reasons [55, 77], largely because the usage of the ECD does not alter the common straightforward preparation technique as when using an awl or a gear shift. Application of the ECD in prior series [10] was shown to be a valuable adjunct particularly for narrow pedicles in pediatric scoliosis [9].
With the ATPS insertion, we analyzed the site of entry into the vertebral body on preoperative CT scans and controlled the selected entry point using fluoroscopy. We did not use fluoroscopically guided screw tract preparation and insertion, although recent studies showed that using the coaxial pedicle axis view the pedicle margins are outlined [13, 70] which we confirmed also for ATPS insertions [36]. In the current study fluoroscopy was not used to elucidate the effect of the ECD on accuracy rates. We yielded an astonishingly high-accuracy rate in both planes for both techniques, that of ATPS being even higher than that of pCPS, but we did not find the accuracy being related to OPW. In our previous study [36], there was a strong negative correlation between accuracy in the axial plane and OPW (P < 0.0001), accuracy in the axial plane and the product of OPH × OPW (P = 0.03) as well as the accuracy sum score and OPW (P = 0.001, r = −0.64) indicating that the OPW had a significant impact on the accuracy in axial plane. These discrepancies might reflect the impact of excluding vertebra with ‘sclerotic dense pedicles’. The concept of excluding dense pedicles for screw insertion seems to be a sound principle within a surgical concept. The success is reflected by our high-accuracy rates. In context with yielding for higher accuracy, significant differences between ATPS and pCPS placement have to be stressed. Our study intended to test the ECD in anterior and posterior pedicle screw insertion, thus, we could confirm the recent considerations [6, 70] that might explain higher accuracy with ATPS compared with pCPS: comparing screw insertion points the safe corridor for insertion of ATPS is larger than that of pCPS including a larger range of error margin as illustrated in Figs. 3, 4.
Although the accuracy rate in the current study was high for ATPS there is potential for further refinement of the technique. For pCPS insertion of C2 Ebraheim [19] demonstrated that direct palpation of the Isthmus is a safer guide for screw insertion than generic trajectory guidelines, being confirmed by large clinical series [21, 60]. In the current study direct pedicle palpation for ATPS insertion through a decompression site was not possible because of the study protocol. But, as Choi [15] demonstrated in pCPS placements, and we test in cadavers also by the authors using ATPS, it might further increase three-dimensional imagination of the screw trajectory and accuracy also in ATPS. In concert with laboratory training, preoperative CT angiography (CTA), CT-based assessment of the pedicle anatomy, exclusion of complete sclerotic pedicles for screw anchorage, and use of a manual fluoroscopically assisted insertion technique [82, 83] with supplement of an ECD, safety with ATPS and pCPS fixation can be further increased. Applications of the ECD, as well as other instrumentation techniques, are not recommended in complete sclerotic pedicles as shown by a preoperative CT scan. One ATPS placed in C3 with massive sclerosis at the pedicle base had a grade 4 placement in both planes. The sclerosis was identified on the preoperative CT scans and noted. According to the study protocol the vertebra was instrumented but not included into statistical analysis. It is of interest that the ECD hinted at the pedicle breach by high-signal frequency in that distinct case which was shown a positive prediction for screw misplacement (Fig. 2).
