Abstract
Bradycardia and rarely cardiac arrest as a complication of cervical spine injury due to reduced sympathetic activity is well known, which usually settles down in 4–6 weeks of injury. There are few case reports in literature of high cervical spinal cord injury requiring permanent cardiac pacemaker due to this complication, but an injury as low as cervico-dorsal junction requiring permanent cardiac pacemaker has never been reported. A 47-year-old male suffered traumatic C7–D1 dislocation and continued to have severe bradycardia with multiple episodes of cardiac arrest till 2 months after injury, which finally warranted permanent cardiac pacemaker as a life saving measure. Following permanent cardiac pacemaker no cardiac arrest occurred and the patient was successfully rehabilitated. The case directs our attention to a rare complication of cardiac arrest occurring in an injury as low as cervico-dorsal junction when all other causes are ruled out and shows importance of using permanent cardiac pacemaker to ensure patient safety in community.
Keywords: Spinal cord injury, Bradycardia, Cardiac arrest, Permanent cardiac pacemaker, Cervico-dorsal dislocation
Introduction
Bradyarrhythmia is a well-established complication of cervical spinal cord injury [3, 4, 7, 10, 12]. In complete cervical spinal cord injury, Lehman et al. [7] reported bradycardia <60 beats/min in 100% patients and cardiac arrest in 15%.
The need for permanent cardiac pacemaker following cardiac arrest in a high cervical tetraplegic has been reported in literature [6, 11] but cardiac arrest occurring as a complication of cervico-dorsal spinal injury has never been reported.
Case report
A 47-year-old male suffered a traumatic C7–D1 dislocation with ASIA-A tetraplegia. Due to breathing difficulty at the time of admission, he was put on ventilator at positive airway pressure mode. Skull traction was applied and dislocation gradually reduced with increasing weight till 16 kg. He was maintaining his blood gas levels with hypotension (100/60 mmHg) and bradycardia (45–55 beats/min). His baseline electrocardiogram and preoperative blood profile were normal (Fig. 1).
Fig. 1.
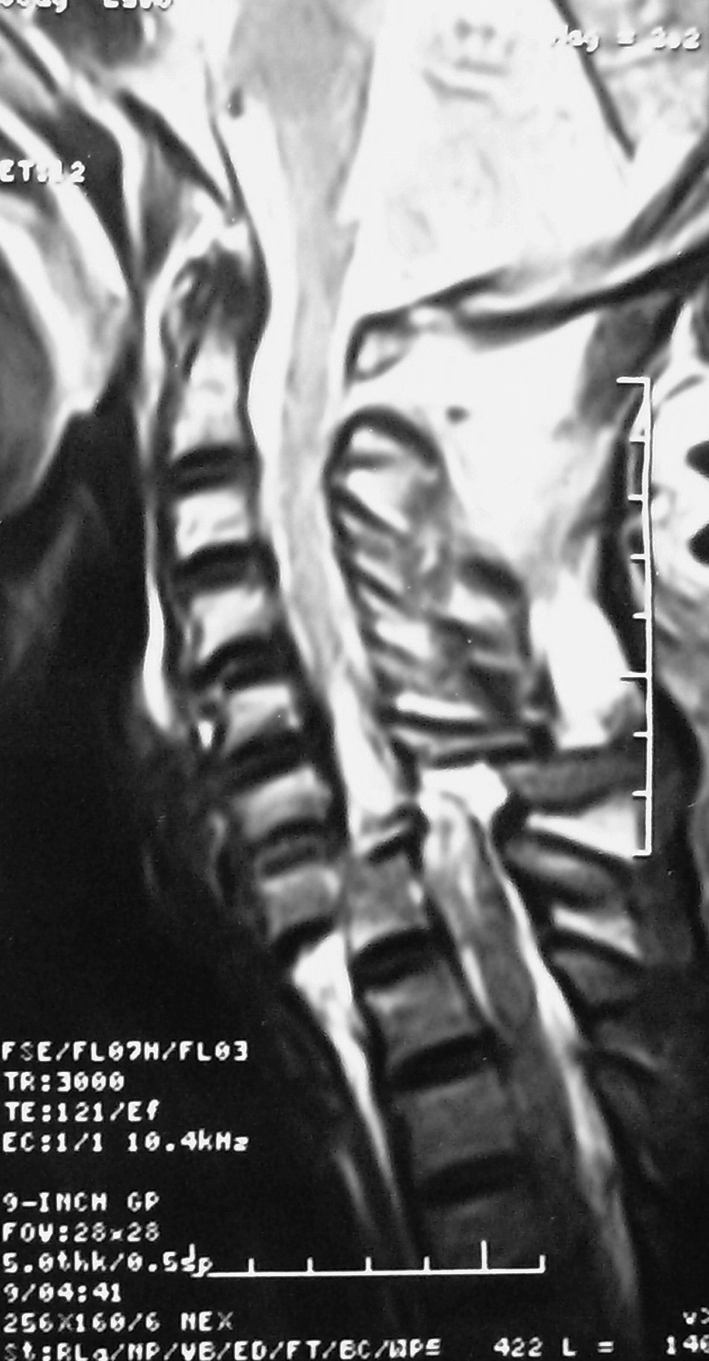
T2 weighted MR Image showing C7–T1 dislocation
On the fourth day, he underwent anterior fusion with tricortical bone graft supplemented with anterior cervical locking plate.