It is important to stress that the concept of a clinically feasible ATPS-plate system incorporates highest precautions for patient safety with respect to a rational risk–benefit analysis. Thus, it emphasizes unilateral transpedicular anchorage of ATPS C3–C6 along common vertebral body screws [37], joining considerations recently presented by Suda et al. [71]. In this concept, a CTA prior to pedicle screw placement is performed intending to detect the dominant VA or a hypoplastic VA. According to the CTA, pedicle screw placement is performed at the side of a non-dominant or hypoplastic VA while lateral mass screws are used on the contralateral sides. Preoperative CTA are crucial [28, 52] for the detection of anomalies and definition of the entrance of the VA into its V2 segment mostly at C6, in 5% at C4–C7 [28, 75]. A recent review of literature [52] identified a reported rate of VAI in anterior cervical spine surgery of 0.2–0.5% compared with 1.3% in posterior surgery using transarticular screws of C1–C2 [50]. In Abumi’s series of 669 pCPS and in Yukawa’s series of 620 pCPS there was only one VAI reported without further sequelae. Even though breaches of the transverse foramen occurred both with pCPS as well as lateral mass screws (up to 8% [76]), the VA is frequently pushed away by the screw traversing the transverse foramen due to the existence of a safe zone [33, 50, 75]. Likewise, new onset nerve root lesions were documented in single cases after pCPS placements [1, 3, 24, 36, 82, 83]. Series on lateral mass screws in 100 patients [18] and in 78 patients [26] reported new radiculopathy symptoms in 4 and 8% of patients, respectively. Luckily many VAI and breaches of the neuroforamen go silent and undetected [2, 24, 35, 50, 52, 73, 80, 81] owing to the existence of a safety zone concerning the cervical pedicle-to-vertebral artery distance as well as larger pedicle heights than pedicle widths. But, VAI occur causing significant sequelae [24, 52] and recently Hasegawa et al. [24] reported in a series of 58 patients undergoing pCPS insertion on two VAI and two other screw-related complications, one causing an upper extremity palsy. Patients (17%) experienced complications related to the pCPS procedure. The authors emphasized the proper selection of indications for pCPS. In this context, a recent series [55] reporting on 27 patients undergoing pCPS fixation deserves comment. There were no neurovascular complications reported and accuracy after 116 pCPS placed ‘satisfactory’ at C2–C7 was reported to be 90% (including grades 1–3 according to our classification). Although computer navigation was used, ‘exact’ pCPS placement was seen in only 55% and ‘sufficient’ pCPS placement was seen in 34%. Critical encroachment by 40–60% of the transverse foramen was noted in 4.3% and the rate of screws at risk was calculated by 15% at C3–C4 and 7% at C5–C7. Postoperative CT scans revealed screw angulations in the axial plane of 22° at C3, 24°–25° at C4–C6 and 17° at C7. The marked difference between the insertion angle and the anatomical pedicle axis were suggested to be caused by limited distraction ability of the neck muscles laterally. Concerning the risk of VAI and nerve root damage, there exist advantages of ATPS. Neck muscles do not hinder large angulations off the sagittal plane and the direction of the pedicle preparation is lateral, off the spinal canal.
The definition of indications for ATPS is crucial for a clinically useful concept. We already stressed clinical scenarios where an ATPS-plate system would be beneficial [36, 37]. There is a current progress with first reports on clinical application of ATPS: Aramoni [6] affixed fibular grafts to cervical pedicles in 9 patients following corpectomies at one to three levels. ATPS were placed with the visualization of the pedicles following corpectomy and k-wire insertions using an in-outside-in fashion as template for subsequent cannulated screws affixing a fibular graft to the pedicles. The screw-fibula constructs were non-constrained, but no displacement of the grafts or neurovascular complications occurred. CT assessment showed that only one screw (4.5%) breached a lateral pedicle wall [6]. A second series on clinical application of ATPS by Y.Yukawa using a non-constrained single screw-plate construct for unstable subaxial injuries is currently in the Journal. Therefore, formulations of indications for ATPS are warranted: literature serves evidence that anteriorly instrumented greater than two-level corpectomy reconstructions should be augmented by posterior instrumentation [4, 11, 17, 23, 25, 37, 38, 45]. Likewise, with the concept of circumferential fusion for greater than two-level corpectomy, deformity needing correction including pseudoarthrosis from prior fusion, and in cases with severe poor bone quality one of the authors [4] reported a 100% success rate in 53 patients with a mean of four levels fused anteriorly (6 posteriorly).
If posterior column insufficiency adjoins the multilevel anteriorly decompressed cervical spine, two-level and even one-level corpectomies warrant circumferential instrumentation or a posterior-only transpedicular construct. Accordingly, indications for ATPS include multilevel neoplastic instability with affection of both the anterior and posterior columns as well as extensive tumor mass resections challenging current instrumentations [38, 44, 46], cervical trauma in patients with ankylosing spondylitis or DISH [46, 66, 79], one- to two-level corpectomy in the setting of fragile edematic/rheumatoid/osteopenic and osteoporotic bone [4, 14, 46], long fusions (≥4 discs) crossing the cervicothoracic junction [4, 7, 51, 65, 67], advanced fracture and discoligamentous cervical instabilities including three-column failure [29, 38, 39], reconstructions in cervical osteomyelitis [59] and revision surgery for cervical non-union following long fusions [12, 65]. Recently, Chen et al. [14] showed that the significant subsidence of mesh cages in two-level corpectomy cause neurologic deterioration from initial recovery after the index surgery in a meaningful number of patients. The anterior approach provides the ability for significant deformity correction through a superior surgical leverage, and technical refinements with rigid instrumentation allow complex anterior reconstructive surgery [45, 51, 61, 68, 69, 78]. Accordingly, correction of cervical kyphosis secondary to degenerative disease, trauma, tumor, surgery, laminectomies or due to systemic arthritis and spondylarthropathies demand increased construct rigidity and transpedicular anchorage techniques [3–5, 45, 51, 68, 69, 74, 78]. Biomechanical studies and clinical series illustrated that increased construct stability by transpedicular anchorage or circumferential stabilization is valuable in many long-segment revision surgeries [4, 29, 38, 45, 55].