Postoperatively, he continued to have ventilator support and hence tracheostomy was done. On the seventh day, he developed asymptomatic bradycardia as low as 38 beats/min and was corrected with single dose of atropine. On the next day, he developed sudden bradycardia followed by arrest which was revived with cardiac massage and single dose of atropine. Patient was awake just before this episode, without hypoxia or any medical procedures being carried out. He continued to be on ventilator support at positive airway pressure. Again, the same day he developed two more episodes of cardiac arrest which were revived successfully with cardiac massage and single dose of atropine. All three episodes that occurred in the day took less than 1 min for revival. Patient had no cardiac problems in the past and his electrocardiogram following all three episodes showed no abnormalities other than sinus bradycardia. His electrolytes were within normal limits throughout the postoperative period. Later Holter studies showed heart rate varying between 44 and 58 beats/min. The dominant rhythm was sinus bradycardia without any critical arrhythmias. Lack of sympathetic cardiac acceleration due to spinal cord injury was diagnosed as the probable aetiology. Low dose ephedrine (0.5 mg/kg every 8 h) was begun. On the tenth day, he again developed sudden cardiac arrest while he was awake and resting quietly. Rhythm strip documented sinus bradycardia of 44 beats/min changing to sinus pause of 30 s. Later it converted first to premature ventricular contractions followed by sinus tachycardia after resuscitation with atropine.
Continual cardiac instability necessitated temporary cardiac pacemaker set at 65 beats/min. The patient continued to be on ventilator for the next 2 weeks during which he was gradually weaned off. Temporary cardiac pacemaker was removed after 3 weeks of insertion (32nd day following injury) due to risk of infection. There was no arrest during this period of temporary cardiac pacemaker. On the third day after removal of temporary cardiac pacemaker, he again suffered cardiac arrest. Oxygen saturation just before the episode was normal. He was revived, but due to low oxygen saturation and breathing difficulty after revival, he was put on ventilator at positive airway pressure mode. His heart rate was between 48 and 54 beats/min without any critical arrhythmias. He once again needed temporary cardiac pacemaker to avoid further episodes of cardiac instability with a broader antimicrobial cover to decrease the risk of infection. He improved again and weaned from ventilator in a week’s time. He was continued on temporary cardiac pacemaker for about 4 weeks after reinsertion, and it was removed (60th day following injury) considering recovery in sympathetic support due to improvement in blood pressure recordings. But two days after removing temporary cardiac pacemaker, he suffered another cardiac arrest which was again revived and decision made on permanent cardiac pacemaker to prevent any long-term sequelae.