Following the implant removals, the anterior spinal segments frequently encounters compromised bone stock often indicating sacrifice of the previously instrumented vertebra. ATPS anchorage would be a valuable option in these cases, particularly in anterior revision at C7 and T1.
Finally, avoiding a posterior approach that is deemed to only augment an anteriorly reconstructed decompression would be valuable, because the addition of an extended posterior approach can cause significant medical and approach-related morbidity [6, 23, 38, 42, 45, 51, 64, 68]. Avoiding morbidity of a second surgery deserves special consideration in the elderly. The number of elderly patients treated for even complex cervical disorders increases carrying a larger number of medical comorbidities and complications [16].
Like Brazenor [11], we stress that there are reasons for attempting to invent an immediately rigid technique of anterior fixation in the cervical spine for selected cases. The concept of an ATPS screw-plate system is not suited to replace common anterior screw-plate systems. It rather resembles an extension of the surgical armamentarium for challenging cervical spine instabilities that are not satisfactorily addressed by current anterior-only strategies. In long instrumented cervical fusions, terminal construct anchors, like pedicle screws, work much like anchors on a suspension bridge, providing robust points of stability to secure the long segmental load of the bridge span [51]. An ATPS-plate system will offer these characteristics.
Limitations
Similar to other studies working on human cadavers, the main limitation of the current study was related to the relatively small number of specimens. The percentage of vertebrae discarded prior to testing varies [8, 27, 72]. In the current study, five pedicles had to be excluded due to the damage during harvesting (n = 2) or revealing a dense cortical pedicle (n = 3). The statistical shortcomings of small sample size and its reasons have been discussed previously [34] and larger laboratory and clinical series are indicated to refine accuracy rates using ATPS. Nonetheless, the current study provides information on technical refinements of ATPS with the use of an ECD as well as on laboratory experience using the ECD for pCPS.
Conclusion
The current study showed that the use of an ECD can be a valuable adjunct in cervical pedicle screw fixation, for both pCPS and ATPS. In this second trial on ATPS refining its insertion technique, the accuracy rate was high, particularly at the biomechanically challenged levels of long cervical fusions, C6–T1. We demonstrated that manual insertion of ATPS supported by an ECD equaled the favorable results obtained with a manual fluoroscopically assisted techniques in our previous laboratory setting. Further studies on ATPS will address whether the combination of both techniques and their net effect will serve for highest accuracy in clinical application of anterior and posterior cervical pedicle screws fixation.
Acknowledgment
The authors did not receive any cent or benefit by the producer of the ‘PediGuard’ for the current investigation.
Conflict of interest statement The authors did not receive any other financial support.
References
- 1.Abumi K, Itoh H, Taneichi H, Kaneda K. Transpedicular screw fixation for traumatic lesions of the middle and lower cervical spine: description of the technique and preliminary report. J Spinal Disord. 1994;7:19–28. doi: 10.1097/00002517-199407010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25:962–969. doi: 10.1097/00007632-200004150-00011. [DOI] [PubMed] [Google Scholar]
- 3.Abumi K, Shono Y, Taneichi H, Itoh M, Kaneda K. Correction of cervical kyphosis using pedicle screw fixation systems. Spine. 1999;24:2456–2462. doi: 10.1097/00007632-199912010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Acosta FL, Jr, Aryan HE, Chou D, Ames CP. Long-term biomechanical stability and clinical improvement after extended multilevel corpectomy and circumferential reconstruction of the cervical spine using titanium mesh cages. J Spinal Disord Tech. 2008;21:165–174. doi: 10.1097/BSD.0b013e3180654205. [DOI] [PubMed] [Google Scholar]
- 5.Acosta AH, Jr, FL Ames CP. Successful outcome of six-level cervicothoracic corpectomy and circumferential reconstruction: case report and review of literature on multilevel cervicothoracic corpectomy. Eur Spine J. 2006;15(Suppl 5):670–674. doi: 10.1007/s00586-006-0203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aramomi M, Masaki Y, Koshizuka Kadota R, Okawa A, Koda M, Yamazaki M. Anterior pedicle screw fixation for multilevel cervical corpectomy and spinal fusion. Acta Neurochir (Wien) 2008;150:575–582. doi: 10.1007/s00701-008-1574-1. [DOI] [PubMed] [Google Scholar]
- 7.Aryan HE, Sanchez-Mejia RO, Ben-Haim S, Ames CP. Successful treatment of cervical myelopathy with minimal morbidity by circumferential decompression and fusion. Eur Spine J. 2007;16:1401–1409. doi: 10.1007/s00586-006-0291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrey C, Mertens P, Rumelhart C, Cotton F, Jund J, Perrin G. Biomechanical evaluation of cervical lateral mass fixation: a comparison of the Roy-Camille and Magerl screw techniques. J Neurosurg (Spine 3) 2004;100:268–276. doi: 10.3171/spi.2004.100.3.0268. [DOI] [PubMed] [Google Scholar]
- 9.Betz R. Clinical application of the pediguard. Geneva/Switzerland: Free Paper Presentation and Work-shop; 2008. [Google Scholar]
- 10.Bolger C, Kelleher MO, McEvoy L, Brayda-Bruno Kaelin A, Lazennec J-Y, Le Huec J-C, Logroscino C, Mata P, Moreta P, Saillant G, Zeller R. Electrical conductivity measurement: a new technique to detect iatrogenic initial pedicle perforation. Eur Spine J. 2007;16:1919–1924. doi: 10.1007/s00586-007-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brazenor GA. Comparison of multisegment anterior cervical fixation using bone graft versus a titanium rod and buttress prosthesis. Spine. 2007;32:63–71. doi: 10.1097/01.brs.0000250304.24001.24. [DOI] [PubMed] [Google Scholar]
- 12.Carreon L, Glassman SD, Campbell MJ. Treatment of anterior cervical pseudoarthrosis: posterior fusion versus anterior revision. Spine J. 2006;6:154–156. doi: 10.1016/j.spinee.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Cha SH, Kim C, Choi BK, Kim HJ, Baek SY. C-arm assessment of cervical pedicle screw: screw coaxial fluoroscopy and oblique view. Eur Spine J. 2007;32:1721–1727. doi: 10.1097/BRS.0b013e3180b9f29b. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Chen D, Guo Y, Wang X, Lu X, He Z, Yuan W. Subsidience of titanium mesh cage: a study based on 300 cases. J Spinal Disord. 2008;21:489–492. doi: 10.1097/BSD.0b013e318158de22. [DOI] [PubMed] [Google Scholar]
- 15.Choi WG, Tsan Y-S, Lee C-J, Lee S-H. Microscopically assisted subaxial cervical pedicle screw fixation. Geneva: Spineweek; 2008. [Google Scholar]
- 16.Cloyd JM, Acosta FL, Ames CP. Effect of age on the perioperative and radiographic complications of multilevel cervicothroacic spinal fusions. Spine. 2008;33:E977–E982. doi: 10.1097/BRS.0b013e31818e2ad7. [DOI] [PubMed] [Google Scholar]
- 17.Daubs M. Early failures following cervical corpectomy reconstruction with titanium mesh cages and anterior plating. Spine. 2005;30:1402–1406. doi: 10.1097/01.brs.0000166526.78058.3c. [DOI] [PubMed] [Google Scholar]
- 18.Deen HG, Nottmeier EW, Reimer R. Early complications of posterior rod-screw fixation of the cervical and upper thoracic spine. Neurosurg. 2006;59:1062–1067. doi: 10.1227/01.NEU.0000245592.54204.D0. [DOI] [PubMed] [Google Scholar]
- 19.Ebraheim N, Rollins JR, Jr, Xu R, Jackson WT. Anatomic consideration of C2 pedicle screw placement. Spine. 1996;21:691–695. doi: 10.1097/00007632-199603150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Epstein NE (2008) What can we learn from a review of medicolegal malpractice suits involving cervical spine surgery. Annual meeting of the CSRS-A, Austin, TX, USA, Paper [DOI] [PubMed]