Permanent cardiac pacemaker was put next day (63rd day following injury), following which patient was weaned from ventilator and did not show further cardiac instability till he was discharged fully rehabilitated at 4 months following injury.
Discussion
Severe bradycardia and cardiac arrest as a complication of acute cervical spinal cord injury is well known [4, 7]. It has been observed that higher the level of injury, more profound the clinical consequences of sympathetic dysfunction and hence the need for cardiovascular interventions [3].
Sympathetic innervation to heart arises from neurons in intermediolateral grey columns of cord segments T1–T4 which is under control of higher centres via cervical spinal cord. Parasympathetic innervation comes via vagus nerve. Following any spinal cord injury above T1, the supraspinal control of sympathetic nervous system is lost, making the functioning spinal cord below the lesion independent of higher centres (known as ‘decentralization’ of sympathetic nervous system), resulting in diminished sympathetic activity, while the parasympathetic control remains intact via vagus nerve, which results in relative parasympathetic dominance causing bradycardia and rarely cardiac arrest.
As the period of spinal shock passes the sympathetic activity returns, resulting in gradual improvement in heart rate to normal within 4–6 weeks after injury [4, 7, 12].
This return in sympathetic activity is mainly due to two reasons [12].
First, following acute spinal cord injury, the preganglionic sympathetic neurons undergo atrophy after deafferentation from loss of excitatory inputs of medullary neurons, causing depression of sympathetic activity. With time, these preganglionic sympathetic neurons regain their normal morphology resulting in gradual return of sympathetic activity.
Second, the peripheral vascular alpha receptors become hyper-responsive resulting in enhanced pressor response but has no direct effect on elevation of heart rate [1].
In our patient, the level of injury was cervico-dorsal junction which apart from creating decentralization of sympathetic nervous system, also leads to direct mechanical insult to preganglionic sympathetic neurons in cord segments T1–T3, which probably may lead to neuronal cell death making them unavailable for later recovery in morphology and function. This would lead to permanent withdrawal of major sympathetic cardioaccelerator support, thereby not letting recovery in bradycardia and recurrent cardiac arrest even at more than 2 months following injury.
Since literature [2, 4–6, 8, 9] describes following mechanisms of bradycardia following spinal cord injury, we considered all of them in our case before attributing it to cardiac sympathetic denervation.
Vagal stimulation following tracheal suctioning is a well-known mechanism of bradycardia in tetraplegic due to dysfunction of sympathetic system [5, 6, 9].
Hypoxic episodes can also trigger bradycardia in a tetraplegic patient on assisted ventilation due to lack of pulmonary inflation reflex [2, 5, 6].
In chronic stage, autonomic hyperreflexia can also trigger reflex bradycardia [4, 6, 8].
However, in our patient, all these three mechanisms were ruled out as all the episodes of bradycardia and cardiac arrest occurred when patient was not being suctioned, had no hypoxia or any trigger for development of autonomic hyperreflexia which again is unlikely at such an acute stage.
Clinical evidence against primary cardiac cause probably comes from the fact that the trial with ephedrine as preventive measure resulted in failure. Above all, he never had a single episode of bradycardia or cardiac arrest after temporary/permanent pacemaker insertion, which again rules out primary cardiac aetiology.
Our patient, having a cervico-dorsal injury was expected to recover his sympathetic support to heart in 4–6 weeks time [4, 7, 12], but in view of continual cardiac instability at 9 weeks and clinical evidence of cardiac stability during the period of temporary cardiac pacemaker forced us to consider implantation of permanent cardiac pacemaker. Despite improvement in serial blood pressure readings at the end of 8 weeks following injury, he continued to have cardiac instability as soon as temporary cardiac pacemaker was removed. This forced us to conclude that although recovery in sympathetic tone due to peripheral alpha receptor hyper-responsiveness and morphological recovery of preganglionic sympathetic neurons in functioning spinal cord segments below the injured level had occurred, the injured cord segments of T1–T3 in a cervico-dorsal injury had stopped giving sympathetic cardiac acceleration resulting in persistent bradycardia and cardiac arrest. The justification of using permanent cardiac pacemaker in our case lies in the fact that, in the absence of cardiac pacing, cardiac instability persisted even at more than 2 months after injury, when spontaneous recovery in bradycardia should have occurred [4, 7, 12]. The patient could be stabilised successfully with permanent cardiac pacemaker and completed his rehabilitation.