- 21.Garces-Ambrossi GL, McGirt MJ, Parker SL, Mehta VA, Sciubba DM, Witham TF, Wolinsky JP, Ziya GK (2008) Trans-laminar versus pedicle screw fixation of C2: comparison of surgical morbidity and accuracy of 313 consecutive screws. Annual meeting of the CSRS-A, Austin, Texas, USA: Paper [DOI] [PubMed]
- 22.Hacker AG, Molloy S, Bernard J. The contralateral lamina: a reliable guide in subaxial, cervical pedicle screw placement. Eur Spine J. 2008;17:1457–1461. doi: 10.1007/s00586-008-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart RA, Tatsumi RL, Hiratzka JR, Yoo JU. Perioperative complications of combined anterior and posterior cervical decompression and fusion crossing the cervico-thoracic junction. Spine. 2008;33:2887–2891. doi: 10.1097/BRS.0b013e318190affe. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa K, Hirano T, Shimoda H, Homma T, Morita O. Indications for cervical pedicle screw instrumentation in nontraumatic lesions. Spine. 2008;33:2284–2289. doi: 10.1097/BRS.0b013e31818043ce. [DOI] [PubMed] [Google Scholar]
- 25.Hee HT, Majd ME, Holt RT, Whitecloud TS, 3rd, Pemkowski D. Complications of multilevel cervical corpectomies and reconstruction with titanium cages and anterior plating. J Spinal Disord Tech. 2003;16:1–9. doi: 10.1097/00024720-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Heller SD, Sutterlin CE (1995) Complications of posterior cervical plating. Spine 20:2442–2448 [DOI] [PubMed]
- 27.Hitchon PW, Brenton MD, Coppes JK, From AM, Torner JC. Factors affecting pullout strength of self-drilling and self-tapping anterior cervical screws. Spine. 2003;28:9–13. doi: 10.1097/00007632-200301010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Hong JT, Dk Park, Lee MJ, Kim SW, An HS. Anatomical variations of the vertebral artery segment in the lower cervical spine. Spine. 2008;33:2422–2426. doi: 10.1097/BRS.0b013e31818938d1. [DOI] [PubMed] [Google Scholar]
- 29.Hostin RA, Wu C, Perra JH, Polly DW, Akesen B, Wroblewski JM. A biomechanical evaluation of three revision screw strategies for failed lateral mass fixation. Spine. 2008;33:2415–2421. doi: 10.1097/BRS.0b013e31818916e3. [DOI] [PubMed] [Google Scholar]
- 30.Isomi T, Panjabi MM, Wang JL, Vaccaro AR, Garfin SR, Patel T. Stabilizing potential of anterior cervical plates in multilevel corpectomies. Spine. 1999;24:2219–2223. doi: 10.1097/00007632-199911010-00008. [DOI] [PubMed] [Google Scholar]
- 31.Ito H, Neo M, Yoshida M, Fujibayashi S, Yoshitomi H, Nakamura T. Efficacy of computer-assisted pedicle screw insertion for cervical instability in RA patients. Rheumatol Int. 2006;27:567–574. doi: 10.1007/s00296-006-0256-7. [DOI] [PubMed] [Google Scholar]
- 32.Ito Y, Sugimoto Y, Tomioka M, Hasegawa Y, Nakago K, Yagata Y. Clinical accuracy of 3D fluoroscopy-assisted cervical pedicle screw insertion. J Neurosurg Spine. 2008;9:450–453. doi: 10.3171/SPI.2008.9.11.450. [DOI] [PubMed] [Google Scholar]
- 33.Jeanneret B, Gebhard JS, Magerl F. Transpedicular screw fixation of articular mass fracture-separation: results of an anatomical study and operative technique. Spine. 1994;19:2529–2539. doi: 10.1097/00002517-199407030-00004. [DOI] [PubMed] [Google Scholar]
- 34.Johnston LT, Karaikovic EE, Lautenschlager EP, Marcu D. Cervical pedicle screws vs. lateral mass screws: uniplanar fatigue analysis and residual pullout strengths. Spine J. 2006;6:667–672. doi: 10.1016/j.spinee.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Kast E, Mohr K, Richter H-P, Börm W. Complications of transpedicular screw fixation in the cervical spine. Eur Spine J. 2006;15:327–334. doi: 10.1007/s00586-004-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koller H, Acosta F, Tauber M, Fox M, Martin H, Forstner R, Augat P, Penzkofer R, Pirich C, Kässmann H, Resch H, Hitzl W. Cervical anterior transpedicular screw fixation (ATPS): Part II. Accuracy of manual insertion and pull-out strenght of ATPS. Eur Spine J. 2008;17:539–555. doi: 10.1007/s00586-007-0573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koller H, Hempfing A, Acosta F, Fox M, Scheiter A, Tauber M, Holz U, Resch H, Hitzl W. Cervical anterior transpedicular screw fixation. Part I: study on morphological feasibility, indications, and technical prerequisites. Eur Spine J. 2008;17:523–538. doi: 10.1007/s00586-007-0572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koller H, Hempfing A, Ferraris L, Meier O, Metz-Stavenhagen P. 4- and 5-level anterior fusions of the cervical spine: review of literature and clinical results. Eur Spine J. 2006;16:2055–2071. doi: 10.1007/s00586-007-0398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koller H, Zenner J, Forstner R, Hempfing A, Maislinger I, Kolb K, Tauber M, Resch H, Mayer M, Hitzl W (2009) Mid- to long-term outcome of instrumented anterior cervical fusion for subaxial injuries. Eur Spine J 18(5):630–653 [DOI] [PMC free article] [PubMed]
- 40.Kotani Y, Abumi K, Ito M, Minami A. Improved accuracy of computer-asisted cervical pedicle screw insertion. J Neurosurg (Spine 3) 2003;99:257–263. doi: 10.3171/spi.2003.99.3.0257. [DOI] [PubMed] [Google Scholar]
- 41.Kwon BK, Vaccaro AR, Grauer JN, Beiner JM. The use of rigid internal fixation in the surgical management of cervical spondylosis. Neurosurg. 2007;60(Suppl 1):S118–S128. doi: 10.1227/01.NEU.0000249222.57709.59. [DOI] [PubMed] [Google Scholar]
- 42.Lu J, Wu X, Li Yonggang, Kong X. Surgical results of anterior corpectomy in the aged patients with cervical myelopathy. Eur Spine J. 2007;17:129–135. doi: 10.1007/s00586-007-0518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier O, Hempfing A, Koller H. Answer to the Letter to the Editor concerning ′4- and 5-level anterior fusions of the cervical spine: “natura non-facit saltus” ‘by Franco JL. Eur Spine J. 2008;17:1121–1122. doi: 10.1007/s00586-008-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melcher RP, Harms J, Boyaci B, Schmelzle R. Resection and reconstruction of destructive lesions of the second cervical vertebra. Eur Spine J. 2008;17:1565. [Google Scholar]
- 45.Mummaneni PV, Dhall SS, Rodts GE, Haid RW. Circumferential fusion for cervical kyphotic deformity. J Neurosurg Spine. 2008;9:515–521. doi: 10.3171/SPI.2008.10.08226. [DOI] [PubMed] [Google Scholar]
- 46.Mummaneni PV, Haid RW, Rodts G., Jr Combined ventral and dorsal surgery for myelopathy and myeloradiculopathy. Neurosurg. 2007;60:S82–S89. doi: 10.1227/01.NEU.0000255376.42099.13. [DOI] [PubMed] [Google Scholar]
- 47.Murakami H, Yamazaki K, Yoshida S, Kazuhiro S, Endo H, Shimamura T. Posterior occipitocervical reconstruction using cervical pedicle screw system for rheumatoid arthritis: comparison with segmental spinal instrumentation. Geneva: Spineweek; 2008. [Google Scholar]
- 48.Namikawa T, Taneichi H, Inami S, Daisaku T, Nakayuki K, Nohara Y. Morphological analysis of the pedicles with risk of screw malplacement in degenerative cervical spine. Geneva: Spineweek; 2008. [Google Scholar]
- 49.Narotam PK, Pauley SM, McGinn GJ. Titanium mesh cages for cervical spine stabilization after corpectomy: a clinical and radiological study. J Neurosurg Spine. 2003;99:172–180. doi: 10.3171/spi.2003.99.2.0172. [DOI] [PubMed] [Google Scholar]
- 50.Neo M, Sakamoto T, Fujibayashi S, Nakamura T. The clinical risk of vertebral artery injury from cervical pedicle screws in degenerative vertebrae. Spine. 2005;30:2800–2805. doi: 10.1097/01.brs.0000192297.07709.5d. [DOI] [PubMed] [Google Scholar]
- 51.O’Shaughnessy BA, Liu JC, Hsieh PC, Koski TR, Ganju A, Ondra SL. Surgical treatment of fixed cervical kyphosis with myelopathy. Spine. 2008;33:771–778. doi: 10.1097/BRS.0b013e3181695082. [DOI] [PubMed] [Google Scholar]