We therefore conclude that despite being a rare scenario of permanent cardiac pacemaker in an injury as low as cervico-dorsal junction, it should be considered whenever all other possible alternatives are ruled out to ensure patient’s safety in community over a long period.
Conflict of interest statement
None of the authors has any potential conflict of interest.
References
- 1.Arnold JM, Feng QP, Delaney GA, Teasell RW. Alpha-adrenoceptor hyperresponsiveness in quadriplegic patients with autonomic dysreflexia. Clin Auton Res. 1995;5:267–270. doi: 10.1007/BF01818891. [DOI] [PubMed] [Google Scholar]
- 2.Berk JL, Levy MN. Profound reflex bradycardia produced by transient hypoxia or hypercapnia in man. Eur Surg Res. 1977;9:75–84. doi: 10.1159/000127928. [DOI] [PubMed] [Google Scholar]
- 3.Bilello JF, Davis JW, Cunningham MA, Groom TF, Lemaster D, Sue LP. Cervical spinal cord injury and the need for cardiovascular intervention. Arch Surg. 2003;138:1127–1129. doi: 10.1001/archsurg.138.10.1127. [DOI] [PubMed] [Google Scholar]
- 4.Campagnold DI, Merli GJ. Autonomic and cardiovascular complications of spinal cord injury. In: Kirshblum S, Campagnold DI, DeLisa JA, editors. Spinal cord Medicine. Philadelphia: Lippincott Williams Wilkins; 2002. pp. 123–134. [Google Scholar]
- 5.Frankel HL, Mathias CJ, Spalding JMK. Mechanisms of reflex cardiac arrest in tetraplegic patients. Lancet. 1975;2:1183–1185. doi: 10.1016/S0140-6736(75)92662-8. [DOI] [PubMed] [Google Scholar]
- 6.Gilgoff IS, Ward SLD, Hohn AR. Cardiac pacemaker in high spinal cord injury. Arch Phys Med Rehabil. 1991;72:601–603. [PubMed] [Google Scholar]
- 7.Lehmann KG, Lane JG, Piepmeier JM, Batsford WP. Cardiovascular abnormalities accompanying acute spinal cord injuries in humans: incidence, time course and severity. J Am Coll Cardiol. 1987;10:46–52. doi: 10.1016/s0735-1097(87)80158-4. [DOI] [PubMed] [Google Scholar]
- 8.Lindan R, Joiner E, Freehafer AA, Hazel C. Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Paraplegia. 1980;18:285–292. doi: 10.1038/sc.1980.51. [DOI] [PubMed] [Google Scholar]
- 9.Mathias CJ. Bradycardia and cardiac arrest during tracheal suction––mechanisms in tetraplegic patients. Eur J Intensive Care Med. 1976;2:147–156. doi: 10.1007/BF00624607. [DOI] [PubMed] [Google Scholar]
- 10.Piepmeier JM, Lehmann KB, Lane JG. Cardiovascular instability following acute cervical spinal cord trauma. Cent Nerv Syst Trauma. 1985;2:153–160. doi: 10.1089/cns.1985.2.153. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Arango AF, Robinson VJ, Sharma VJ. Characteristics of patients with cervical spinal injuries requiring permanent pacemaker implantation. Cardiol Rev. 2006;14(4):e8–e11. doi: 10.1097/01.crd.0000184453.29079.0f. [DOI] [PubMed] [Google Scholar]
- 12.Teasell RW, Arnold JMO, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506–516. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]