- 52.Peng CW, Chou BT, Bendo JA, Spivak JM. Vertebral artery injury in cervical spine surgery: anatomical considerations, management and preventive measures. Spine J. 2008;9:70–76. doi: 10.1016/j.spinee.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Pitzen T, Barbier D, Tintinger F, Steudel WI, Strowitzki M. Screw fixation to the posterior cortical shell does not influence peak torque and pullout in anterior cervical plating. Eur Spine J. 2002;11:494–499. doi: 10.1007/s00586-002-0447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pitzen T, Drumm J, Steudel WI, Allam Y, Branea I, Sova L. The complication rate in multisegmental cervical spine surgery is at least doubled when compared to routine cervical spine procedures. Eur Spine J. 2008;17:1621. [Google Scholar]
- 55.Rath SA, Moszko S, Schäffner PM, Cantone G, Braun V, Richter HP, Antoniadis G. Accuracy of pedicle screw insertion in the cervical spine for internal fixation using frameless stereotactic guidance. J Neurosurg Spine. 2008;8:237–245. doi: 10.3171/SPI/2008/8/3/237. [DOI] [PubMed] [Google Scholar]
- 56.Reinhold M, Magerl F, Rieger M, Blauth M. Cervical pedicle screw placement: feasibility and accuracy of two new insertion techniques based on morphometric data. Eur Spine J. 2006;16:47–56. doi: 10.1007/s00586-006-0104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Resnick DK, Trost GR. Use of ventral plates for cervical arthrodesis. Neurosurg. 2007;60(Suppl 1):112–117. doi: 10.1227/01.NEU.0000215350.95209.CA. [DOI] [PubMed] [Google Scholar]
- 58.Roh JS, Teng AL, Rice JA, Huang RC, Davis JA (2004) Accurate cervical pedicle screw placement using laser-guided fluoroscopy: the ‘perfect pedicle’ technique. 32nd Annual Meeting, Boston
- 59.Ruf M, Stoltze D, Merk HR, Ames M, Harms J. Treatment of vertebral osteomyelitis by radical debridement and stabilization using titanium mesh cages. Spine. 2007;32:E275–E280. doi: 10.1097/01.brs.0000261034.83395.7f. [DOI] [PubMed] [Google Scholar]
- 60.Russo A, Nguyen Q, Bellabarba C, Brnasford R, Chapman JR (2008) Posterior C2 instrumentation: complications and risk. Annual meeting of the CSRS-A, Austin, TX, USA
- 61.Sakamoto T, Neo M, Nakamura T. Transpedicular screw placement evaluated by axial computed tomography of the cervical pedicle. Spine. 2004;22:2510–2514. doi: 10.1097/01.brs.0000144404.68486.85. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt R, Wilke HJ, Claes L, Puhl W, Richter M. Effect of constrained posterior screw and rod systems for primary stability: biomechanical in vitro comparison of various instrumentations in a single-level corporectomy model. Eur Spine J. 2005;14:372–380. doi: 10.1007/s00586-004-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt R, Wilke HJ, Claes L, Puhl W, Richter M. Pedicle screws enhance primary stability in multilevel cervical corporectomies: biomechanical in vitro comparison of different implants including constrained and nonconstrained posterior instruments. Spine. 2003;16:1821–1828. doi: 10.1097/01.BRS.0000083287.23521.48. [DOI] [PubMed] [Google Scholar]
- 64.Shamji MF, Cook C, Pietrobon R, Tackett S, Brown C, Isaacs RE. Impact of surgical approach on complications and resource utilization of cervical spine fusion: a nationwide perspective to the surgical treatment of diffuse cervical spondylosis. Spine J. 2008;9:10–16. doi: 10.1016/j.spinee.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Shen HX, Buchowski JM, Yeom JS, Liu G, Riew KD (2008) Pseudoarthrosis in multi-level anterior cervical fusion with rhBMP-2 and allograft: analysis of 127 cases with minimum 2-year follow-up. IMAST, Hongong/China, Paper [DOI] [PubMed]
- 66.Smajic S, Krauspe R, Muhr G, Schinkel C (2008) Zervikale Wirbelfrakturen bei Patienten mit ankylosierender Spondylitis. Annual Meeting of the German Societies for Trauma and Orthopedic Surgery, Berlin, FRG, Paper
- 67.Steinmetz MP, Miller J, Warbel A, Krishnaney AA, Bingaman W, Benzel EC. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4:278–284. doi: 10.3171/spi.2006.4.4.278. [DOI] [PubMed] [Google Scholar]
- 68.Steinmetz MP, Stewart TJ, Kager CD, Benzel EC, Vaccaro AR. Cervical deformity correction. Neurosurg. 2007;60(Suppl):S90–S97. doi: 10.1227/01.NEU.0000249237.43354.D9. [DOI] [PubMed] [Google Scholar]
- 69.Stewart TJ, Steinmety MP, Benzel EC. Techniques for the ventral correction of postsurgical cervical kyphotic deformity. Neurosurg. 2005;56(1 Suppl):191–195. doi: 10.1227/01.NEU.0000144496.36844.7B. [DOI] [PubMed] [Google Scholar]
- 70.Su P, Li C, Liu S, Huan D. Pedicle screw fixation of the cervical spine. Clin Orthop Relat Res. 2007;462:99–104. doi: 10.1097/BLO.0b013e3180ebe4e5. [DOI] [PubMed] [Google Scholar]
- 71.Suda K, Kajino T, Moridaira H, Limoto S, Taneichi H. How to avoid fatal vascular complications caused by pedicle screws: surgical strategy for safe screw placement. Geneva: Spineweek; 2008. [Google Scholar]
- 72.Takahashi J, Shono Z, Nakamura I, Hirabayashi H, Kamimura M, Ebara S, Kato H. Computer-assisted screw insertion of cervical disorders in rheumatoid arthritis. Eur Spine J. 2006;16:485–494. doi: 10.1007/s00586-006-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taneichi H, Suda K, Kajino T, Kaneda K. Traumatically induced vertebral artery occlusion associated with cervical spine injuries: prospective study using magnetic resonance angiography. Spine. 2005;30:1955–1962. doi: 10.1097/01.brs.0000176186.64276.d4. [DOI] [PubMed] [Google Scholar]
- 74.Tay B, Campos Daziano C, Geula D, Chi J, Deviren V. Circumferential osteotomy for fixed cervical kyphosis: a novel surgical technique. Geneva: Spineweek; 2008. [Google Scholar]
- 75.Tomasino A, Parikh K, Koller H, Zink W, Tsiouris AJ, Steinberger J, Härtl R (2009) The vertebral artery and the cervical pedicle: morphometrical analysis of a critical neighborhood. J Neurosurg (in review process personal communication) [DOI] [PubMed]
- 76.Varel T, Hamdan M, Rivero Ara, Bosch DA, Layun D. Posterior cervical fusion with lateral mass screws: CT analysis. Eur Spine J. 2008;17:3–25. doi: 10.1007/s00586-008-0617-x. [DOI] [Google Scholar]
- 77.Verlaan JJ. Reviewer’s comment on ‘Placement of C2 laminar screws using three-dimensional fluoroscopy-based image guidance’ by E. Nottmeier and A. Foy. Eur Spine J. 2008;17:616–617. doi: 10.1007/s00586-007-0574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang VY, Aryan H, Ames CP. A novel anterior technique for simultaneous single-stage anterior and posterior cervical release for fixed kyphosis: technical note. J Neurosurg Spine. 2008;8:594–599. doi: 10.3171/SPI/2008/8/6/594. [DOI] [PubMed] [Google Scholar]
- 79.Westerveld LA, Verlaan JJ, Oner FC. Spinal fractures in patients with ankylosing spinal disorders: a systematic review of the literature on treatment, neurological status and complications. Eur Spine J Eur Spine J: 2008;14:5–156. doi: 10.1007/s00586-008-0764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeom JS, Buchowski JM, Park K-W, Chang B-S, Lee C-K, Riew KD. Undetected vertebral artery groove and foramen violations during C1 lateral mass and C2 pedicle screw placement. Spine. 2008;33:E942–E949. doi: 10.1097/BRS.0b013e3181870441. [DOI] [PubMed] [Google Scholar]
- 81.Yoshimoto H, Sato S, Hyakumachi T, Yanagibashi Y, Masuda T. Spinal reconstruction using a cervical pedicle screw system. Clin Orthop Relat Res. 2005;431:111–119. doi: 10.1097/01.blo.0000150321.81088.ab. [DOI] [PubMed] [Google Scholar]
- 82.Yukawa Y, Kato F, Ito K, Horie Y, Nakashima H, Ito Z-y, Wakao N. Placement and complications of cervical pedicle screw in 144 cervical trauma patients using pedicle axis view techniques. Geneva: Spineweek; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yukawa Y, Kato F, Yoshirara H, Yanase M, Ito K. Cervical pedicle screw fixation in 100 cases of unstable cervical injuries: pedicle axis views obtained using fluoroscopy. J Neurosurg. 2006;5:488–493. doi: 10.3171/spi.2006.5.6.488. [DOI] [PubMed] [Google Scholar]